Published online May 28, 2011. doi: 10.3748/wjg.v17.i20.2500
Revised: April 19, 2011
Accepted: April 26, 2011
Published online: May 28, 2011
Clinical observations have demonstrated that excessive chronic alcohol use negatively affects human immunodeficiency virus (HIV) infection and contributes to the liver manifestations of the disease, even in HIV mono-infection. HIV/hepatitis C virus (HCV) co-infection is associated with increased progression of HVC liver disease compared to HCV infection alone, and both of these are negatively affected by alcohol use. Recent data suggest that alcohol use and HIV infection have common targets that contribute to progression of liver disease. Both HIV infection and chronic alcohol use are associated with increased gut permeability and elevated plasma levels of lipopolysaccharide; a central activator of inflammatory responses. Both alcoholic liver disease and HIV infection result in non-specific activation of innate immunity, proinflammatory cytokine cascade upregulation, as well as impaired antigen presenting cell and dendritic cell functions. Finally, alcohol, HIV and antiretroviral therapy affect hepatocyte functions, which contributes to liver damage. The common targets of alcohol and HIV infection in liver disease are discussed in this mini-review.
- Citation: Szabo G, Zakhari S. Mechanisms of alcohol-mediated hepatotoxicity in human-immunodeficiency-virus-infected patients. World J Gastroenterol 2011; 17(20): 2500-2506
- URL: https://www.wjgnet.com/1007-9327/full/v17/i20/2500.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i20.2500
The life expectancy of patients infected with human immunodeficiency virus (HIV) has been prolonged, therefore, liver diseases have assumed far greater importance as a cause of morbidity and mortality in these patients. Antiretroviral therapy (ART) used by patients who have HIV infection is often hepatotoxic. In addition, patients who have HIV often are co-infected with hepatotropic viruses such as hepatitis B and C viruses; factors that are damaging to the liver. Furthermore, chronic alcohol consumption causes injury to the liver and may result in more rapid progression to cirrhosis, end-stage liver disease, and hepatocellular carcinoma in co-infected patients. This review addresses the interactions between these factors that result in furthering liver damage.
The natural history of HIV infection has drastically changed since the discovery of its clinical manifestation, AIDS in advanced disease[1-3]. HIV affects about 40 million people worldwide, with most of the infections in developing countries. As a blood-borne pathogen, HIV infection is transmitted via blood, sexual contact, and maternal-newborn transfer. HIV primarily infects immune cells and it replicates in T lymphocytes and monocytes[4-7]. HIV mono-infection results in minimal hepatitis, however, co-infection with hepatitis virus results in increased liver damage. Hepatitis B virus (HBV) infection affects 370 million people and it is estimated that an additional 170 million suffer from hepatitis C virus (HCV) infection[8-13]. Both HBV and HCV can lead to chronic hepatitis and as a result, progressive liver inflammation and fibrosis lead to cirrhosis and liver failure. An additional insult to the liver is excessive alcohol consumption that is an ongoing social and medical problem in people with and without HIV infection.
Several studies have found significant alcohol problems in HIV-infected individuals. In a primary care setting, physicians have reported that alcohol consumption is a common habit in patients initiating care for HIV disease[14-16]. Heavy drinking among people with HIV infection under medical care has been found to be almost double that of the general population[17]. It has also been shown that heavy alcohol consumption has a negative impact on CD4 cell count in HIV-infected persons who are not receiving ART[18].
The interaction of HIV and HCV infection has received increasing attention in recent years. It has been well documented that liver disease progression is accelerated in HIV/HCV co-infected patients compared with HIV or even HCV mono-infection[11,13,19]. Furthermore, chronic heavy alcohol consumption acts as an additional insult in all of these forms of chronic hepatitis. With the new era of highly active antiretroviral therapy (HAART) that provides sufficient control of HIV replication, it has become evident that, in HIV/HCV co-infected patients, progression of HCV infection causes more lethality than HIV infection itself[11,13,19].
