Published online May 28, 2011. doi: 10.3748/wjg.v17.i20.2580
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: May 28, 2011
Non-cirrhotic portal hypertension is a poorly understood condition characterized by portal hypertension in the absence of conventional hepatic cirrhosis and described in association with blood coagulation disorders, myeloproliferative and immunological diseases and with exposure to toxic drugs. Very recently, precise classification criteria have been proposed in order to define four distinct subcategories. The present case highlights how the clinical presentation, the confounding results from imaging studies, and the difficulties in the histological evaluation often render cases of non-cirrhotic portal hypertension a real diagnostic challenge. It also underscores the classification problems which can be faced once this diagnosis is performed. Indeed, the different subcategories proposed result from the prevalent subtypes in a spectrum of hepatic regenerative responses to a variety of injuries determining microcirculatory disturbances. More flexibility in classification should derive from this etiopathogenic background.
- Citation: Gentilucci UV, Gallo P, Perrone G, Vescovo RD, Galati G, Spataro S, Mazzarelli C, Pellicelli A, Afeltra A, Picardi A. Non-cirrhotic portal hypertension with large regenerative nodules: A diagnostic challenge. World J Gastroenterol 2011; 17(20): 2580-2584
- URL: https://www.wjgnet.com/1007-9327/full/v17/i20/2580.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i20.2580
Non-cirrhotic portal hypertension (NCPH) is a poorly understood condition characterized by the presence of portal hypertension in the absence of conventional hepatic cirrhosis (defined as diffuse nodules totally surrounded by fibrous septa). Its greatest prevalence has been observed in India, where it represents 20%-25% of the cases that bleed due to esophageal varices[1]. It has been described in association with blood coagulation disorders and myeloproliferative diseases[2,3], with exposure to toxic substances or to drugs[4,5], and with immunological diseases[6,7]. Most of the cases present with thrombosis or sclerosis of the intrahepatic portal venous system, with variable involvement of the prehepatic portal system[8]. Several clinical and pathological pictures are included within this syndrome.
The liver morphology of patients with NCPH shows greatly varying alterations which frequently include architectural distortion associated with irregular hyperplastic lesions[9,10]. Considering the features of architectural distortion, hyperplastic nodules and fibrosis, an attempt was made to classify each liver into one of 4 diagnostic categories, following the terminology for hepatic nodular lesions recommended by an International Working Party and the definitional criteria used by Nakanuma et al[11,12]: idiopathic portal hypertension, diffuse nodular regenerative hyperplasia (NRH), partial nodular transformation, and incomplete septal cirrhosis. Differential diagnosis between these categories is often difficult because single cases frequently do not match the estimated definitions, suggesting that each case of NCPH represents the peculiar pattern of expression of a spectrum of lesions with a common or similar etiopathogenetic background[10].
Here we report a case of NCPH with large regenerative nodules, which highlights the difficulties both in reaching the diagnosis and in classifying the single case into one of the proposed categories.
In July 2009, a 62-year-old Italian man was admitted to the Clinical Medicine and Hepatology Unit of our Hospital for recurrent melena.
He had been in good general condition until November 2008, when a first episode of melena occurred in the absence of hematemesis and abdominal pain. Therefore, he was admitted to another hospital, where a severe anemia was found (hemoglobin 5.5 g/dL). A pancolonscopy was negative. Upper intestinal endoscopy showed esophageal varices (not better classified), portal hypertensive gastropathy, and the presence of coagulated blood in the stomach. Upper abdominal ultrasonography revealed hepatomegaly, with caudate lobe hypertrophy, a diffusely dishomogenous echotexture due to the presence of multiple hypoechoic nodules, mainly in the left lobe, the biggest of which was 3 cm in diameter, and mild splenomegaly (transverse diameter: 13.2 cm). Since the patient had no history of chronic liver disease and presented normal liver enzymes and function tests, a transjugular catheterism was performed in order to measure the hepatic pressure venous gradient (HPVG), which confirmed severe portal hypertension (23 mmHg), and to obtain a transjugular liver biopsy, which was negative for cirrhosis-the report of which was the following: “Mild hepatocyte regenerative activity”. The patient had been discharged without a definite diagnosis and with the indication to a strict clinical, biochemical and radiological follow-up.
