Published online Jan 14, 2011. doi: 10.3748/wjg.v17.i2.219
Revised: September 9, 2010
Accepted: September 16, 2010
Published online: January 14, 2011
AIM: To investigate the expression and potential prognostic role of vascular endothelial growth factor (VEGF) and endoglin in gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
METHODS: Microvessel density (MVD) in GEP-NETs was evaluated using endoglin and CD31 immunohistochemistry. In addition, tissue levels of endoglin and VEGF were determined in homogenates by ELISA.
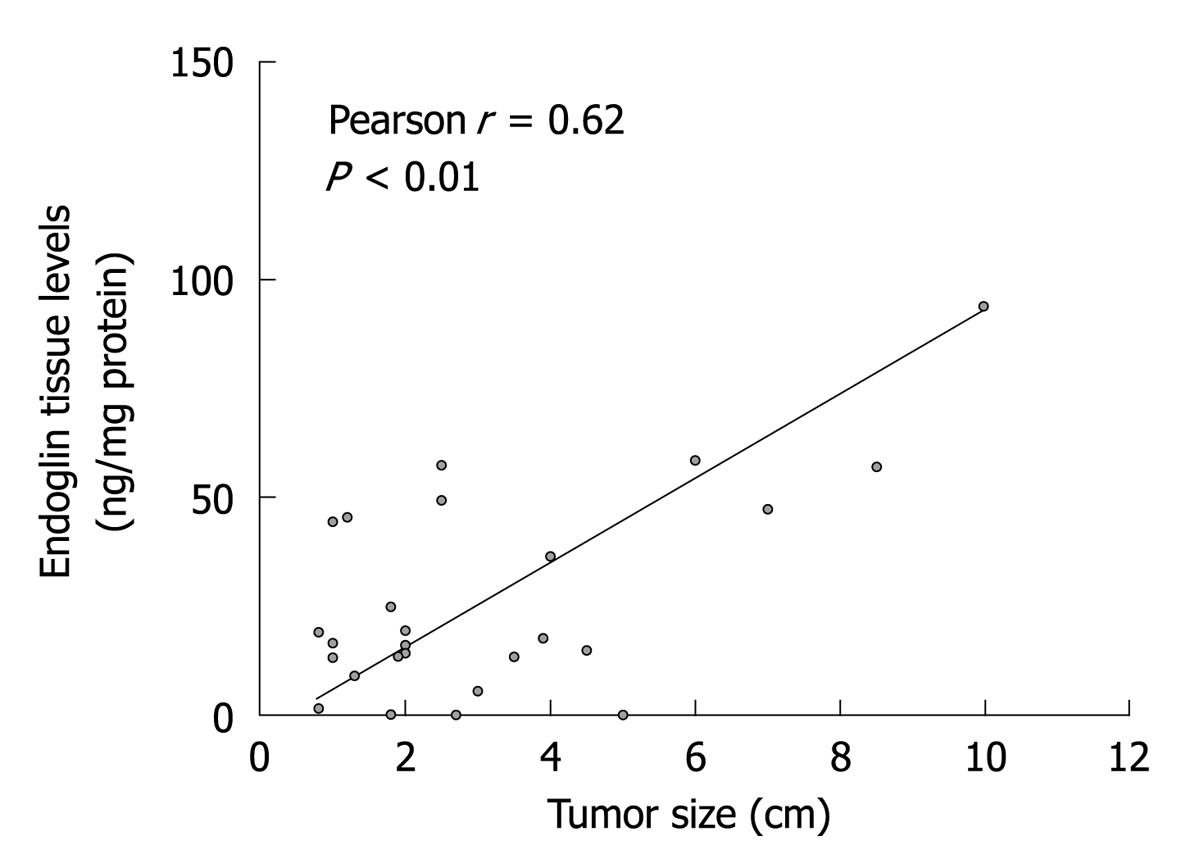
RESULTS: Endoglin was highly expressed on tumor endothelial cells. CD31 MVD in GEP-NETs was significantly higher compared to endoglin MVD (P < 0.01). Two- to four-fold higher tissue levels of endoglin and VEGF were seen in tumors compared to associated normal tissue. This increased endoglin tissue expression in tumors was significantly related to tumor size (P < 0.01), presence of metastases (P = 0.04), and a more advanced tumor stage (P = 0.02), whereas expression of VEGF was not.
CONCLUSION: We suggest that endoglin is a potential marker to indicate and predict metastases, which might be useful in the post-resection therapeutic approach of patients with GEP-NETs.