Excessive alcohol consumption is associated with fatty liver, and if persistent, it can lead to alcoholic steatohepatitis, liver fibrosis and cirrhosis[20]. Clinical studies have demonstrated that excessive alcohol consumption in individuals with comorbid conditions such as chronic HCV or HBV infection accelerates liver damage and progression to liver cirrhosis. In a study of a large cohort of HIV-positive and negative US veterans, investigators have found a trend toward increased liver injury manifested as inflammation and fibrosis, in patients with hazardous or binge drinking[21]. The same study has shown that alcohol abuse and dependence significantly increase the risk of advanced fibrosis and cirrhosis in HIV-mono-infected patients, as well as in those with HIV/HCV co-infection[21]. A cross-sectional study has investigated the impact of alcohol on liver fibrosis using aspartate aminotransferase (AST)-to-platelet ratio index (APRI) as a measure of liver function, and has found significant liver disease with an APRI > 1.5. This study also has found that hazardous alcohol drinking is an independent, modifiable risk factor for fibrosis[21], and the same investigators have concluded that the problem of alcohol abuse is not adequately addressed by health care providers in patients with HIV positivity[21].
In HIV-infected patients, 17% of death was related to end-stage liver disease; of those, 75% of patients had HIV/HCV co-infection and 48% heavy alcohol use history[22]. Alcohol use in HIV-infected patients was also shown to be associated with decreased adherence to ART, and not surprisingly, reduced HIV suppression[23].
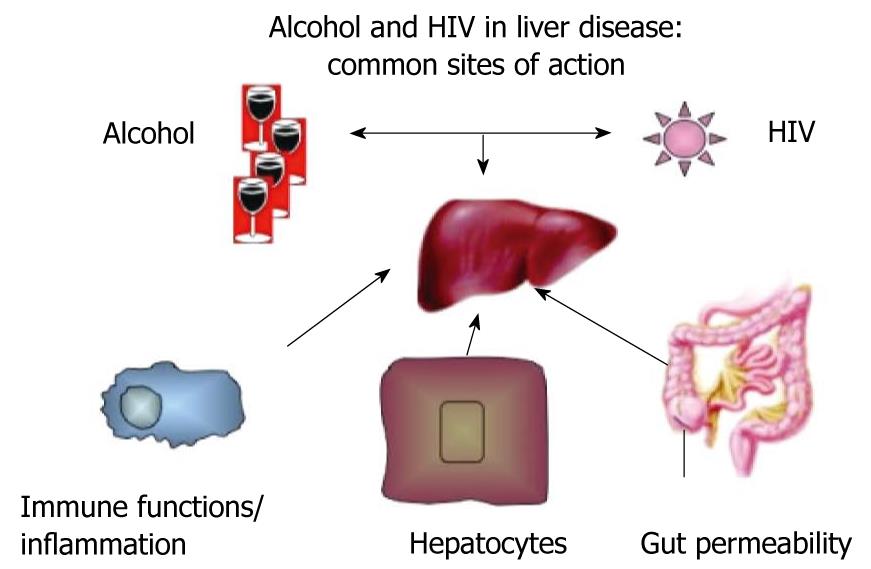
Although the pathological mechanisms of alcoholic liver disease are relatively well explored[24-26], the mechanisms by which HIV infection affects the liver remain somewhat elusive. Based on current knowledge, several elements in the pathological mechanisms of alcohol-induced liver disease can by exacerbated by HIV infection, which results in the potentiation of the individual negative effects. These major categories of mutual actions and modulating effects of both alcohol and HIV infection are on gut permeability and gut-liver homeostasis, immune functions and inflammation, and alterations in hepatocyte functions and survival (Figure 1).
The important role of gut-derived lipopolysaccharide (LPS) has been demonstrated in alcoholic liver disease in humans, as well as in animal models of chronic alcohol administration[27,28]. Gut-derived LPS has been shown to have a causative role in alcoholic liver injury as demonstrated by experiments in which gut sterilization with antibiotics prevented alcohol-induced liver damage[29]. This was associated with increased intestinal permeability in rats[30]. Alcohol feeding in rats and mice increases serum endotoxin levels after 1-2 wk, and these increases remain after continued alcohol administration[31]. Studies in mice as well as in vitro have demonstrated that alcohol damages the intestinal epithelial barrier by downregulating tight junction proteins[32-34]. The importance of the gut-liver axis has been implicated in alcoholic liver diseases and in other etiologies of liver injury[27,28].
