INTRODUCTION
Alcoholic liver disease (ALD) caused by chronic alcohol consumption shows increased mortality rates worldwide[1,2]. As an adverse risk factor of alcohol abuse, ALD includes a broad spectrum of liver diseases, ranging from steatosis (fatty liver), steatohepatitis, fibrosis, and cirrhosis to hepatocellular carcinoma[3,4]. Generally, steatosis is considered to be a mild or reversible condition, whereas steatohepatitis is a pathogenic condition, which has the potential to progress into more severe diseases, such as liver fibrosis/cirrhosis, insulin resistance, and metabolic syndrome in rodents and humans[5-7]. For the past decade, evidence has suggested that the innate immune cells of liver and hepatic stellate cells (HSCs) play crucial roles in ALD. For example, previous studies demonstrated that alcoholic liver steatosis was induced by HSC-derived endocannabinoid and its hepatic CB1 receptor, and alcoholic liver fibrosis was accelerated due to abrogated antifibrotic effects of natural killer (NK) cells/interferon-γ (IFN-γ) against activated HSCs via the upregulation of transforming growth factor-β (TGF-β) and suppressor of cytokine signaling 1 (SOCS1)[8,9]. However, the molecular and cellular mechanisms underlying ALD remain controversial[4,6,10]. Therefore, in the present review, we briefly describe the innate immunity of liver and HSCs, summarize the roles of these in ALD (with particular emphasis on alcoholic liver steatosis, steatohepatitis and liver fibrosis), and provide better strategies for the prevention and treatment of ALD.
INNATE IMMUNITY AND HSC IN LIVER
The innate immune system is the first line of defense against pathogenic microbes and other dangerous insults, such as tissue injury, stress, and foreign bodies[11]. It consists of three sub-barriers: physical (e.g. mucous membrane and skin), chemical (e.g. secreted enzymes for antimicrobial activity and stomach HCL), and cellular barriers (e.g. humoral factors, phagocytic cells, lymphocytic cells, etc), which immediately respond to the pathogens entering the body. Most body defense cells have pattern recognition receptors (PRRs) that recognize the overall molecular patterns of pathogens, known as pathogen associated molecular patterns. The examples of PRRs are toll-like receptors (TLR), nucleotide-binding oligomerization domain-like receptors, and the retinoic acid-induced gene I-like helicases[12].
When extraneous molecules enter the human body, they have to be processed by the liver, either by metabolism or detoxification. Therefore, the liver is considered as a barrier against pathogens, toxins, and nutrients absorbed from the gut via the portal circulation system. Consequently, the liver is enriched in innate immune system including humoral factors (e.g. complement and interferon), phagocytic cells (e.g. Kupffer cells and neutrophils), and lymphocytes [e.g. NK cells, natural killer T (NKT) cells and T cell receptor γδ T cells][11,13-15]. In a healthy liver, the principal phagocytic cells, the Kupffer cells, representing 20% of the non-parenchymal cells (NPC), assist in the clearance of wastes via phagocytosis in the body[15,16]. However, when the liver is injured, Kupffer cells elicit immune and inflammatory responses (e.g. hepatitis, fibrosis, and regeneration) by producing several mediators, including tumor necrosis factor-α (TNF-α), TGF-β, interleukin-6 (IL-6), and reactive oxygen species (ROS)[17-19]. Among these, TGF-β plays a crucial role in the transdifferentiation of quiescent HSCs into fibrogenic activated HSCs, via the suppression of their degradation and the stimulation of the production of extracellular matrix (ECM), especially in collagen fibers[19-21]. In a healthy liver, liver lymphocytes constitute about 25% of the NPC. Mouse liver lymphocytes contain 5%-10% NK cells and 30%-40% NKT cells, whereas rat and human liver lymphocytes consist of approximately 30%-50% NK cells and 5%-10% NKT cells[11,13,15,16]. These distributions of NK and NKT cells are quite abundant compared with those in peripheral blood, which contains 2% of NKT cells and 13% of NK cells[13]. Previously, NK/NKT cells were regarded to assume a crucial role in mediating the immune responses against tumor and microbial pathogens. However, recent studies have suggested that they contribute significantly to liver injury, regeneration, and fibrosis[22-25].
