Published online Jan 14, 2011. doi: 10.3748/wjg.v17.i2.267
Revised: September 26, 2010
Accepted: October 3, 2010
Published online: January 14, 2011
Sorafenib, a multitargeted tyrosine kinase inhibitor, has been shown to improve survival in patients with advanced hepatocellular carcinoma (HCC). As the clinical use of sorafenib increases, many adverse effects have been reported, such as hand-foot skin reaction, diarrhea, anorexia, asthenia, alopecia, weight loss, hypertension and arterial thromboembolism. However, there are no prior reports of splenic infarction as an adverse effect of sorafenib. Here, a case of splenic infarction in a patient with HCC who was treated with sorafenib is reported. The patient had no other predisposing factors to explain the splenic infarction except for the administration of sorafenib. The splenic infarction improved after sorafenib was discontinued; however, the HCC progressed.
- Citation: Kim SO, Han SY, Baek YH, Lee SW, Han JS, Kim BG, Cho JH, Nam KJ. Splenic infarction associated with sorafenib use in a hepatocellular carcinoma patient. World J Gastroenterol 2011; 17(2): 267-270
- URL: https://www.wjgnet.com/1007-9327/full/v17/i2/267.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i2.267
Sorafenib (Nexavar®, Bayer) is an oral vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI) that has been approved by the United States Food and Drug Administration for the treatment of hepatocellular carcinoma (HCC). Many studies regarding the adverse effects of this drug have been reported, which include hand-foot skin reaction, diarrhea, anorexia, asthenia, alopecia, weight loss, hypertension and arterial thromboembolism. The rate of arterial thromboembolic events following the use of sorafenib has been reported to be about 3.8%[1]. However, most cases have been cardiac or cerebrovascular events. There have been no clinical reports of splenic infarction associated with sorafenib, to date. We report a patient who developed a spontaneous infarction of the spleen after treatment with sorafenib used for the treatment of HCC.
A 69-year-old female with a history of HCC presented to the gastrointestinal department with left upper quadrant (LUQ) discomfort. The patient was previously treated with percutaneous ethanol injection therapy (PEIT) for primary HCC in 1999, and subsequently transarterial chemoembolization (TACE) for local recurrent HCC in 2008 and 2009. Recently, two cycles of hepatic arterial infusion (HAI) chemotherapy with FUdR (0.3 mg/kg) and sorafenib (400 mg po bid) were provided for intrahepatic metastases. Initially, this combination chemotherapy was started the day after an HAI catheter was inserted. During these treatments, intermittent abdominal pain and itching of both palms were observed. At that time, endoscopic findings showed acute gastromucosal lesions; therefore, treatment with sucralfate and a proton pump inhibitor was started. The abdominal pain improved. However, after several days other characteristics of abdominal discomfort returned. The patient reported a sudden onset of dull, left-sided upper quadrant abdominal pain. The patient stopped her medications including sorafenib and the symptoms gradually improved.
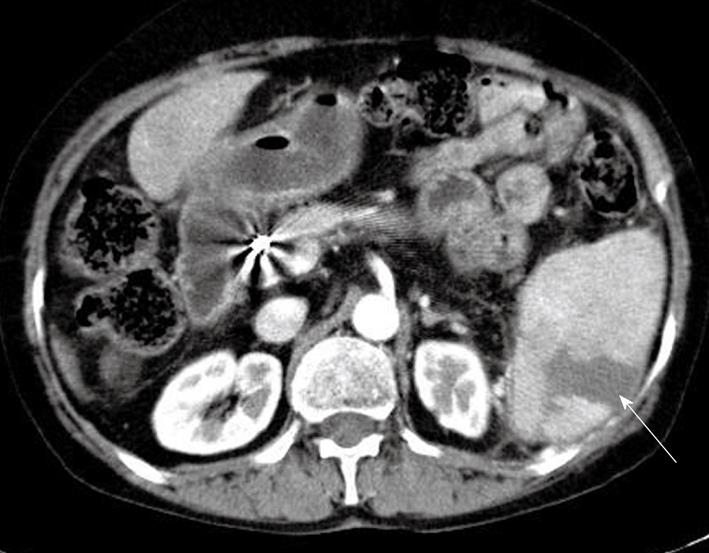
A physical examination was unremarkable except for mild LUQ tenderness. The laboratory investigations including complete blood counts, electrolytes, liver and kidney function tests were all within normal limits. EKG, chest X-ray and cardiac enzymes were also within normal range. An abdominal computed tomography (CT) scan showed a wedge-shaped opacity in the spleen suggestive of an acute infarction (Figure 1). Further evaluations were performed to determine the possible cause of the spontaneous splenic infarction such as infectious endocarditis, atrial fibrillation, hematologic disorders, autoimmune disease or other possible infectious diseases. Additional laboratory tests of the peripheral blood smear, i.e. protein C and S, antithrombin III, lipid profile, homocysteine and autoantibodies including antinuclear antibody, antineutrophil cytoplasmic antibodies (ANCA), rheumatoid factor, antiphospholipid antibodies, and complement levels, were not specific. Tumor markers of α-fetoprotein (316.18 ng/mL) and PIVKA-II (393 mAU/mL) were elevated. Although she had underlying liver cirrhosis, the absence of encephalopathy and ascites, and the normal prothrombin time and albumin resulted in a Child-Pugh A functional class. The 2D echocardiogram and venous Doppler were unremarkable.