- Citation: Kuiper P, Hawinkels LJ, Jonge-Muller ES, Biemond I, Lamers CB, Verspaget HW. Angiogenic markers endoglin and vascular endothelial growth factor in gastroenteropancreatic neuroendocrine tumors. World J Gastroenterol 2011; 17(2): 219-225
- URL: https://www.wjgnet.com/1007-9327/full/v17/i2/219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i2.219
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs), including gastrointestinal carcinoids and pancreatic neuroendocrine tumors (PNETs), comprise a very heterogeneous group of neoplasia, with respect to tumor biology, histocytopathology and prognosis[1]. Despite a slow-growing nature, they are primarily malignant[2]. Angiogenesis, the formation of new blood vessels from the existing vascular bed, is a crucial process in tumor progression. When tumors reach a size of 1-2 mm, they become dependent on neovascularization, not only to provide them with nutrients and oxygen, but also as an exit route for metabolic waste products, further growth of the primary tumor, and eventually, metastatic spread[3]. One of the key factors in angiogenesis is vascular endothelial growth factor (VEGF), which has numerous effects on endothelial cells (ECs), including induction of migration and differentiation[4]. Several studies have addressed the prognostic implications of VEGF in patients with GEP-NETs, and trials investigating the action of the anti-VEGF antibody bevacizumab in patients with GEP-NETs are ongoing[5,6].
Another important growth factor, with a pivotal role in angiogenesis is transforming growth factor (TGF)-β1, a multifunctional cytokine that is involved in numerous physiological and pathological processes[7]. Endoglin (CD105) is a co-receptor for TGF-β1. As a result of its principal expression on ECs of newly formed blood vessels, several studies have suggested that endoglin is a specific marker of neovascularization in various cancer types[8-10]. In pancreatic carcinomas, high endoglin microvessel density (MVD) has been found to be related to shorter survival, and therefore, is suggested to be a prognostic marker[11]. In colorectal cancer, the vessel count by positive endoglin staining is able to identify patients at high risk of metastases[12].
In the present study, we assessed the tissue expression and levels of two key players in the process of angiogenesis, namely endoglin and VEGF, to assess their potential clinical implications in patients with GEP-NETs.
After surgical removal, tumor tissues were collected at the Department of Gastroenterology, Leiden University Medical Centre (LUMC), Leiden, and either frozen at -80°C for protein extraction and/or embedded in paraffin for immunohistochemical staining.
Sixty-eight homogenates (27 tumor samples and 41 normal samples) of 27 patients were available for the determination of tissue levels of endoglin. For the measurement of VEGF levels, one tumor sample was exhausted, therefore, the total number of tumor samples comprised 26. For CD31 and endoglin immunostaining, 50 and 49 samples, respectively, of 39 patients, were available. For most patients, but not all, both homogenates and paraffin slides were available. In total, 41 patients with GEP-NETs were included. GEP-NETs comprised PNETs and gastrointestinal neuroendocrine tumors, which were also referred to as carcinoids.
Clinicopathological information was obtained by evaluation of patients’ medical files and pathology reports, when available. According to the classification of the World Health Organization for GEP-NETs, tumors were categorized into well-differentiated neuroendocrine tumor (NET), well-differentiated neuroendocrine carcinoma (NEC), or poorly differentiated NEC[13]. From some patients, the WHO classification was not assessable due to lack of specified classification. All studies were performed according to the guidelines of the LUMC medical ethics committee, in compliance with the Helsinki Declaration.
Immunohistochemistry was performed as follows. Tissues were fixed in formalin, embedded in paraffin, and cut into 5-μm sections. After deparaffinization and rehydration, endogenous peroxidases were blocked in methanol containing 0.3% H202 (Merck, Darmstadt, Germany). Antigen retrieval was performed by boiling in 0.01 mol/L citrate buffer, pH 6.0, for 10 min. Slides were incubated overnight at room temperature (RT) with primary antibodies: biotinylated goat anti-human endoglin (1:200; R&D Systems Europe, Abingdon, UK), or mouse monoclonal anti-CD31 (1:400; Dako, Glostrup, Denmark) diluted in PBS with 1% bovine serum albumin (BSA), as described previously[14]. Immunodetection was performed with a biotinylated goat anti-mouse antibody (for CD31) and horseradish peroxidase (HRP)-streptavidin complex (both Dako) for 30 min at RT. Staining was visualized using 0.05% 3,3’-diaminobenzidine (Sigma, Darmstadt, Germany) that contained 0.0038% H202. Colon carcinomas were used as positive controls. Negative controls were included by omitting the primary antibodies. Representative photomicrographs were taken with an Olympus BX-51TF microscope equipped with a DP23-3-5 camera.
The endoglin and CD31 MVD in the tumor-bearing area were quantified by computerized analysis. Four representative tumor areas for either endoglin or CD31 were selected and photographed at 100 × magnification. Images were binarized and the extent of staining was quantified using ImageJ 1.43u (National Institutes of Health, Bethesda, MD, USA). Finally, the average MVD out of four photographs was taken. The microvessel quantification was performed blinded, that is, without knowledge of patients or tumor characteristics, and expressed as the number of pixels per field × 1000.
Tissues were homogenized and protein concentrations were determined according to Lowry et al[14,15]. Endoglin levels were determined in tissue homogenates, using a commercially available quantitative immunoassay (ELISA) for human endoglin, performed according to the manufacturer’s instructions (R&D Systems), as described before[14]. VEGF tissue levels were determined using a commercially available duoset (R&D Systems) as described before[16].