More interestingly, there are enigmatic cells in the liver that were previously called Ito cells or sinusoidal fat-storing cells, but are now standardized as HSCs[21]. HSCs comprise up to 30% of NPC in the liver and are located in specialized spaces called Disse, between hepatocytes and sinusoidal endothelial cells. In addition, quiescent HSCs store retinol (vitamin A) lipid droplets and regulate retinoid homeostasis in healthy livers. However, they become activated and transformed into myofibroblastic cells that have special features with retinol (vitamin A) loss and enhanced collagen expression when liver injuries occur[19,21,26]. For several decades, activated HSCs have been considered to be major cells that induce liver fibrosis via the production of ECM and inflammatory mediators (e.g. TGF-β) in humans and rodents[19-21]. However, recent studies have suggested that the novel roles of HSCs are closely associated with other diseases, such as alcoholic liver steatosis and immune responses, by producing endocannabinoids and presenting antigen molecules, respectively[8,27,28]. Moreover, HSCs can directly interact with immune cells, such as NK cells, NKT cells and T cells, via the expression of retinoic acid early inducible-1 (RAE1), CD1d, and major histocompatibility complex (MHC) I and II[22,28,29]. During HSC activation, they metabolize the retinols into retinaldehyde (retinal) via alcohol dehydrogenase (ADH), and the retinal is further metabolized into retinoic acid (RA) via retinaldehyde dehydrogenase (Raldh)[3,29]. Surprisingly, activated HSCs express an NK cell activating ligand known as RAE1; however, RAE1 expression is absent in quiescent HSCs. This suggests that the activation processes of HSCs are necessary for the expression of a NK cell activated ligand, RAE1. Furthermore, several TLRs have also been identified in HSCs[30]. Taken together, HSCs might be important not only in liver fibrosis, but also in other liver diseases related to immune responses.
ALCOHOLIC LIVER STEATOSIS BY INNATE IMMUNITY AND HSCS
Alcoholic liver steatosis has long been considered as a mild condition; however, increasing evidence suggests that it is a potentially pathologic state, which progresses into a more severe condition in the presence of other cofactors, such as the sustained consumption of alcohol, viral hepatitis, diabetes, and drug abuse[31,32]. It is believed that fat accumulation in the hepatocytes is a result of an imbalanced fat metabolism, such as decreased mitochondrial lipid oxidation and enhanced synthesis of triglycerides. Several underlying mechanisms of these processes indicate that it might be related to an increased NADH⁄NAD+ ratio[33,34], increased sterol regulatory element-binding protein-1 (SREBP-1) activity[35,36], decreased peroxisome proliferator-activated receptor-α activity[37,38], and decreased AMP-activated protein kinase (AMPK) activity[8,36].
Moreover, recent studies have suggested the involvement of innate immune cells, particularly Kupffer cells, in alcoholic liver steatosis[39,40]. Generally, alcohol intake increases gut permeabilization, which allows an increased uptake of endotoxin/lipopolysaccharide (LPS) in portal circulation[18]. Kupffer cells are then activated in response to LPS via TLR4 signaling cascade, leading to the production of several types of pro-inflammatory mediators such as TNF-α, IL-1, IL-6, and ROS[3,4,39]. Of these mediators, the increased expression of TNF-α and enhanced activity of its receptor (TNF-α R1) have been observed in alcoholic liver steatosis in mice[39-42]. In addition, it has been reported that TNF-α has the potential to increase mRNA expression of SREBP-1c, a potent transcription factor of fat synthesis, in the liver of mice and to stimulate the maturation of SREBP-1 in human hepatocytes[43,44]. Furthermore, a recent report demonstrated that alcohol-mediated infiltration of macrophages decreased the amount of adiponectin (known as anti-steatosis peptide hormone) production of adipocytes, leading to alcoholic liver steatosis[45]. Therefore, Kupffer cells/macrophages might contribute to the development of alcoholic liver steatosis via the upregulation of the SREBP1 activity in hepatocytes and the downregulation of the production of adiponectin in adipocytes. In contrast, IL-6 produced by Kupffer cells/macrophages is a positive regulator in protecting against alcoholic liver steatosis via activation of signal transducer and activator of transcription (STAT)3, consequently inhibiting of SREBP1 gene expression in hepatocytes[46-48].