The novel finding, that HIV infection is associated with increased plasma endotoxin levels was first reported by Douek’s group in 2006[35]. Plasma LPS was significantly increased in patients with chronic HIV infection, as well as in patients with AIDS compared to uninfected controls or to those with acute infection[35]. LPS in the serum is bound to lipoprotein binding protein (LBP) and soluble CD14 (sCD14) that modulate the biological availability of LPS. The same authors have found increased plasma sCD14 and LBP levels even in acute HIV infection, as well as in those with progressive HIV infection[35]. Subsequent studies have evaluated the effect of ART on plasma LPS levels and have found that patients with ART have a moderate decrease in plasma LPS levels[36]. This is also associated with an improvement in plasma tumor necrosis factor (TNF)α levels, which suggests attenuation of inflammatory cell activation[36-38]. The source of the increased plasma LPS in HIV infection is presumably the gut. It has been speculated that HIV infection in the intestinal epithelial and immune environment permits increased translocation of gut microbiota and their components such as LPS[39].
Increased serum LPS levels have been noted in patients with chronic HCV infection, even in the absence of advanced liver disease[40]. Furthermore, in patients with HCV and HIV co-infection, serum LPS levels are higher compared to those in patients with HIV infection alone[41].
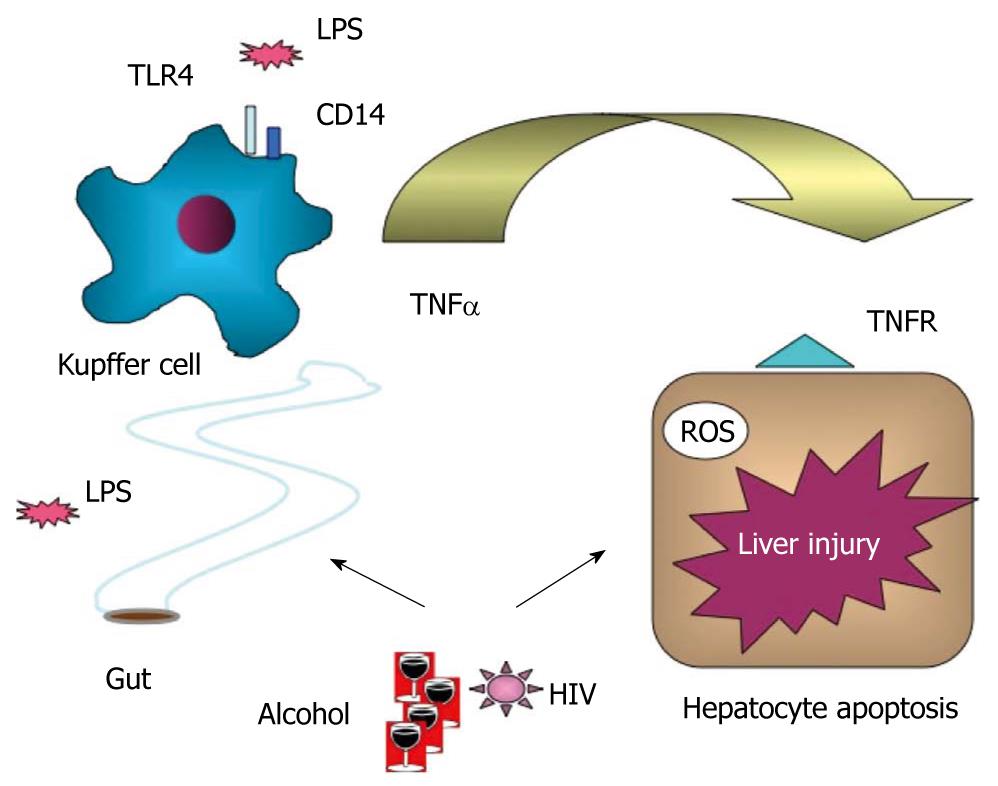
The biological significance of the elevated serum or plasma LPS in HIV infection is yet to be fully understood. It is generally accepted that chronic LPS exposure of immune and inflammatory cells results in a non-specific inflammation that may “reset” thresholds for specific immune responses[42]. It is tempting to speculate that constant and repeated exposure of innate immune cells, particularly tissue macrophages (Kupffer cells) in the liver from the gut-derived LPS in the portal blood, could result in loss of LPS tolerance, which predisposes to increased inflammatory responses[43]. We have found loss of Toll-like receptor (TLR) tolerance in circulating monocytes of HCV-infected patients, and this is associated with increased production of proinflammatory signals including TNFα[40].