Statistical analysis was performed using SPSS version 16 and GraphPad Prism version 5. Unpaired t test and one-way ANOVA were used to compare mean levels of endoglin and VEGF between various data sets. Orthogonal regression analysis and Pearson’s correlation (r) were used to explore the relationship between two variables. Survival curves were plotted using the method of Kaplan and Meier. Results are reported as mean ± SE. A P value of < 0.05 was considered statistically significant.
Overall, 41 patients with NETs were included (Table 1), of which, the majority were female. Most patients (28/41) had a solitary primary tumor, while 13/41 patient had multiple primaries. Primary tumors of 23/41 patients were localized in the pancreas, 5/41 in the duodenum, 10/41 in the small bowel, 1/41 in the appendix, 1/41 in the sigmoid, and in one patient, the exact primary tumor location was unknown. Functional tumors were mainly insulinomas (42.1%) and gastrinomas (52.6%). Tumor size was significantly different between the groups (P = 0.01), with a smaller tumor size for functional PNETs. Metastases were seen in the majority of patients, with an almost equal distribution of lymph node or liver location. Angio-invasion was present in only 18.3% of the tumors.
| Patients (n = 41) | |
| Age (yr) | |
| mean ± SD | 47 ± 14 |
| Range | 20-77 |
| Sex | |
| Male | 17 (41.5) |
| Female | 24 (58.5) |
| Tumor type | |
| Carcinoid | 12 (29.3) |
| Functional PNET | 19 (46.3) |
| Non-functional PNET | 10 (24.4) |
| Tumor grade | |
| Well-differentiated NET | 13 (31.7) |
| Well-differentiated NEC | 26 (63.4) |
| Poorly differentiated NEC | 1 (2.4) |
| Unknown | 1 (2.4) |
| Metastases | |
| Present | 26 (63.4) |
| Lymph node only | 9 (34.6) |
| Liver only | 7 (26.9) |
| Both | 10 (38.5) |
| Absent | 15 (36.6) |
| Tumors (n = 60) | |
| Primary or metastatic tissues | |
| Primary | 45 (75.0) |
| Metastasis | 15 (25.0) |
| Angio-invasion | |
| Present | 11 (18.3) |
| Absent | 49 (81.7) |
| Tumor size (mean ± SD, cm) | |
| Carcinoids | 3.4 ± 2.7 |
| Functional PNETs | 1.9 ± 1.7 |
| Non-functional PNETs | 3.6 ± 2.4 |
Endoglin and VEGF tissue levels were measured in 27 tumor samples from 18 patients with GEP-NETs. Endoglin and VEGF levels were significantly increased in tumors compared to (associated) normal tissues (Table 2). However, among the various types of GEP-NETs, both endoglin and VEGF levels were comparable. Metastatic tumors showed significantly higher endoglin levels compared to those in primary lesions. VEGF levels were also increased in metastases, although not significantly. Furthermore, well-differentiated NECs showed significantly higher endoglin levels compared to well-differentiated NETs. Again, this difference in VEGF levels was not statistically significant, although levels in well-differentiated NECs were also increased. Of particular interest, we observed that primary tumor tissues of patients who had developed lymph node or liver metastases displayed significantly higher endoglin levels than from those without metastases. Neither endoglin nor VEGF levels were significantly related to other clinicopathological parameters, including patients’ age, sex, hormonal status (i.e. functional or non-functional) of the PNETs, or the presence of angio-invasion. Endoglin tissue levels, but not tissue levels of VEGF, were found to increase with tumor size (Figure 1). Finally, endoglin tumor levels showed no significant correlation with VEGF tumor levels (r = 0.11 with P = 0.59).