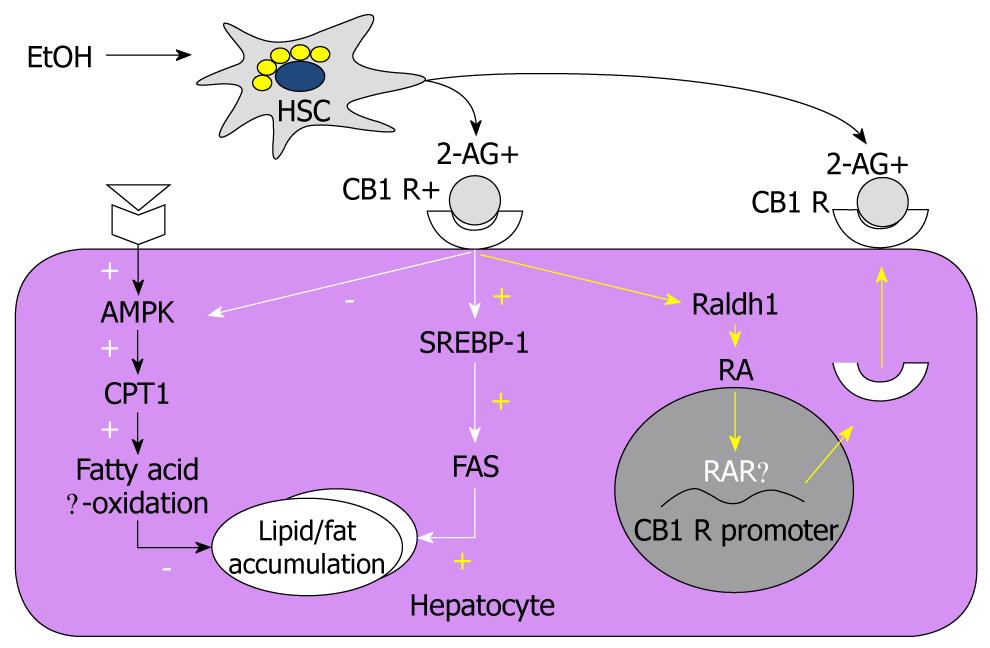
Endocannabinoids, endogenous cannabinoids, are lipid mediators that interact with cannabinoid receptors (CB1 and CB2) to produce effects similar to those of marijuana[49]. There are the two main endocannabinoids, arachidonoyl ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG). Recently, an intriguing report suggested that alcoholic liver steatosis is mediated mainly through HSC-derived endocannabinoid and its hepatocytic receptor[8]. The study suggested that chronic alcohol consumption stimulated HSC to produce 2-AG, and the interaction with the CB1 receptor upregulated the expression of lipogenic genes SREPB1c and fatty acid synthase but downregulated the activities of AMPK and carnitine palmitoyltransferase 1. Consequently fat is accumulated in the hepatocyte. More recently, a related study reported that the increased expression of CB1 receptors on hepatocytes because of alcohol consumption was mediated by RA acting via a RA receptor (RAR)-γ[27]. This study also showed that 2-AG treatment in mouse hepatocytes increased the production of RA by Raldh1, the catalytic enzyme of retinaldehyde into RA. RA then binds with RAR-γ, increasing the expression of CB1 receptor mRNA and protein, and consequently exacerbating the alcohol-mediated fat accumulation via enhanced endocannabinoid and lipogenic signaling pathways[27]. Reports stating that alcohol consumption simultaneously elevated the expression of RAR and the production of retinol metabolites, including RA, in mouse and rat liver, supported these findings[50-52]. Moreover, hepatocytes and HSCs are major sources of retinoids, including retinol and RA, in the body[26,53]. In contrast to the CB1 receptors, the association of CB2 receptors with the development of hepatic steatosis has not yet been studied in depth. One study showed that the expression of CB2 receptors was increased in the livers of patients with non-alcoholic fatty liver disease[54]. In an animal model, however, feeding of high-fat diet for 15 wk induced severe fatty liver in wild-type mice, but not in hepatic CB2 knockout mice[55]. The involvement of endocannabinoid, RA, and their receptors has been integrated in Figure 1.