At the admission to our hospital, laboratory tests showed anemia and leucopenia (hemoglobin 9.9 g/dL, hematocrit 32.9%, leucocyte count 2680/μL, platelets 162 000/μL), a good renal function (urea 36 mg/dL, creatinine 0.78 mg/dL), and normal liver enzymes and hepatic function tests: AST 24 U/L [upper normal limit (u.n.l.) 35 U/L], ALT 21 U/L (u.n.l. 45 U/L), alkaline phosphatase 59 U/L (u.n.l. 120 U/L), GGT 18 U/L (u.n.l. 55 U/L), total bilirubin 0.7 mg/dL, total protein 6.6 g/dL, albumin 58%, gamma-globulins 17.1%, INR 1.1. The patient had no history of associated diseases and was not taking any medication. The blood tests performed in the last few years were also reassessed and showed only impaired glucose tolerance and intermittent mild elevation of the GGT (1.5-2 X u.n.l.).
Viral serology was negative for hepatitis B surface antigen and antibodies to hepatitis C virus. To rule out autoimmune hepatitis, primary biliary cirrhosis, α-1-antitrypsin deficiency, Wilson’s disease, and hemochromatosis, anti-nuclear, anti-mitochondrial, anti-smooth muscle, and anti-liver and kidney antibodies, α-1-antityripsin, serum iron, transferrin and ferritin, ceruloplasmin, cupremia and cupruria were assessed and found to be negative or within the normal ranges. Also serum α-fetoprotein level was within the normal ranges (1.7 ng/mL, u.n.l. 8.1 ng/mL). Upper intestinal endoscopy confirmed esophageal varices (F2 blue, without cherry red spots, hematocystic spots or diffuse redness, but with red wale markings ++/+++), and severe portal hypertensive gastropathy.