| Endoglin (ng/mg) | VEGF (pg/mg) | |||||||
| n | mean | SE | P | n | mean | SE | P | |
| Tissues | ||||||||
| Normal | 38 | 12.1 | 2.0 | < 0.012 | 38 | 75.0 | 9.5 | < 0.012 |
| Tumor | 27 | 26.8 | 4.5 | 26 | 316.8 | 46.0 | ||
| Tumor type | ||||||||
| Carcinoid | 8 | 35.3 | 11.4 | 0.37 | 8 | 354.9 | 72.0 | 0.67 |
| Functional PNET | 14 | 25.4 | 4.7 | 13 | 274.4 | 46.7 | ||
| Non-functional PNET | 5 | 16.8 | 8.7 | 5 | 366.2 | 186.8 | ||
| Origin | ||||||||
| Primary tumors | 19 | 18.8 | 3.9 | < 0.012 | 18 | 293.2 | 52.0 | 0.45 |
| Metastatic tumors | 8 | 45.7 | 9.0 | 8 | 369.9 | 95.8 | ||
| WHO classification | ||||||||
| Well-differentiated NETs | 6 | 7.6 | 5.2 | 0.0212 | 6 | 200.2 | 52.8 | 0.211 |
| Well-differentiated NECs | 20 | 32.9 | 4.0 | 19 | 328.5 | 60.2 | ||
| Poorly-differentiated NECs | 1 | 19.0 | ND | 1 | 795.0 | ND | ||
| Primary tumors: metastases | ||||||||
| Present | 12 | 24.8 | 5.2 | 0.042 | 11 | 339.5 | 76.4 | 0.28 |
| Absent | 7 | 8.5 | 3.5 | 7 | 220.6 | 54.8 | ||
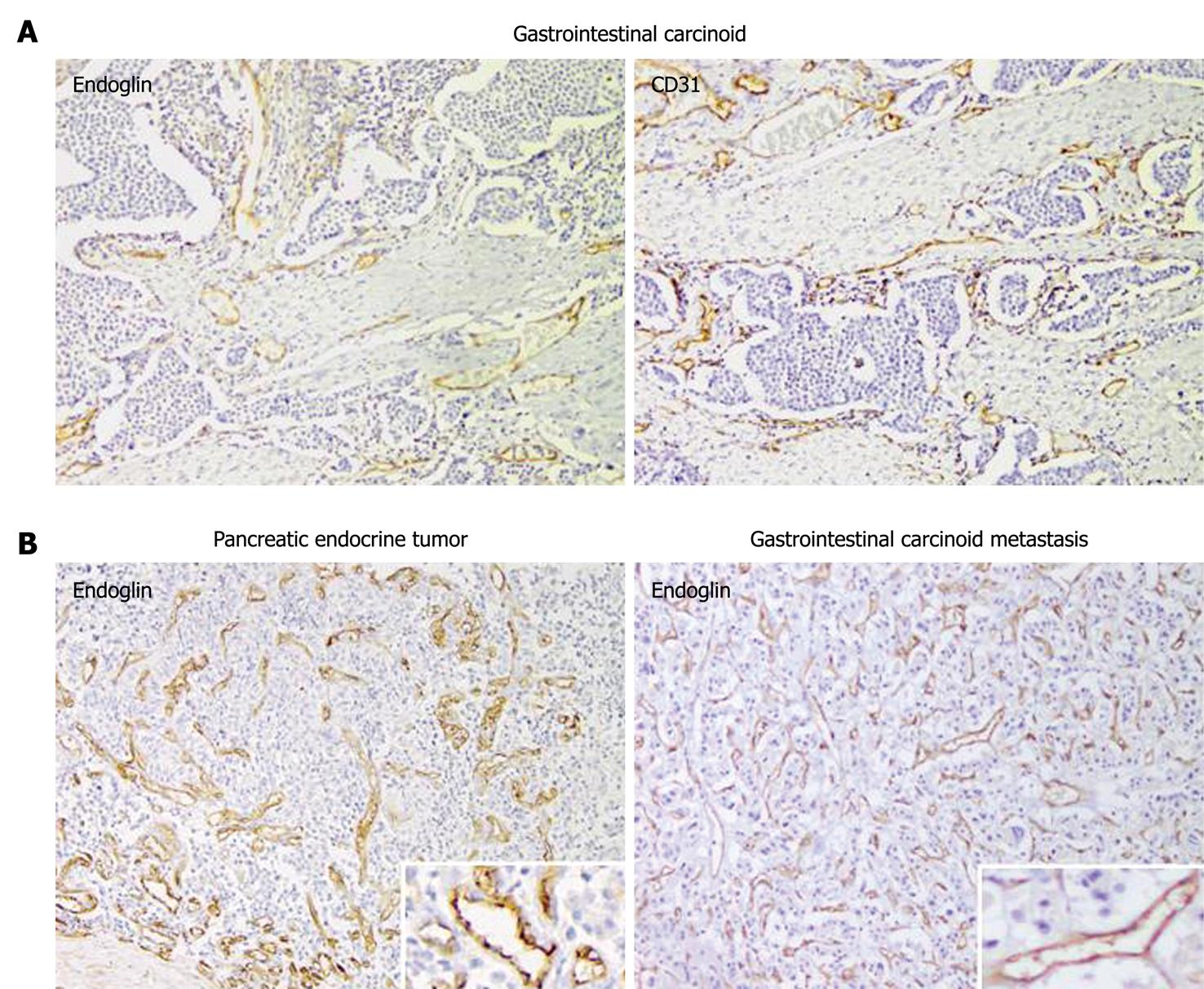
The immunohistochemical expression of endoglin and CD31 was analyzed in 39 patients with GEP-NETs. All tumors showed expression for CD31 and endoglin on intratumor vascular ECs. Endoglin expression was mainly observed on ECs of small tumor-associated blood vessels, whereas its expression in normal, non-tumorous tissue was weak or negative, in contrast to CD31 staining (Figure 2). The CD31 MVD was found to be significantly higher than the endoglin MVD in 73% of the tumor samples (P < 0.01). No significant differences in endoglin and CD31 MVD were observed between carcinoids and PNETs (Table 3). Furthermore, endoglin and CD31 MVD were not significantly related to clinicopathological parameters such as patients’ age, sex, tumor size, functionality, and angio-invasion.