Figure 1 Regulatory mechanisms of the hepatic lipogenesis and CB1 receptor expression via hepatic stellate cell-derived endocannabinoids/CB1 receptors and retinoic acid/retinoic acid receptor-γ in hepatocytes, respectively.
CB1 R: CB1 receptor; AMPK: AMP-activated protein kinase; HSC: Hepatic stellate cell; 2-AG: 2-arachidonoylglycerol; SREBP-1: Sterol regulatory element-binding protein-1; FAS: Fatty acid synthase; RA: Retinoic acid; RAR: Retinoic acid receptor.
Interestingly, in contrast with previous reports that endocannabinoids activated HSCs to induce liver fibrosis and alcoholic liver steatosis[8,56], Siegmund et al reported that HSCs’ sensitivity to anandamide (AEA)-induced cell death was because of low expression of fatty acid amide hydrolase and that 2-AG also induced apoptotic death of HSCs via ROS induction[57-59]. These data indicated that endocannabinoids might play negative roles in liver fibrosis. Therefore, the functions of endocannabinoids to HSCs are still unclear and need to be studied further.
ALCOHOLIC STEATOHEPATITIS BY INNATE IMMUNITY AND HSCS
Alcoholic steatohepatitis has a mixed status with fat accumulation and inflammation in the liver, which has the potential to progress into more severe pathologic states such as alcoholic liver fibrosis, cirrhosis, and hepatocellular carcinoma. In response to alcohol uptake, many hepatic cells participate in the pathogenesis of alcoholic steatohepatitis. However, as described above, mainly Kupffer cells and HSCs initiate and maintain hepatic inflammation and steatosis[4,8,60-63]. Considering their specific location at the interface between the portal and systemic circulation, Kupffer cells are the central players in orchestrating the immune response against endotoxin (LPS) via TLR4 signaling pathways[62,64]. TLR4 initiates two main pathways, and when TLR4 binds LPS, TIR domain-containing adaptor protein and myeloid differentiation factor 88 (MyD88) are recruited, resulting in the early-phase activation of nuclear factor-κB (NF-κB). The activation of NF-κB leads to the production of pro-inflammatory cytokines, including TNF-α, IL-6, and monocyte chemotatic protein-1 (MCP-1). Meanwhile, TIR-domain containing adaptor inducing IFN-β (TRIF) and TRIF-related adaptor molecule activate interferon regulatory factor 3 (IRF3), leading to the production of type I IFN and late activation of NF-κB[62,65]. Recent studies reported that alcohol-mediated liver injury and inflammation were primarily induced by in a TLR4-dependent, but MyD88-independent, manner in NPCs (Kupffer cells and macrophages), whereas IRF3 activation in parenchymal cells (hepatocytes) rendered protective effects to ALD[66,67]. In addition, the importance of gut-derived endotoxin/LPS in ALD was suggested by experiments where animals were treated with either antibiotics or lactobacilli to remove or reduce the gut microflora provided protection from the features of ALD[68]. Among pro-inflammatory cytokines, TNF-α primarily contributes to the development of ALD, and its levels are increased in patients with alcoholic steatohepatitis[39] and in the liver of alcohol-fed animals[40,69]. Moreover, Kupffer cells secrete other important cytokines, including IL-8, IL-12, and IFNs, which contribute to the intrahepatic recruitment and activation of granulocytes that are characteristically found in severe ALD, and influence immune system polarization[70]. Interestingly, TLR4 is expressed not only on innate immune cells, such as Kupffer cells and recruited macrophages, but also on hepatocytes, sinusoidal endothelial cells, and HSCs in the liver[30].
In addition to LPS, oxidative stress-mediated cellular responses also play an important role in activations of innate immune cells and HSCs. Furthermore, Kupffer cells represent a major source of ROS in response to chronic alcohol exposure[71,72]. One important ROS is the superoxide ion, which is mainly generated by the enzyme complex NADPH oxidase. Underlining the important role of ROS in mediating ethanol damage, treatment with antioxidants and deletion of the p47phox subunit of NADPH oxidase in ethanol-fed animals reduced oxidative stress, activation of NF-κB, and TNF-α release in Kupffer cells, thus preventing liver injury[71,73]. Moreover, NADPH oxidase induces TLR2 and TLR4 expression in human monocytic cells[74], and direct interaction of NADPH oxidase isozyme 4 with TLR4 is involved in LPS-mediated ROS generation and NF-κB activation in neutrophils[75].