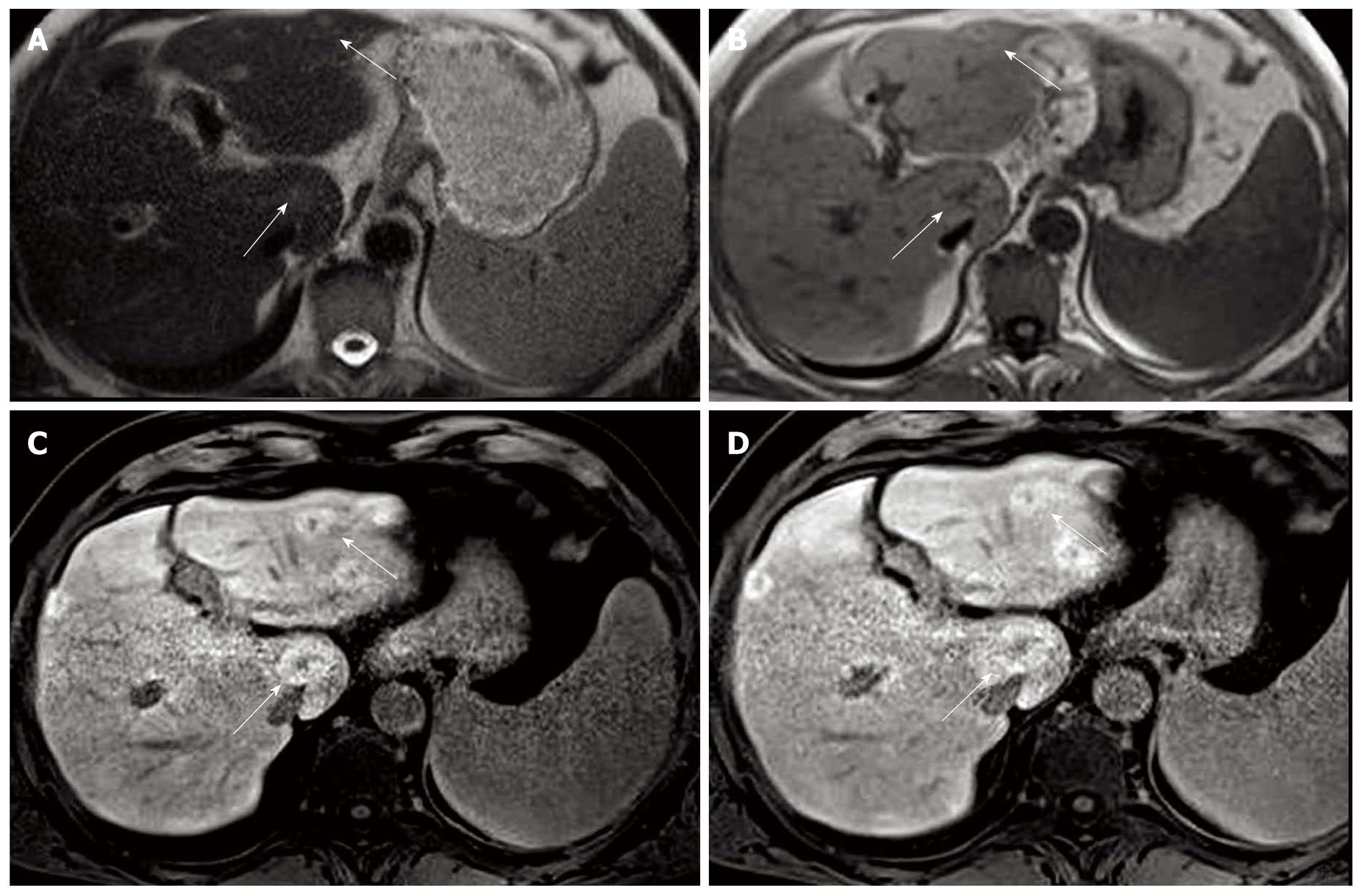
Contrast-enhanced computed tomography (CT) and magnetic resonance (MR) of the upper abdomen were performed. On CT, several slightly hypodense liver lesions (almost 10, on both hepatic lobes) were visualized, showing an inhomogeneous contrast uptake due to the presence of a hypodense central core. On MR, liver morphology was not that of a cirrhotic liver. In basal conditions, the hepatic parenchyma was heterogeneous due to the presence of multiple nodules, the biggest of which was 3 cm in diameter, hypointense on T1-weighted sequences and slightly hyperintense on T2-weighted images (Figure 1). Nodules were better represented after gadolinium injection, showing mild signal hyperintensity. The very first radiological impression was that of multiple liver metastases from endocrine tumor, melanoma or renal cell carcinoma. The lesions were also compatible with large regenerative nodules in a radiologically noncirrhotic liver. The hypothesis of hepatocellular carcinoma was also considered, but not supported by the poor hypervascularization in the arterial phase and by the lack of hypointensity in portal venous and delayed phases.