In alcoholic liver disease, there is a large body of evidence in support of increased sensitivity of Kupffer cells to LPS and proinflammatory cytokine activation[44,45] (Figure 2). Early studies have demonstrated that elimination of Kupffer cells with gadolinium chloride in rats ameliorates alcohol-induced liver damage and that LPS/CD14 receptor signaling is involved[44]. Recently, we have shown that deficiency in TLR4, the receptor for LPS, or deficiency in the TLR4 downstream signaling by interferon regulatory factor 3, are crucial for development of alcohol-induced liver damage[31,46]. The combined effect of alcohol and HIV infection on LPS signaling in the liver awaits further investigation.
Activation of the inflammatory cascade and increased proinflammatory cytokine production is a hallmark of both alcoholic steatohepatitis and chronic HIV infection[26,45]. The immunological consequences of such proinflammatory cascade activation are not fully understood. The clinical observations of increased susceptibility to infections in alcoholic steatohepatitis and chronic HIV infection demonstrate that, although non-specific proinflammatory activation is present, pathogen-specific innate immune responses are defective. Furthermore, antigen-specific immune responses as well as antigen presenting function of monocytes and dendritic cells are severely dampened by alcohol exposure, as well as in chronic HIV infection[47-49]. Our previous studies have demonstrated that acute alcohol consumption in humans inhibits the antigen presenting function in human monocyte-derived dendritic cells and monocytes via mechanisms that involve decreased interleukin (IL)-12 and co-stimulatory molecule expression and/or impaired dendritic cell differentiation/maturation[48-50]. The effects of prolonged alcohol also inhibit myeloid dendritic cell functions in humans as well as mouse models[51].
Defects in myeloid and plasmacytoid dendritic cell function have been extensively studied in HIV infection. It has been shown that HIV infection results in impaired differentiation and maturation of monocyte-derived dendritic cells in humans and that these dendritic cells have impaired antigen presentation capacity. Chronic HCV infection is also associated with decreased antigen presenting function of dendritic cells and HIV and HCV infection affect immune responses[52,53]. Although the combined effects of alcohol exposure and HIV infection are yet to be evaluated on dendritic cell functions, we have previously demonstrated that alcohol treatment has an additive inhibitory effect on the already dampened accessory cell function in dendritic cells of HCV-infected patients[54]. This is associated with reduced expression of the T cell co-stimulatory molecules, CD80 and CD86, low IL-12 and increased IL-10 production[54]. The role of dendritic cells in HIV/HCV co-infection in relation to the liver disease needs further exploration. However, the importance of dendritic cells in liver tolerance and inflammation is emerging[42,43].
There are multiple mechanisms by which alcohol, HIV infection and even ART could directly affect hepatocytes. As summarized in Table 1, reactive oxygen radical production, mitochondrial damage and steatosis can all be induced independently by alcohol, HIV or HCV infections alone or in combination with HAART[55]. However, these factors together will most likely cause combined damage in exposed hepatocytes. Some of the mechanisms involved include depletion of the intracellular glutathione stores by chronic alcohol and HIV infection that predisposes to increased reactive oxygen species (ROS) production and increased susceptibility to hepatocyte death[55]. In addition, alcohol, HCV, and HAART cause mitochondrial damage, fatty liver, and increased levels of proinflammatory cytokines, particularly, TNFα, which results in hepatocyte death. This mechanism has been investigated in alcoholic liver damage as well as in HIV infection, and HIV/HCV co-infection[55].
| Effect | HIV | HCV | Alcohol | HAART |
| Increase ROS | √ | √ | √ | |
| Increase proinflammatory cytokines | √ | √ | √ | |
| Oxidative stress | √ | √ | √ | √ |
| Lipid peroxidation | √ | √ | √ | |
| Mitochondrial damage | √ | √ | √ | √ |
| Steatosis | √ | √ | √ | √ |
| Glutathione depletion | √ | √ | √ | |
| Proteasome dysfunction | √ | √ |
Although the introduction of HAART for HIV infection has resulted in a significant decrease in the mortality rate of AIDS, a sizable proportion of patients receiving HAART develop liver toxicity. For example, patients treated with the antiretroviral nucleoside reverse-transcriptase inhibitors (NRTIs) zidovudine, zalcitabine, didanosine and stavudine have been reported to develop hepatic steatosis[56,57]. Heavy alcohol consumption by HIV patients on HAART results in liver decompensation in about 2% of cases[58], and increases the risk of liver injury 5.8 times in 10% of patients receiving NRTI or non-NRTI regimens[55]. The enhanced liver toxicity due to alcohol, HCV, and HAART could result from shared mechanisms such as fat accumulation and mitochondrial damage.