| MVD-endoglin | MVD-CD31 | |||||||
| n | mean1 | SE1 | P | n | mean1 | SE1 | P | |
| Tumor type | ||||||||
| Carcinoid | 11 | 55 | 107 | 0.30 | 13 | 123 | 23 | 0.75 |
| Functional PNET | 24 | 65 | 8 | 23 | 106 | 18 | ||
| Non-functional PNET | 14 | 85 | 18 | 14 | 100 | 17 | ||
| Origin | ||||||||
| Primary tumors | 36 | 66 | 8 | 0.58 | 37 | 111 | 13 | 0.69 |
| Metastatic tumors | 13 | 75 | 15 | 13 | 101 | 24 | ||
| WHO classification | ||||||||
| Well-differentiated NETs | 13 | 69 | 18 | 0.932 | 13 | 76 | 12 | 0.082 |
| Well-differentiated NECs | 33 | 67 | 7 | 34 | 121 | 15 | ||
| Poorly-differentiated NECs | 1 | 212 | 1 | 82 | ||||
| Primary tumors: metastases | ||||||||
| Present | 19 | 66 | 9 | 0.96 | 20 | 138 | 18 | 0.053 |
| Absent | 17 | 67 | 14 | 17 | 88 | 15 | ||
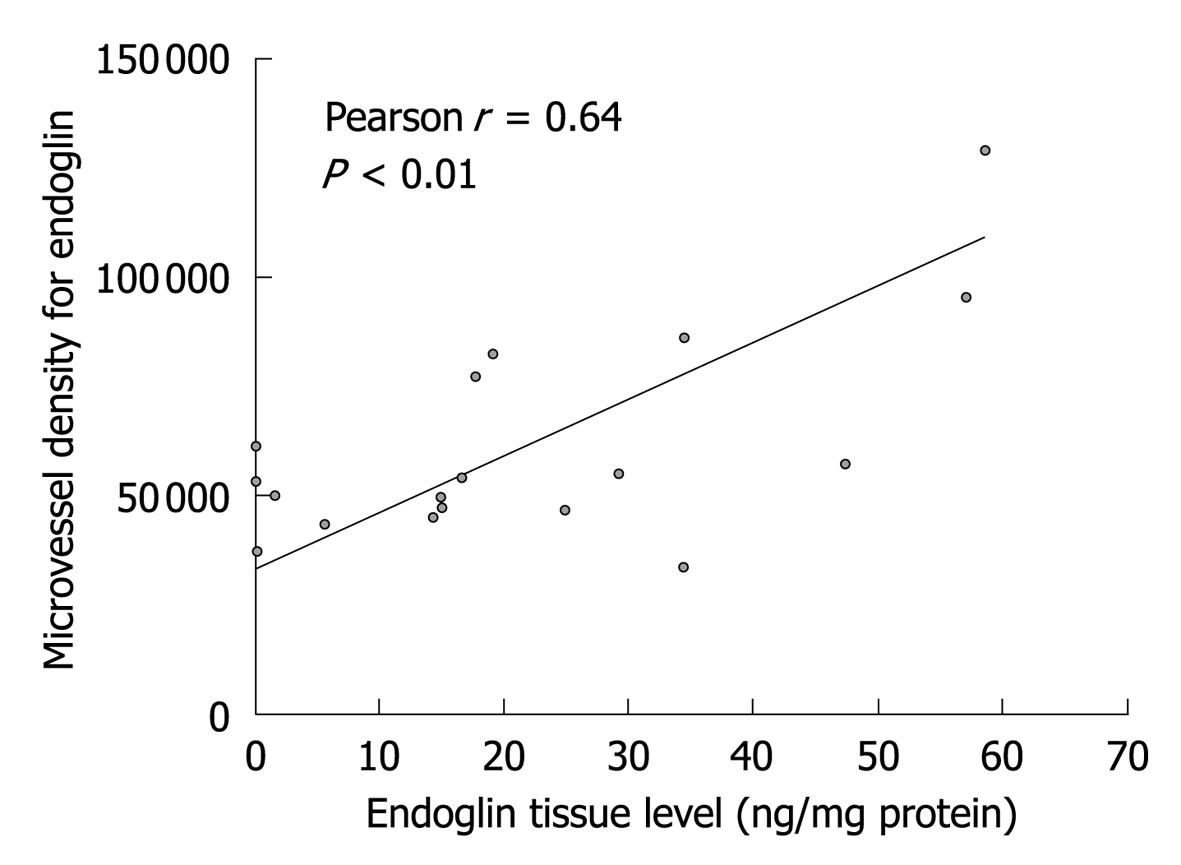
Endoglin and CD31 MVD were significantly correlated with endoglin tumor levels; r = 0.64 with P < 0.01 (Figure 3) and r = 0.58 with P < 0.01, respectively. VEGF tumor levels were not correlated with endoglin MVD (r = 0.28 with P = 0.25), but were borderline significantly correlated with CD31 MVD (r = 0.43 with P = 0.07).