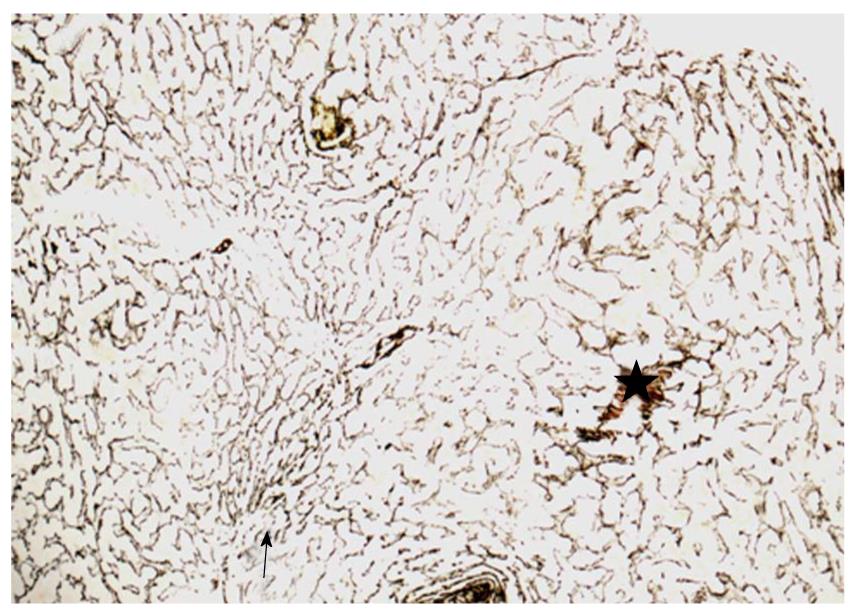
In order to rule out malignancy, a US-guided liver biopsy was performed on the 2-cm-sized lesion in the second hepatic segment. Histologically, small foci of necrosis of isolated hepatocytes with focal accumulation of macrophages and other inflammatory cells, and infiltration of portal tracts by scattered mononuclear cells, were observed. The inflammatory infiltrates slightly involved some portal tracts, and were not accompanied by piecemeal necrosis or fibrosis. As recommended in cases of non-cirrhotic portal hypertension, the silver impregnation stain was performed and did not show any architectural alteration. The final histological diagnosis was nonspecific mild chronic hepatitis. In addition, sections from the transjugular liver biopsy performed in the first Hospital were obtained and reassessed by silver impregnation stain (Figure 2). A vaguely nodular arrangement of liver parenchyma was detected, in which areas with hyperplastic hepatocytes, arranged in plates more than one cell thick, were alternated with areas in which the trabeculae were compressed and atrophic. The interface between nodules was not defined by fibrous septa. There was only minimal inflammation and no evidence of degenerative changes of hepatocytes. Histologically, the diagnosis was NRH. Clinically, the diagnosis was NCPH, in the form of NRH associated with large regenerative nodules.
As NCPH has been described in association with blood coagulation disorders, myeloproliferative diseases, immunological alterations, systemic or intra-abdominal infections and exposure to toxic substances or to drugs, all these conditions were evaluated and excluded by questioning the patient and performing the appropriate investigations. In particular, the complete panel for hereditary thrombotic risk factors was performed. Lupus anticoagulant, protein-C, protein-S, antithrombin, homocysteine, cardiolipin IgG and IgM antibodies, and anti-β2-glycoprotein-I antibodies were in the normal range. The molecular study was negative for factor V H1299R and G1691A, methylenetetrahydrofolate reductase C677T and A1298C, Plasminogen Activator Inhibitor-1 4G/5G, and for prothrombin G20210A gene polymorphisms. In order to rule out myeloproliferative diseases, the V617F Janus kinase-2 gene mutation was investigated and found to be negative. Bone marrow aspiration was not performed due to the lack of sufficient clinical suspicion justifying the procedure.
A first-degree atrioventricular block partially contraindicated beta-blocker therapy and the patient underwent band ligation of esophageal varices. An upper intestinal endoscopy was repeated after one month, revealing persistence of two F1 varices with red wale markings +/+++, which were band-ligated again. A third endoscopic control, performed after another month, was finally negative.
At present, after nearly 1 year of follow-up, the patient is in good clinical condition. The last image studies, MR in January 2010 and ultrasonography in June 2010, did not show significant modifications of the hepatic picture. The last upper intestinal endoscopy, performed in August 2010, showed only one F1 esophageal varix without red wale markings, hematocystic spots or diffuse redness. A long-term clinical, radiological and endoscopic follow-up is warranted.
The present case highlights how the clinical presentation, the confounding results from imaging studies, and the difficulties in the histological evaluation often render cases of NCPH a real diagnostic challenge. It also underscores the classification problems which can be faced once the diagnosis of NCPH is performed.