Besides Kupffer cells, HSCs also contribute to alcoholic steatohepatitis by producing endocannabinoids and releasing proinflammatory cytokines and chemokines, such as TNF-α, IL-6, MCP-1, and macrophage inflammatory protein-2[63,76-78]. Moreover, Kupffer cells activated by alcohol stimulate the proliferation and activation of HSCs via IL-6 and ROS-dependent mechanisms in a co-culturing system[17,79]. Furthermore, retinol metabolites of HSCs activate latent TGF-β, leading to suppression of apoptosis of HSCs[80-82]. Recently, an intriguing review provided novel roles for HSCs in liver immunology, where HSCs, depending on their activation status, can produce several mediators, including TGF-β, IL-6, and RA, which are important components in naïve T cell differentiation into regulatory T cells (Treg cells) or IL-17 producing T cells (Th-17 cells)[83]. Based on this review, it can be hypothesized that HSCs regulate hepatic inflammation via modulation of T cell differentiation into Treg or Th-17 cell under certain circumstances. However, this remains an unclear proposition; therefore, further studies on the role of HSCs in hepatic inflammatory diseases, including alcoholic steatohepatitis and viral hepatitis, are necessary.
ALCOHOLIC LIVER FIBROSIS BY INNATE IMMUNITY AND HSCS
Chronic alcohol drinking is one of major causes of liver fibrosis, which is characterized by the excessive accumulation of ECM components because an imbalanced ECM degradation and production[6]. However, only 10%-40% of heavy drinkers develop alcoholic liver fibrosis[1,3]. Although the underlying mechanisms of alcoholic liver fibrosis are not yet completely understood, several suggestions have been made in the literature. First, acetaldehyde and ROS generated by hepatic alcohol metabolism activate the production of collagen and TGF-β1 in HSCs through a paracrine mechanism[84,85]. Secondly, hepatocyte apoptotic bodies induced by alcohol are phagocytosed in Kupffer cells and HSCs, resulting in the production of TGF-β1 and subsequently activating