Alcohol and HCV-induced fatty liver is well documented. Several mechanisms have been reported for alcohol-induced fatty liver including increased hepatic uptake of fatty acids and de novo lipogenesis, impaired peroxisome proliferator-activated receptor α signaling, reduced mitochondrial fatty acid oxidation and reduced secretion of triglycerides[59-61]. These effects could be attributed to reduced adiponectin secretion by the adipose tissue and elevated expression of TNFα[62-64].
Some AIDS patients develop HAART-associated lipodystrophy (LD). A study by Sutinen et al[65] has shown that, in comparison to HIV-negative subjects, HAART+LD patients show: (1) higher liver fat content (regardless of alcohol consumption); (2) for a given amount of liver fat, serum-free insulin concentration correlated with liver fat, which suggests that fat accumulation in the liver is crucial for development of insulin resistance; and (3) significantly lower leptin concentrations than the other two groups. Others have also demonstrated low plasma leptin concentrations in patients with HAART-associated LD[66]. Hypoleptinemia and subsequent insulin resistance could favor fatty liver accumulation in patients suffering from NRTI-induced lipoatrophy[67-69].
Although Sutinen and co-workers did not observe lactic acidosis, others have reported lactic acidosis and hepatic steatosis in some patients using HAART, which was attributed to mitochondrial toxicity induced by nucleoside analogs[70,71]. In addition, co-infection with HCV increases the risk for severe liver damage during HAART[72], and end-stage liver disease is the primary cause of death in HIV/HCV co-infected patients under HAART[73,74]. Seth[75] has reported that, in HIV co-infected patients, the HCV load is higher by an average of 0.5-1.0 log than the mono-infected patients due to immune depression. Studies on liver fibrosis show conflicting results. Although some studies have found that early HAART in HIV/HCV co-infected patients may slow liver fibrosis progression[76], others have found that HAART regimens including nevirapine accelerate liver fibrosis progression in HIV/HCV co-infected patients[77].
Alcohol and HCV can induce mitochondrial DNA (mtDNA) damage through the production of ROS and/or reactive metabolites. It has been reported that prolonged administration of antiretroviral NRTIs increases the risk of mitochondrial damage in the liver[78]. NRTIs can cause the accumulation of the oxidized base 8-hydroxydeoxyguanosine (8-OH-dG) in liver mtDNA[79,80]. In addition, NRTIs can cause mtDNA point mutations in some patients, which may result from interaction with mtDNA replication (via misreading of 8-OH-dG by DNA polymerase c) and/or impairment of polymerase c repair capacity[81]. It has been reported that NRTIs inhibit mtDNA replication by undergoing phosphorylation as the cognate endogenous nucleosides, which are subsequently incorporated within the mitochondrial genome by DNA polymerase c[81].
Although increasing evidence suggests enhanced combined effects of alcohol and HIV infection on the liver with and without viral hepatitis, a number of questions remain unanswered. What are the interactions between alcohol use, HIV, HCV and HBV infections? Is the crosstalk between organs, such as the homeostasis between the liver and gut, affected by combined insults of alcohol, HIV and viral hepatitis? What is the effect of the cumulative effects of alcohol and HIV infection on cross-regulation between various cell types in the liver? How can new therapeutic targets be developed in the light of knowledge about the interactive effects of HIV and alcohol on the liver? All of these questions are pertinent to the status and defects observed in immune functions, gut permeability, Kupffer cell activation, hepatocyte function and liver fibrosis/stellate cell function in patients with HIV infection and excessive alcohol use and/or viral hepatitis.