Even if the exact prevalence and clinical load of NCPH are not known, there are a number of non-cirrhotic patients with long-standing portal hypertension of unknown etiology. Several clinical and pathological pictures are included within this syndrome. Among these, there are some that are morphologically very similar and, differing from country to country and author to author, they have been called idiopathic portal hypertension[13], hepatoportal sclerosis[14], and non-cirrhotic portal fibrosis[15]. In them, the hepatic architecture is mildly distorted due to the existence of irregular distances between the portal venous and hepatic venous structure[16], and nodular regenerative foci are frequently found[17]. In other types of NCPH, greater architectural distortion and prominent regenerative nodulation are found[12]. Only very recently, precise classification criteria have been proposed in order to define only 4 distinct subcategories: idiopathic portal hypertension, diffuse NRH, partial nodular transformation, and incomplete septal cirrhosis[11,12].
The clinical presentation of our case is typical of NCPH, bleeding from esophageal varices, and laboratory data correspond, since liver enzymes are usually normal or slightly elevated and hepatic function is preserved. As in the present case, in needle biopsies of the liver the changes of regeneration and atrophy may be very subtle on routine hematoxylin-eosin stains, and any case of suspected NCPH should be investigated further using reticulin stains[18]. Even the lack of coexisting diseases which are usually associated with NCPH is not infrequent[10]. However, the dominant finding in all the imaging studies performed in our case, i.e. multiple nodules ranging from a few millimetres to 3 cm in diameter, should not be considered typical of NCPH. Indeed, this picture is neither consistent with partial nodular transformation, where macronodules are concentrated around the hepatic ilus[12], nor with NRH, where nodules are usually too small to be radiologically detectable[19]. NRH is characterized by a diffuse nodular transformation of the hepatic parenchyma, without fibrosis separating the nodules. These histological features, consisting of diffuse hyperplastic nodules composed of cells resembling normal hepatocytes, and little or no inflammation or cholestasis, are concordant with the histological findings in our case. However, nodules characterizing NRH are usually 1-3 mm in size, and, although clusters of nodules up to 10 mm in diameter centered on a large portal tract are occasionally found[11], many nodules found in the liver of our patient are significantly bigger. Therefore, in the present case, on the histological background of NRH, nodules much larger than the typical NRH ones were detected by imaging studies.
It has been suggested that all the categories constituting NCPH could represent the same lesional spectrum, the different stages of the same disease or the final stage common to different diseases with varying etiologies. Probably, personal anatomy, patterns of vascularization and damage location influence the final picture. The nomenclature proposed, in many cases, provides an inadequate description of the hepatic morphology, and combinations of morphological patterns are often reported in literature. For example, as Nakanuma et al[12] evidenced in their study, nodular parenchymal hyperplasia without fibrous rim was prominent in all cases of NRH but was found also in one fourth of idiopathic portal hypertension cases, in all partial nodular transformation cases, and in one third of incomplete septal cirrhosis cases, in which it was present in a mild degree and with a focal distribution. Moreover, Ibarrola et al[10] were able to classify only 3 out of their 9 NCPH patients according to accepted terminology, using combined terms to describe the hepatic morphology of another 3 cases, and coined the descriptive terms of “irregular architectural transformation” for the last 3 cases, which showed very grossly deformed livers with irregularly distributed nodules of variable size.
In conclusion, the present case highlights the typical difficulties encountered in the diagnostic and classification processes of NCPH. The different categories of NCPH represent the prevalent subtypes of hepatic regenerative response to a variety of injuries determining microcirculatory disturbances. More flexibility in classification should derive from this etiopathogenic background.