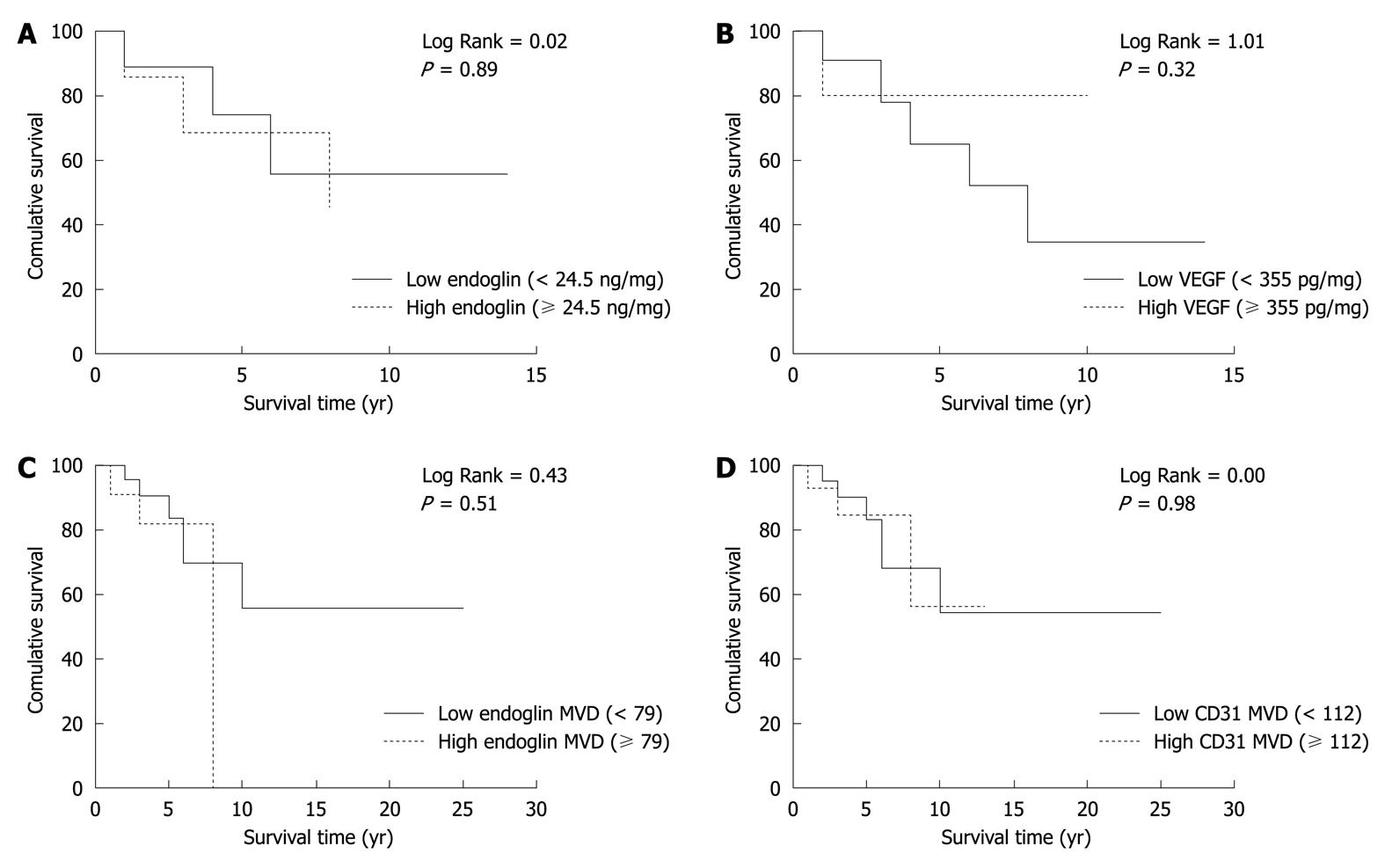
To evaluate the prognostic potential of endoglin and VEGF tissue levels, Kaplan-Meier survival analysis was performed (Figure 4) by dividing the patients into two groups (i.e. low vs high) using the mean value of endoglin and VEGF tumor levels (Table 2). Both endoglin and VEGF tissue levels were not significantly related to patient survival. Furthermore, patients were divided into two groups based on the MVD of endoglin and CD31. Both parameters were not significantly correlated with overall survival of these patients.
In this study, we observed that the expression of the angiogenic cell marker endoglin was related to tumor size, aggressiveness and metastatic potential in patients with GEP-NETs, whereas expression of another key player in angiogenesis, namely VEGF, was not.
In general, GEP-NETs are highly vascularized. In recent years, it has become clear that angiogenesis has important effects on tumor progression in several cancers, and the therapeutic role of angiogenesis inhibitors in the treatment of cancers is increasing[17,18]. In this study, we investigated whether endoglin and VEGF were related to any clinicopathological characteristics of GEP-NETs, and evaluated their potential prognostic implications.