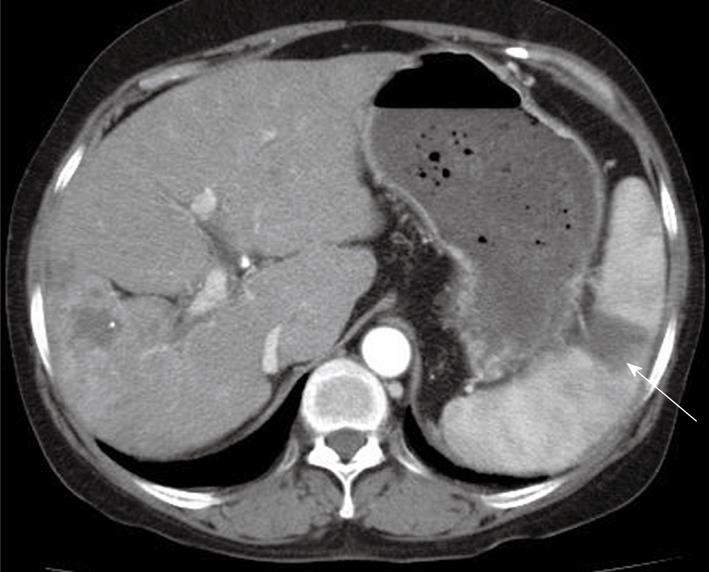
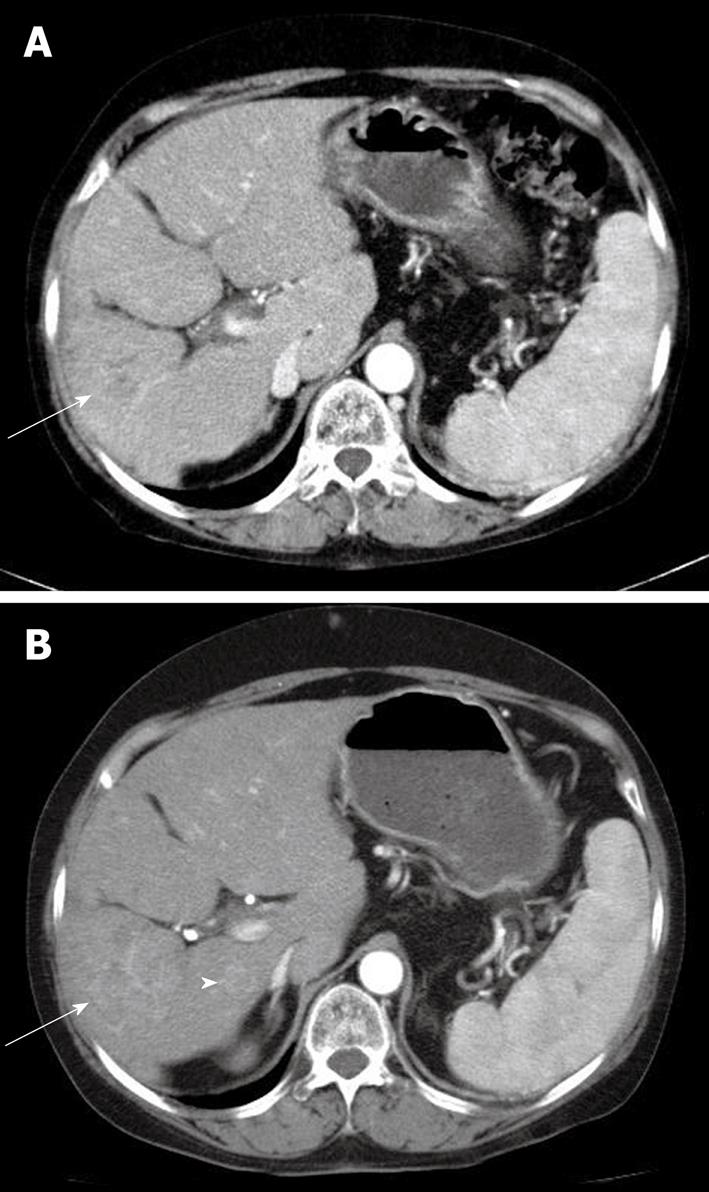
During the evaluation, the patient continued HAI chemotherapy with FUdR according to schedule without sorafenib. At discharge, sorafenib was administered again, because the symptoms had gradually improved; however, a few days later, the sorafenib was discontinued due to a second attack of LUQ pain. One month later, the follow up CT scan showed another wedge-shaped lesion in the spleen without aggravation of the HCC (Figure 2). The follow up laboratory data showed no significant changes, including for platelet count. Intra-arterial chemotherapy without sorafenib was provided. One month later, a CT scan showed diminished infarction size in the spleen; however, the HCC progressed (Figure 3).