Peer reviewers: Julian Swierczynski, MD, PhD, Professor, Department of Biochemistry, Medical University of Gdansk, 80-211 Gdansk, Poland; Luis Bujanda, PhD, Professor, Departament of Gastroenterology, CIBEREHD, University of Country Basque, Donostia Hospital, Paseo Dr. Beguiristain s/n, 20014 San Sebastián, Spain
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Schneider MF, Gange SJ, Williams CM, Anastos K, Greenblatt RM, Kingsley L, Detels R, Mu ñoz A. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984-2004. AIDS. 2005;19:2009-2018. |
| 2. | Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853-860. |
| 3. | Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27-34. |
| 4. | Chang JJ, Altfeld M. Innate immune activation in primary HIV-1 infection. J Infect Dis. 2010;202 Suppl 2:S297-S301. |
| 5. | Mogensen TH, Melchjorsen J, Larsen CS, Paludan SR. Innate immune recognition and activation during HIV infection. Retrovirology. 2010;7:54. |
| 6. | Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33. |
| 7. | Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep. 2011;8:4-11. |
| 8. | Sherman KE, Peters M, Koziel MJ. HIV and liver disease forum: conference proceedings. Hepatology. 2007;45:1566-1577. |
| 9. | Castellares C, Barreiro P, Martín-Carbonero L, Labarga P, Vispo ME, Casado R, Galindo L, García-Gascó P, García-Samaniego J, Soriano V. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat. 2008;15:165-172. |
| 10. | Maida I, Núñez M, Ríos MJ, Martín-Carbonero L, Sotgiu G, Toro C, Rivas P, Barreiro P, Mura MS, Babudieri S. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr. 2006;42:177-182. |
| 11. | Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74-80. |
| 12. | Martínez E, Milinkovic A, Buira E, de Lazzari E, León A, Larrousse M, Loncá M, Laguno M, Blanco JL, Mallolas J. Incidence and causes of death in HIV-infected persons receiving highly active antiretroviral therapy compared with estimates for the general population of similar age and from the same geographical area. HIV Med. 2007;8:251-258. |
| 13. | Woreta TA, Sutcliffe CG, Mehta SH, Brown TT, Higgins Y, Thomas DL, Torbenson MS, Moore RD, Sulkowski MS. Incidence and risk factors for steatosis progression in adults coinfected With HIV and hepatitis C virus. Gastroenterology. 2011;140:809-817. |
| 14. | Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20:151-155. |
| 15. | López-Diéguez M, Montes ML, Pascual-Pareja JF, Quereda C, Von Wichmann MA, Berenguer J, Tural C, Hernando A, González-García J, Serrano L. GESIDA 37/03-FIPSE 36465/03-NEAT IG5 Study Group. The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. AIDS. 2011;25:899-904. |
| 16. | Bertholet N, Cheng DM, Samet JH, Quinn E, Saitz R. Alcohol consumption patterns in HIV-infected adults with alcohol problems. Drug Alcohol Depend. 2010;112:160-163. |
| 17. | Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179-186. |
| 18. | Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194-199. |
| 19. | Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8:1002-1012. |
| 20. | O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51:307-328. |
| 21. | Lee KC, Lim WW, Lee SS. High prevalence of HCV in a cohort of injectors on methadone substitution treatment. J Clin Virol. 2008;41:297-300. |
| 22. | Salmon-Ceron D, Rosenthal E, Lewden C, Bouteloup V, May T, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C. Emerging role of hepatocellular carcinoma among liver-related causes of deaths in HIV-infected patients: The French national Mortalité 2005 study. J Hepatol. 2009;50:736-745. |
| 23. | Barve S, Kapoor R, Moghe A, Ramirez J, Eaton J, Gobejishvili L, Joshi-Barve S, McClain C. Focus On The Live: Alcohol use, highly active antiretroviral therapy, and liver disease in HIV-infected patients. NIAAA. 2010;33:229. |
| 24. | Immune mechanisms in alcoholic liver disease. Genes Nutr. 2009;Epub ahead of print. |
| 25. | Tavio M, Grossi P, Baccarani U, Scudeller L, Pea F, Berretta M, Adani G, Vivarelli M, Riva A, Tirelli U. HIV-Infected Patients and Liver Transplantation: Who, When and Why. Curr HIV Res. 2011;9:120-127. |
| 26. | Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258-1266. |
| 27. | Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321-1329. |
| 28. | Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304-1313. |