HSCs[86,87]. Thirdly, alcohol-mediated activation of Kupffer cells, such as LPS/TLR4 signaling, also activates HSCs via release of cytokines, chemokines, and ROS[17,63,88]. Moreover, TLR4/MyD88 signaling in HSCs enhances TGF-β signaling, inducing liver fibrosis via down-regulation of a transmembrane TGF-β receptor inhibitor, Bambi[89]. Furthermore, it is reported that NADPH oxidase–mediated ROS production contributes to liver fibrosis[90]. However, recent studies have inferred another possibility - that chronic alcohol consumption predisposes NK/NKT cells to decrease in function, which accelerates the development of liver fibrosis[9,91].
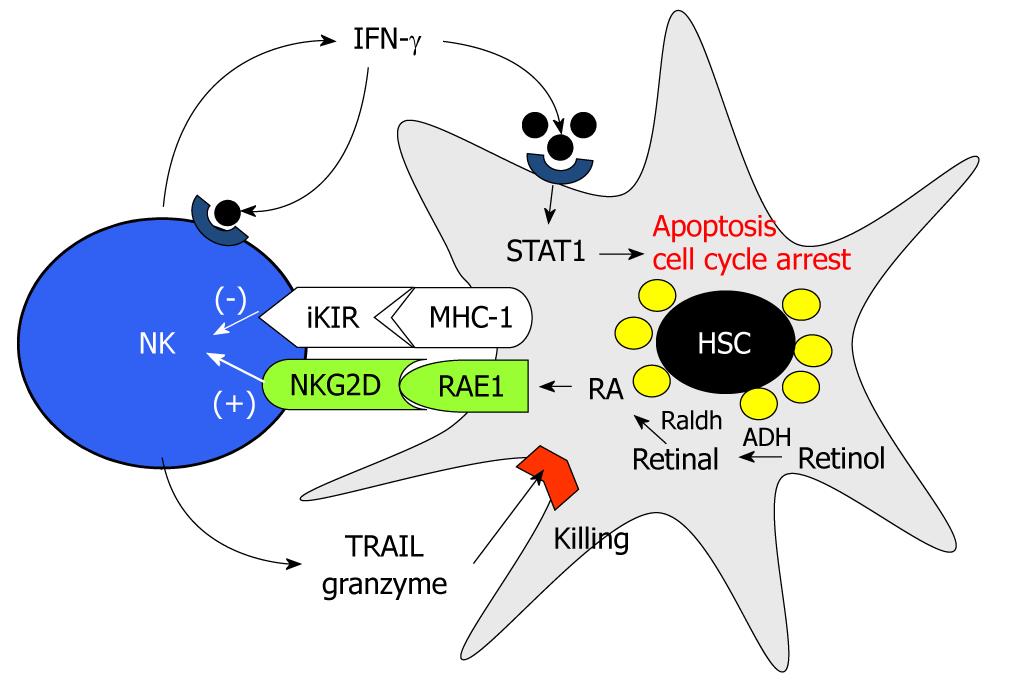
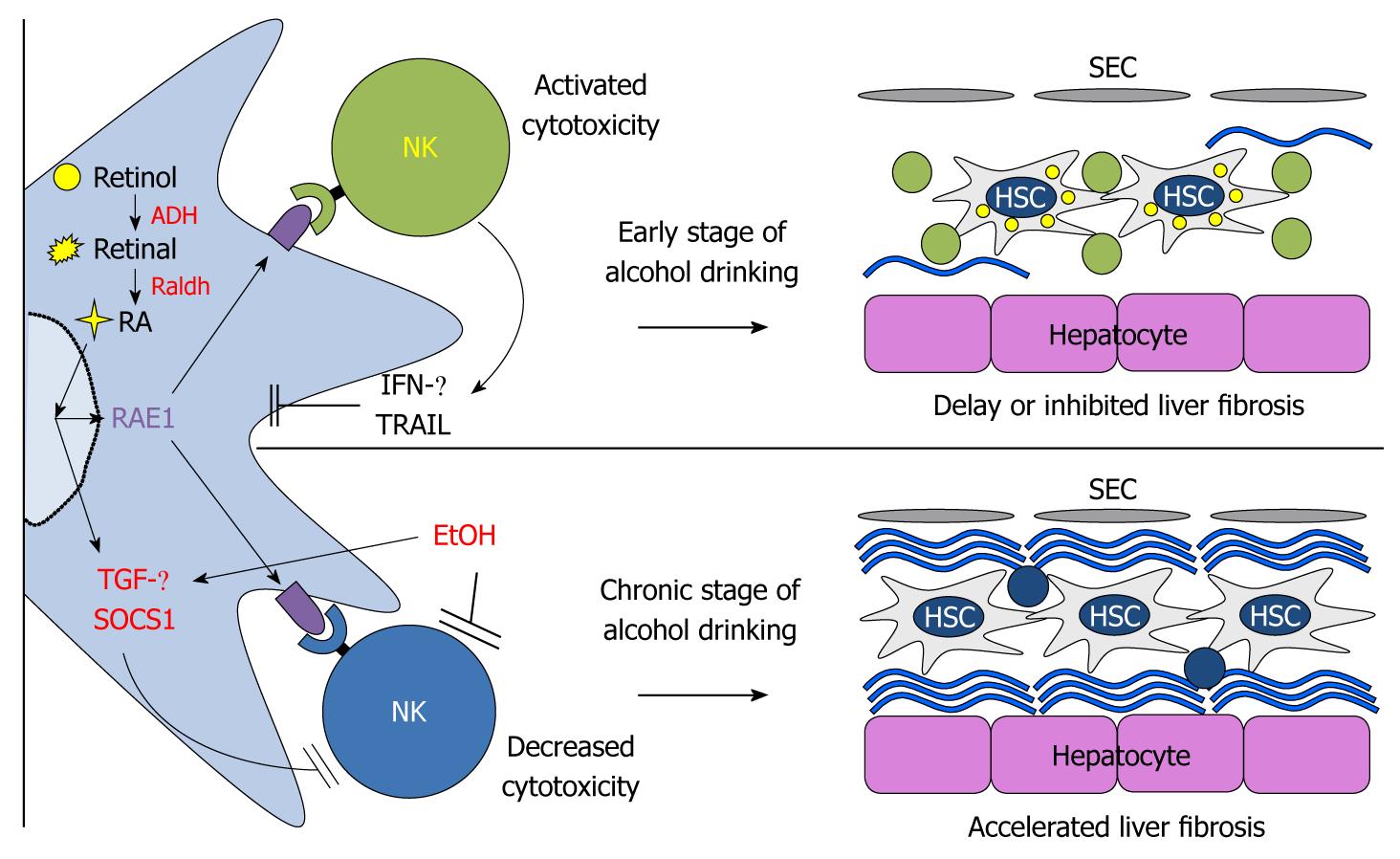
Originally, as we depicted in Figure 2, NK cells have anti-fibrotic effects via several mechanisms. First, NK cells can directly kill activated HSCs by NKG2D- and TNF-related apoptosis, dependent on the induction TRAIL ligand, whereas NK cells cannot induce apoptosis of quiescent HSCs[24,92]. This is because early activated HSCs express NK cell-activating ligand RAE-1, which is an activating ligand of NKG2D on NK cells, by RA and TRAIL receptors, but they express decreased MHC-I, an NK cell-inhibitory ligand[29,92]. Second, NK cells can suppress liver fibrosis via production of IFN-γ, which can induce HSC cell cycle arrest and apoptosis in a STAT1-dependant manner and induce autocrine activation of NK cells[93,94]. Similar to NK cells, NKT cells (invariant NKT cells) can also suppress HSC activation via direct killing and IFN-γ production; however, the anti-fibrotic effects of NKT cells are beneficial only at the onset stage of liver fibrosis because of iNKT depletion tolerance[22]. In contrast, strong activation of iNKT cells by a single injection of α-galactosylceramide adversely enhanced liver fibrosis via highly increased IFN-γ-mediated hepatocyte apoptosis[22]. However, in alcoholic liver fibrosis, it is now accepted that chronic alcohol consumption accelerates liver fibrosis because of the suppressed