By immunohistochemistry, we observed high endoglin expression on vascular ECs in tumor tissues of GEP-NETs. In contrast to CD31, immunopositivity of endoglin was mainly observed on newly formed blood vessels, which indicates that endoglin is more representative of tumor neovascularization than the pan-endothelial marker CD31.
Furthermore, we found that endoglin tissue levels were significantly higher in tumors compared to normal tissues. We observed that increased endoglin expression was indicative of metastatic disease. Endoglin levels were higher in metastases compared to primary tumors, and primary tumors with metastases showed higher endoglin levels compared to tumors without metastases. Additionally, endoglin levels were increased in well-differentiated NECs compared to well-differentiated NETs, and higher endoglin levels were related to larger tumor size in patients with GEP-NETs. In several cancers, the extent of tumor angiogenesis was shown to reflect their potency to become invasive and form metastases[19,20]. Our data indicate that tissue endoglin can serve as a potential assessment marker for tumor aggressiveness (i.e. NEC vs NET) and the presence of metastases following tumor resection. In the context of anticancer therapy, anti-endoglin treatment might provide a new effective anti-angiogenic strategy for GEP-NETs, but more research is needed. However, several promising in vivo and in vitro studies using anti-endoglin antibodies for anti-cancer treatment have recently been published[21].
In the present study, we did not evaluate the immunohistochemical expression of VEGF. High immunoexpression of VEGF on GEP-NETs has already been shown by others, but opposing results regarding the prognostic role of VEGF in these tumors have been reported. Takahashi et al[22] found no correlation of VEGF-A immunoexpression with growth of blood vessels, hematogenous spread or tumor growth in pancreatic endocrine tumors. In contrast, Zhang et al[23] have revealed that strong expression of VEGF was associated with increased angiogenesis and poor prognosis in patients with GEP-NETs. However, we determined tissue VEGF expression in GEP-NETs and found that VEGF tissue levels showed a similar pattern to endoglin, but were not significantly related to any clinicopathological parameter. Therefore, we assume that, although VEGF is most likely to be involved in the process of neoplastic blood vessel formation in GEP-NETs, this key mediator of angiogenesis is not the appropriate prognostic marker in these tumors. In contrast, our data suggest that endoglin can function as a predictive marker for the development of metastases in GEP-NETs. Endoglin is a co-receptor for TGF-β1. Among the various members of the TGF-β family, TGF-β1 is mostly involved in cancer, and has been shown to stimulate angiogenesis[24]. Endoglin is an important modulator of the TGF-β response; particularly in tumor pathogenesis[25]. In another study by our group, strongly increased tissue levels of endoglin were observed in colorectal cancer, whereas premalignant lesions displayed endoglin levels comparable to those in normal tissues, which supports the pivotal role of endoglin in tumor progression[14].
The fact that neither endoglin nor VEGF levels were associated with patient survival might be due to the relatively good prognosis of the patients. Gastrointestinal carcinoids show a 5-year survival rate of about 70%, whereas PNETs have a reported 5-year survival rate ranging from 25% to 100%, even in the case of (unresectable) liver metastases[26,27]. In our study cohort, 10/18 patients in whom endoglin or VEGF levels were determined were still alive at the end of the study (median survival 8 years), which makes it unlikely to use one of these parameters as a predictor of outcome or survival marker. However, our data support a role for endoglin in identifying patients with GEP-NETs at risk for metastasis.
It is worth reiterating that the current study involved a relatively small number of patients. Nevertheless, GEP-NETs are a rare disease with a low incidence, which leads to general scarcity of patients and samples. However, we believe that the significant differences observed here are representative and illustrate the differential expression pattern of endoglin and VEGF among GEP-NETs.
In conclusion, we suggest that endoglin is a potential marker to predict present and future metastases, which might help to optimize the therapeutic approach in patients with GEP-NETs.
Angiogenesis is required for tumor growth and progression and development of metastases. Vascular endothelial growth factor (VEGF) and endoglin both play an important role in angiogenesis. Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are rare and heterogeneous. Although markers for GEP-NETs exist, sensitive and specific markers that indicate tumor growth and behavior are lacking.
The aim of the present study was to evaluate the expression and potential prognostic role of VEGF and endoglin in GEP-NETs.
From other studies it is already known that GEP-NETs are highly vascularized tumors. Although several studies have investigated the immunohistochemical expression VEGF in GEP-NETs, VEGF tissue levels or endoglin expression have not been studied in these tumors before. Therefore, this study is believed to be the first to investigate tissue expression and levels of VEGF and endoglin in GEP-NETs, to determine the clinical impact of these angiogenic factors in patients with GEP-NETs.
Based on our findings, we suggest that endoglin is a potential marker to indicate the presence of metastases in GEP-NETs. By demonstrating that increased endoglin expression on tumors is related to tumor aggressiveness (including grade of differentiation, size and presence of metastases), this study could present a future target for post-resection therapeutic intervention in the treatment of patients with GEP-NETs.
Angiogenesis is the process of new blood vessel formation. This process is induced by several growth factors, including VEGF, and transforming growth factor (TGF)-β1. Endoglin is a co-receptor for TGF-β1 and a marker for angiogenic endothelial cells.