| 29. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. |
| 30. | Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538-547. |
| 31. | Petrasek J, Dolganiuc A, Nath B, Hritz I, Kodys K, Catalano , Kurt-Jones E, Mandrekar P, Szabo G: Hepatocyte-specific IRF3 and type I interferons are protective in alcohol-induced liver injury I mice via cross-talk with macrophages. Hepatology. 2011;In press. |
| 32. | Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836-1846. |
| 33. | Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220-1230. |
| 34. | Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355-364. |
| 35. | Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365-1371. |
| 36. | Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, Alfano M, Poli G, Rossouw T. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723-733. |
| 37. | Nowroozalizadeh S, Månsson F, da Silva Z, Repits J, Dabo B, Pereira C, Biague A, Albert J, Nielsen J, Aaby P. Microbial translocation correlates with the severity of both HIV-1 and HIV-2 infections. J Infect Dis. 2010;201:1150-1154. |
| 38. | Trøseid M, Nowak P, Nyström J, Lindkvist A, Abdurahman S, Sönnerborg A. Elevated plasma levels of lipopolysaccharide and high mobility group box-1 protein are associated with high viral load in HIV-1 infection: reduction by 2-year antiretroviral therapy. AIDS. 2010;24:1733-1737. |
| 39. | Douek D. HIV disease progression: immune activation, microbes, and a leaky gut. Top HIV Med. 2007;15:114-117. |
| 40. | Dolganiuc A, Norkina O, Kodys K, Catalano D, Bakis G, Marshall C, Mandrekar P, Szabo G. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627-1636. |
| 41. | Baum M, Sales S, Jayaweera D, Lai S, Bradwin G, Rafie C, Page J, Campa A. Coinfection with hepatitis C virus, oxidative stress and antioxidant status in HIV-positive drug users in Miami. HIV Med. 2011;12:78-86. |
| 42. | Biasin M, Piacentini L, Lo Caputo S, Naddeo V, Pierotti P, Borelli M, Trabattoni D, Mazzotta F, Shearer GM, Clerici M. TLR activation pathways in HIV-1-exposed seronegative individuals. J Immunol. 2010;184:2710-2717. |
| 43. | Medvedev AE, Lentschat A, Wahl LM, Golenbock DT, Vogel SN. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J Immunol. 2002;169:5209-5216. |
| 44. | Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453-460. |
| 45. | Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22 Suppl 1:S53-S56. |
| 46. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. |
| 47. | Lau AH, Szabo G, Thomson AW. Antigen-presenting cells under the influence of alcohol. Trends Immunol. 2009;30:13-22. |
| 48. | Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87:609-620. |
| 49. | Donaghy H, Stebbing J, Patterson S. Antigen presentation and the role of dendritic cells in HIV. Curr Opin Infect Dis. 2004;17:1-6. |
| 50. | Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398-3407. |
| 51. | Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220-232. |
| 52. | Szabo G, Dolganiuc A. Hepatitis C and innate immunity: recent advances. Clin Liver Dis. 2008;12:675-692, x. |
| 53. | Kim AY, Chung RT. Coinfection with HIV-1 and HCV--a one-two punch. Gastroenterology. 2009;137:795-814. |
| 54. | Dolganiuc A, Kodys K, Kopasz A, Marshall C, Mandrekar P, Szabo G. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin Exp Res. 2003;27:1023-1031. |
| 55. | Núñez M, Lana R, Mendoza JL, Martín-Carbonero L, Soriano V. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:426-431. |
| 56. | Lai KK, Gang DL, Zawacki JK, Cooley TP. Fulminant hepatic failure associated with 2’,3’-dideoxyinosine (ddI). Ann Intern Med. 1991;115:283-284. |
| 57. | Le Bras P, D’Oiron R, Quertainmont Y, Halfon P, Caquet R. Metabolic, hepatic and muscular changes during zidovudine therapy: a drug-induced mitochondrial disease? AIDS. 1994;8:716-717. |
| 58. | Fabris P, Tositti G, Manfrin V, Giordani MT, Vaglia A, Cattelan AM, Carlotto A. Does alcohol intake affect highly active antiretroviral therapy (HAART) response in HIV-positive patients? J Acquir Immune Defic Syndr. 2000;25:92-93. |
| 59. | Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34:35-38. |
| 60. | Donohue TM. Alcohol-induced steatosis in liver cells. World J Gastroenterol. 2007;13:4974-4978. |
| 61. | Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45:717-724. |
| 62. | Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology. 2008;47:867-879. |