The vascular endothelial growth factors (VEGFs) play a critical role in angiogenesis and stimulate endothelial cell proliferation, migration and tube formation[2]. Sorafenib was developed because of its anti-angiogenic action in inhibiting tyrosine kinase of the VEGF receptor; it has been shown to improve the clinical outcome of patients with HCC in a large phase III trial[3]. The side effects associated with this agent are mostly mild to moderate. Rash, exfoliative dermatitis, hand-foot skin reaction, diarrhea and fatigue are the most common adverse events, occurring in 33%-38% of patients[4]. However, unexpected toxicities have also been reported, including thrombotic events[5]. Most of the adverse events are downstream effects of the suppression of VEGF signaling in endothelial cells of normal organs. The thrombotic events are also associated with anti-VEGF effects. VEGF not only stimulates endothelial cell proliferation, but also promotes endothelial cell survival and helps maintain vascular integrity. Inhibition of VEGF could thereby diminish the regenerative capacity of endothelial cells and cause abnormalities that expose pro-coagulant phospholipids of the luminal plasma membrane or underlying matrix, leading to thrombosis[6]. In addition, VEGF increases production of NO and prostacyclin, and inhibits proliferation of vascular smooth muscle cells. Reduction in NO and prostacyclin, after inhibition of VEGF signaling, may predispose to thromboembolic events[7]. Moreover, VEGF inhibition may also increase the risk of thrombosis by increasing the hematocrit and blood viscosity via overproduction of erythropoietin[8]. Other concurrent pathological findings in a patient might also play a central role. There are some reports of anti-VEGF agent-related thromboembolic events such as myocardial infarction and cerebrovascular accidents. To date, thrombotic risks with intravenous bevacizumab have been studied extensively, in contrast to oral VEGFR TKIs where data on arterial events have not yet been evaluated. Recently, Choueiri et al[9] reported the relative risk of arterial thrombotic events with bevacizumab and a VEGFR TKI (sorafenib or sunitinib); a two-fold and three-fold increase was reported, respectively. This risk did not depend on the type of malignancy.
In a meta-analysis of bevacizumab-treated patients, the major underlying risk factors of arterial thrombotic events were advanced age, hypertension, diabetes, and a prior history of thrombotic events. The treatment duration for the incidence of thrombotic events was within the first 3 mo of therapy; however, the data regarding the occurrence of the events, during the course of the trial, were frequently not reported[10]. In the case reported here, the patient was of advanced age but had no predisposing factors. The thromboembolic event was a splenic infarction with the clinical symptom of LUQ pain. The symptoms developed 2 mo after administration of sorafenib. Most prior reports of TKI-associated arterial thrombotic events have shown myocardial infarctions and/or cerebrovascular accidents; a splenic artery infarction has not been previously reported. Possible causes of splenic infarction were investigated. The physical examination, laboratory findings, imaging studies and other drug history did not suggest any other possible causes except for the sorafenib administration. Although the patient had a history of other therapeutic procedures such as PEIT, TACE, and HAI chemotherapy, the last TACE was administered 16 mo previously and the HAI chemotherapy was continued without LUQ pain. The HAI catheter was inserted at the correct hepatic arterial level, and the distal end was connected to the port at the right femoral artery. Anatomically this should not contribute to occlusion of the splenic artery. Furthermore, the patient tolerated HAI chemotherapy without the sorafenib. The time interval from the initial administration of sorafenib to onset of LUQ pain, and the fact that discontinuing sorafenib was associated with resolution of symptoms, suggested that the sorafenib was the cause of the acute splenic infarction. Two months later, the follow up CT scan showed improvement of the splenic lesion; however, the HCC had progressed.
In this case, discontinuing sorafenib resolved the splenic infarction, however, at the expense of the HCC. Perhaps if the sorafenib had been continued with other pain medications and anticoagulants, the outcome would have been better. The use of low-dose aspirin for the prophylaxis of arterial thromboembolic events in high-risk patients is supported by an extensive body of literature[11] and is recommended as the standard of care[12]. Therefore, if a patient has a good response to sorafenib, it might be better to continue the sorafenib with the addition of low-dose aspirin to prevent events such as a splenic infarction, which is not life threatening.
Peer reviewer: Yuichiro Eguchi, MD, Department of Internal Medicine, Saga Medical School, 5-1-1 Nabeshima, Saga, 849-8501, Japan
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
| 1. | Vaklavas C, Lenihan D, Kurzrock R, Tsimberidou AM. Anti-vascular endothelial growth factor therapies and cardiovascular toxicity: what are the important clinical markers to target? Oncologist. 2010;15:130-141. |
| 2. | Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039. |
| 3. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. |
| 4. | Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788-1795. |
| 5. | Zangari M, Fink LM, Elice F, Zhan F, Adcock DM, Tricot GJ. Thrombotic events in patients with cancer receiving antiangiogenesis agents. J Clin Oncol. 2009;27:4865-4873. |
| 6. | Kilickap S, Abali H, Celik I. Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol. 2003;21:3542; author reply 3543. |
| 7. | Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280:C1375-C1386. |
| 9. | Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280-2285. |
| 10. | Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232-1239. |