Peer reviewer: Edoardo G Giannini, Assistant Professor, Department of Internal Medicine, Gastroenterology Unit, Viale Benedetto XV, no. 6, Genoa, 16132, Italy
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 2. | Wanless IR, Godwin TA, Allen F, Feder A. Nodular regenerative hyperplasia of the liver in hematologic disorders: a possible response to obliterative portal venopathy. A morphometric study of nine cases with an hypothesis on the pathogenesis. Medicine (Baltimore). 1980;59:367-379. |
| 3. | Wanless IR, Peterson P, Das A, Boitnott JK, Moore GW, Bernier V. Hepatic vascular disease and portal hypertension in polycythemia vera and agnogenic myeloid metaplasia: a clinicopathological study of 145 patients examined at autopsy. Hepatology. 1990;12:1166-1174. |
| 4. | Solis-Herruzo JA, Vidal JV, Colina F, Santalla F, Castellano G. Nodular regenerative hyperplasia of the liver associated with the toxic oil syndrome: report of five cases. Hepatology. 1986;6:687-693. |
| 5. | Vernier-Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, Cadiot G, Bouhnik Y, De Vos M, Boureille A. Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine. Gut. 2007;56:1404-1409. |
| 6. | Sekiya M, Sekigawa I, Hishikawa T, Iida N, Hashimoto H, Hirose S. Nodular regenerative hyperplasia of the liver in systemic lupus erythematosus. The relationship with anticardiolipin antibody and lupus anticoagulant. Scand J Rheumatol. 1997;26:215-217. |
| 7. | Matsumoto T, Kobayashi S, Shimizu H, Nakajima M, Watanabe S, Kitami N, Sato N, Abe H, Aoki Y, Hoshi T. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver. 2000;20:366-373. |
| 8. | Nayak NC. Phlebothrombotic nature of non-cirrhotic portal fibrosis. In Okuda K, Omata M, editors. Idiopathic portal hypertension. University of Tokyo Press. 1983;291-302. |
| 9. | Dhiman RK, Chawla Y, Vasishta RK, Kakkar N, Dilawari JB, Trehan MS, Puri P, Mitra SK, Suri S. Non-cirrhotic portal fibrosis (idiopathic portal hypertension): experience with 151 patients and a review of the literature. J Gastroenterol Hepatol. 2002;17:6-16. |
| 10. | Ibarrola C, Colina F. Clinicopathological features of nine cases of non-cirrhotic portal hypertension: current definitions and criteria are inadequate. Histopathology. 2003;42:251-264. |
| 11. | Terminology of nodular hepatocellular lesions. International Working Party. Hepatology. 1995;22:983-993. |
| 12. | Nakanuma Y, Hoso M, Sasaki M, Terada T, Katayanagi K, Nonomura A, Kurumaya H, Harada A, Obata H. Histopathology of the liver in non-cirrhotic portal hypertension of unknown aetiology. Histopathology. 1996;28:195-204. |
| 13. | Okuda K, Nakashima T, Okudaira M, Kage M, Aida Y, Omata M, Sugiura M, Kameda H, Inokuchi K, Bhusnurmath SR. Liver pathology of idiopathic portal hypertension. Comparison with non-cirrhotic portal fibrosis of India. The Japan idiopathic portal hypertension study. Liver. 1982;2:176-192. |
| 14. | Mikkelsen WP, Edmondson HA, Peters RL, Redeker AG, Reynolds TB. Extra- and intrahepatic portal hypertension without cirrhosis (hepatoportal sclerosis). Ann Surg. 1965;162:602-620. |
| 15. | Aikat BK, Bhusnurmath SR, Chhuttani PN, Mitra SK, Dutta DV. The pathology of noncirrhotic portal fibrosis: a review of 32 autopsy cases. Hum Pathol. 1979;10:405-418. |
| 16. | Okuda K, Kono K, Ohnishi K, Kimura K, Omata M, Koen H, Nakajima Y, Musha H, Hirashima T, Takashi M. Clinical study of eighty-six cases of idiopathic portal hypertension and comparison with cirrhosis with splenomegaly. Gastroenterology. 1984;86:600-610. |
| 17. | Nakanuma Y, Nonomura A, Hayashi M, Doishita K, Takayanagi N, Uchida T, Obata Y, Noma K, Ikoma J, Yoshikawa K. Pathology of the liver in "idiopathic portal hypertension" associated with autoimmune disease. The Ministry of Health and Welfare Disorders of Portal Circulation Research Committee. Acta Pathol Jpn. 1989;39:586-592. |
| 18. | Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology. 2006;44:7-14. |
| 19. | Ames JT, Federle MP, Chopra K. Distinguishing clinical and imaging features of nodular regenerative hyperplasia and large regenerative nodules of the liver. Clin Radiol. 2009;64:1190-1195. |