activity of NK cells (as shown in patients and mice)[9,91,95]. In patients with alcoholic liver cirrhosis, the number and cytolytic activity of peripheral blood NK cells were significantly decreased compared to those of patients without liver disease[95]. In parallel with this report, decreased numbers and cytotoxicity of liver NK cells against HSCs and tumor cells were observed in chronically alcohol-fed mice[9,91]. In addition, direct IFN-γ treatment failed to increase activities of NK cells and to suppress activated HSCs in chronically alcohol-fed mice, showing no beneficial effects of IFN-γ in alcoholic liver fibrosis[9]. These results are possibly due to increased expression and production of TGF-β and SOCS1 by monocytes and activated HSCs[9,96]. We have integrated these findings in Figure 3, and in the case of NKT cells, they seem to contribute to alcoholic liver injury because the activation of NKT cells accelerate alcoholic liver injury while NKT deficiency delays the process[97,98]. Nevertheless, reports on the effects of alcohol on NK/NKT cell functions are still controversial. Therefore, further studies of the effect of alcohol on NK/NKT functions are necessary.
Figure 2 Mechanism of natural killer cell cytotoxicity against activated hepatic stellate cells.
STAT: Signal transducer and activator of transcription; IFN: Interferon; NK: Natural killer; HSC: Hepatic stellate cell; MHC: Major histocompatibility complex; RAE1: Retinoic acid early inducible-1; RA: Retinoic acid; ADH: Alcohol dehydrogenase.
Figure 3 A model for chronic alcohol acceleration of liver fibrosis via inhibition of natural killer cell killing against hepatic stellate cells and suppressor of cytokine signaling 1 suppression of interferon-γ signaling in hepatic stellate cells.
SEC: Sinusoidal endothelial cell; ADH: Alcohol dehydrogenase; HSC: Hepatic stellate cell; RA: Retinoic acid; RAE1: Retinoic acid early inducible-1; IFN: Interferon; NK: Natural killer; TGF: Transforming growth factor; SOCS1: Suppressor of cytokine signaling 1.