| 63. | Chen X, Sebastian BM, Tang H, McMullen MM, Axhemi A, Jacobsen DW, Nagy LE. Taurine supplementation prevents ethanol-induced decrease in serum adiponectin and reduces hepatic steatosis in rats. Hepatology. 2009;49:1554-1562. |
| 64. | Zeng T, Xie KQ. Ethanol and liver: recent advances in the mechanisms of ethanol-induced hepatosteatosis. Arch Toxicol. 2009;83:1075-1081. |
| 65. | Sutinen J, Häkkinen AM, Westerbacka J, Seppälä-Lindroos A, Vehkavaara S, Halavaara J, Järvinen A, Ristola M, Yki-Järvinen H. Increased fat accumulation in the liver in HIV-infected patients with antiretroviral therapy-associated lipodystrophy. AIDS. 2002;16:2183-2193. |
| 66. | Estrada V, Serrano-Ríos M, Martínez Larrad MT, Villar NG, González López A, Téllez MJ, Fernández C. Leptin and adipose tissue maldistribution in HIV-infected male patients with predominant fat loss treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;29:32-40. |
| 67. | Igoudjil A, Abbey-Toby A, Begriche K, Grodet A, Chataigner K, Peytavin G, Maachi M, Colin M, Robin MA, Lettéron P. High doses of stavudine induce fat wasting and mild liver damage without impairing mitochondrial respiration in mice. Antivir Ther. 2007;12:389-400. |
| 68. | Brennan AM, Lee JH, Tsiodras S, Chan JL, Doweiko J, Chimienti SN, Wadhwa SG, Karchmer AW, Mantzoros CS. r-metHuLeptin improves highly active antiretroviral therapy-induced lipoatrophy and the metabolic syndrome, but not through altering circulating IGF and IGF-binding protein levels: observational and interventional studies in humans. Eur J Endocrinol. 2009;160:173-176. |
| 69. | Mulligan K, Khatami H, Schwarz JM, Sakkas GK, DePaoli AM, Tai VW, Wen MJ, Lee GA, Grunfeld C, Schambelan M. The effects of recombinant human leptin on visceral fat, dyslipidemia, and insulin resistance in patients with human immunodeficiency virus-associated lipoatrophy and hypoleptinemia. J Clin Endocrinol Metab. 2009;94:1137-1144. |
| 70. | Sundar K, Suarez M, Banogon PE, Shapiro JM. Zidovudine-induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature. Crit Care Med. 1997;25:1425-1430. |
| 71. | Lonergan JT, Behling C, Pfander H, Hassanein TI, Mathews WC. Hyperlactatemia and hepatic abnormalities in 10 human immunodeficiency virus-infected patients receiving nucleoside analogue combination regimens. Clin Infect Dis. 2000;31:162-166. |
| 72. | Monforte Ade A, Bugarini R, Pezzotti P, De Luca A, Antinori A, Mussini C, Vigevani GM, Tirelli U, Bruno R, Gritti F. Low frequency of severe hepatotoxicity and association with HCV coinfection in HIV-positive patients treated with HAART. J Acquir Immune Defic Syndr. 2001;28:114-123. |
| 73. | Rosenthal E, Pialoux G, Bernard N, Pradier C, Rey D, Bentata M, Michelet C, Pol S, Perronne C, Cacoub P. Liver-related mortality in human-immunodeficiency-virus-infected patients between 1995 and 2003 in the French GERMIVIC Joint Study Group Network (MORTAVIC 2003 Study). J Viral Hepat. 2007;14:183-188. |
| 74. | Pineda JA, García-García JA, Aguilar-Guisado M, Ríos-Villegas MJ, Ruiz-Morales J, Rivero A, del Valle J, Luque R, Rodríguez-Baño J, González-Serrano M. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46:622-630. |
| 75. | Seth AK. Management of hepatitis C in HIV infected and other immunocompromised individuals. Trop Gastroenterol. 2006;27:111-117. |
| 76. | Mariné-Barjoan E, Saint-Paul MC, Pradier C, Chaillou S, Anty R, Michiels JF, Sattonnet C, Ouzan D, Dellamonica P, Tran A. Impact of antiretroviral treatment on progression of hepatic fibrosis in HIV/hepatitis C virus co-infected patients. AIDS. 2004;18:2163-2170. |
| 77. | Macías J, Castellano V, Merchante N, Palacios RB, Mira JA, Sáez C, García-García JA, Lozano F, Gómez-Mateos JM, Pineda JA. Effect of antiretroviral drugs on liver fibrosis in HIV-infected patients with chronic hepatitis C: harmful impact of nevirapine. AIDS. 2004;18:767-774. |
| 78. | Kovari H, Ledergerber B, Battegay M, Rauch A, Hirschel B, Foguena AK, Vernazza P, Bernasconi E, Mueller NJ, Weber R. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis b or c virus co-infection. Clin Infect Dis. 2010;50:502-511. |
| 79. | de la Asunción JG, del Olmo ML, Sastre J, Pallardó FV, Viña J. Zidovudine (AZT) causes an oxidation of mitochondrial DNA in mouse liver. Hepatology. 1999;29:985-987. |
| 80. | Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1-28. |