This is a well-written paper that describes a study that evaluated the expression and potential prognostic role of VEGF and endoglin in a small sample of GEP-NETs patients, and is of considerable interest.
Peer reviewer: De-Liang Fu, Professor, Department of General Surgery, Pancreatic Disease Institute, 12 Wulumuqi Road, Shanghai 200040, China
S- Editor Sun H L- Editor Kerr C E- Editor Zheng XM
| 1. | Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11:1-18. |
| 2. | Poncet G, Villaume K, Walter T, Pourreyron C, Theillaumas A, Lépinasse F, Hervieu V, Cordier-Bussat M, Scoazec JY, Roche C. Angiogenesis and tumor progression in neuroendocrine digestive tumors. J Surg Res. 2009;154:68-77. |
| 3. | Folkman J. Tumor angiogenesis. Adv Cancer Res. 1974;19:331-358. |
| 4. | Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9:211-220. |
| 5. | Strosberg JR, Kvols LK. A review of the current clinical trials for gastroenteropancreatic neuroendocrine tumours. Expert Opin Investig Drugs. 2007;16:219-224. |
| 6. | Yao JC, Phan A, Hoff PM, Chen HX, Charnsangavej C, Yeung SC, Hess K, Ng C, Abbruzzese JL, Ajani JA. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316-1323. |
| 7. | Hawinkels LJ, Verspaget HW, van Duijn W, van der Zon JM, Zuidwijk K, Kubben FJ, Verheijen JH, Hommes DW, Lamers CB, Sier CF. Tissue level, activation and cellular localisation of TGF-beta1 and association with survival in gastric cancer patients. Br J Cancer. 2007;97:398-404. |
| 8. | Li C, Guo B, Bernabeu C, Kumar S. Angiogenesis in breast cancer: the role of transforming growth factor beta and CD105. Microsc Res Tech. 2001;52:437-449. |
| 9. | Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22:6557-6563. |
| 10. | Zijlmans HJ, Fleuren GJ, Hazelbag S, Sier CF, Dreef EJ, Kenter GG, Gorter A. Expression of endoglin (CD105) in cervical cancer. Br J Cancer. 2009;100:1617-1626. |
| 11. | Yoshitomi H, Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Miyazaki M. Specific expression of endoglin (CD105) in endothelial cells of intratumoral blood and lymphatic vessels in pancreatic cancer. Pancreas. 2008;37:275-281. |
| 12. | Romani AA, Borghetti AF, Del Rio P, Sianesi M, Soliani P. The risk of developing metastatic disease in colorectal cancer is related to CD105-positive vessel count. J Surg Oncol. 2006;93:446-455. |
| 13. | Rindi G, Klöppel G. Endocrine tumors of the gut and pancreas tumor biology and classification. Neuroendocrinology. 2004;80 Suppl 1:12-15. |
| 14. | Hawinkels LJ, Kuiper P, Wiercinska E, Verspaget HW, Liu Z, Pardali E, Sier CF, ten Dijke P. Matrix metalloproteinase-14 (MT1-MMP)-mediated endoglin shedding inhibits tumor angiogenesis. Cancer Res. 2010;70:4141-4150. |
| 15. | Lowry OH, Rosebrough NJ, Farr AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. |
| 16. | Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, Fontijn RD, David G, Hommes DW, Lamers CB. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 2008;44:1904-1913. |
| 17. | Quesada AR, Muñoz-Chápuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 2006;26:483-530. |
| 18. | Nussenbaum F, Herman IM. Tumor angiogenesis: insights and innovations. J Oncol. 2010;2010:132641. |
| 19. | Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401-409. |
| 20. | Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1-8. |
| 21. | Fonsatti E, Nicolay HJ, Altomonte M, Covre A, Maio M. Targeting cancer vasculature via endoglin/CD105: a novel antibody-based diagnostic and therapeutic strategy in solid tumours. Cardiovasc Res. 2010;86:12-19. |
| 22. | Zhang J, Jia Z, Li Q, Wang L, Rashid A, Zhu Z, Evans DB, Vauthey JN, Xie K, Yao JC. Elevated expression of vascular endothelial growth factor correlates with increased angiogenesis and decreased progression-free survival among patients with low-grade neuroendocrine tumors. Cancer. 2007;109:1478-1486. |
| 23. | Takahashi Y, Akishima-Fukasawa Y, Kobayashi N, Sano T, Kosuge T, Nimura Y, Kanai Y, Hiraoka N. Prognostic value of tumor architecture, tumor-associated vascular characteristics, and expression of angiogenic molecules in pancreatic endocrine tumors. Clin Cancer Res. 2007;13:187-196. |
| 24. | Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506-520. |
| 25. | Bernabeu C, Lopez-Novoa JM, Quintanilla M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim Biophys Acta. 2009;1792:954-973. |
| 26. | Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, Caplin M, Delle Fave G, Kaltsas GA, Krenning EP. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61-72. |
| 27. | Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469-1492. |