Although the underlying mechanisms of liver fibrosis are not clear, alcohol consumption in patients with hepatitis C virus (HCV) infection may accelerate the process. This is because HCV triggers dysfunction and apoptosis of lymphocytes, such as T cells, NK cells, and NKT cells, via NADPH oxidase-derived oxygen radicals, which might be enhanced by alcohol-mediated apoptosis of hepatocyte and ROS production, and subsequently accelerating liver fibrosis[99,100]. In addition, HCV core and nonstructural proteins either induce TLR4 expression in hepatocytes and B cells, leading to enhanced production of IFN-β and IL-6, or enhance the secretion of TGF-β1 and the expressions of procollagen α(I) or α-smooth muscle actin in human-activated HSCs and LX-2 cells[101,102]. Therefore, all these factors and findings may be promoting the effect of alcohol on liver fibrosis in patients with HCV infection.
TREATMENT STRATEGY FOR ALD
In alcoholic patients, the best therapeutic is to reduce ethanol intake significantly, subsequently avoiding further liver injury[1]. However, abstinence is very difficult to achieve. The alternative option is liver transplantation, but donors are relatively scarce[2]. For these reasons, many studies have been performed to determine targets or strategies for treating ALD. Regarding the critical role of TNF-α and ROS in animal models with ALD, several drugs have been developed and are currently available for clinical trial. To suppress the inflammatory responses, phosphodiesterase inhibitor (Pentoxifylline) and corticosteroid therapies were also administered and resulted in reductions of TNF-α, IL-8, and soluble and membranous forms of intracellular adhesion molecule 1 in patients with ALD, via inhibition of activator protein 1 and NF-κB[103-106]. Even though treatments with antioxidants have shown inhibitory effects on alcohol-mediated oxidative stress in animal models, studies of treatment with antioxidants (S-adenosylmethionine, vitamin E, and silymarin, the active element in milk thistle) had no beneficial effects in either patients with alcoholic hepatitis or those with alcoholic cirrhosis[107,108]. In addition, other treatments, such as antifibrotics (colchicines) and nutritional therapies, have been tried, but the effects were minimal. Based on this discrepancy between animal studies and clinical trials, therapeutic strategies should be reconstituted to overcome ALD. For example, treatments for the amelioration of ALD should be targeted simultaneously to HSCs and innate immune cells (e.g. Kupffer cells and NK cells), because these cells can produce endocannabinoid (e.g. 2-AG), inflammatory mediators (e.g. TNF-α, ROS), pro-fibrotic cytokines (e.g. TGF-β), and negative regulators against NK cells (e.g. TGF-β, SOCS1) concurrently in response to chronic alcohol consumption. Thus, we need novel orchestrated strategies, which are capable of enhancing NK cell cytotoxicity while simultaneously suppressing the activation of HSCs and Kupffer cells.
CONCLUSION
The present review summarized the pathogenesis of ALD, in which NK cells, Kupffer cells and HSCs are highly involved. Alcohol-mediated activation of Kupffer cells appears to be required for the development of alcoholic steatohepatitis via LPS-TLR4 signaling pathways. In addition, alcohol-induced paracrine activation of HSC-derived endocannabinoid in hepatocytes might be a major factor in the induction of alcoholic steatosis. Furthermore, both Kupffer cells and HSCs play important roles in alcoholic liver fibrosis via the suppression of the antifibrotic effects of NK cells. Therefore, the interactions among them should be simultaneously considered when developing therapeutics for ALD. For example, even though Kupffer cells are appropriately suppressed by a certain drug, alcohol-activated HSCs still might enhance the accumulation of fat in the liver, leading to lipotoxicity, which in turn generates oxidative stress and inflammation, subsequently restoring steatohepatitis. Besides, functions of NK cells are abrogated or suppressed by alcohol-induced ROS and high levels of TGF-β in the liver. Thus, additional antioxidant and neutralizing TGF-β1 antibody treatment may have beneficial effects in slowing down ALD. Conclusively, further studies to elucidate the roles of innate immunity and HSCs might aid in the development of novel therapeutic targets for the treatment of ALD.
Peer reviewers: Ekihiro Seki, MD, PhD, Department of Medicine, University of California SanDiego, Leichag Biomedical Research Building Rm 349H, 9500 Gilman Drive MC#0702, La Jolla, CA 92093-0702, United States; Atsushi Masamune, MD, PhD,Division of Gastroenterology, Tohoku University Graduate School of Medicine,1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan
S- Editor Tian L L- Editor Stewart GJ E- Editor Zheng XM