Published online Jan 14, 2011. doi: 10.3748/wjg.v17.i2.213
Revised: October 19, 2010
Accepted: October 26, 2010
Published online: January 14, 2011
AIM: To study the effect of circulating cell-free oxyhemoglobin (FHb) on intestinal microcirculation and intestinal epithelial injury in a rat model.
METHODS: To induce elevated intravascular circulating FHb, male Sprague-Dawley rats received water or FHb infusion. Microcirculatory changes in jejunum, ileum and colon were evaluated using fluorescent microspheres. Intestinal injury was quantified as plasmatic release of ileal lipid binding protein (iLBP) and verified by histological analysis of the ileum.
RESULTS: Water and FHb infusions resulted, when compared with saline infusion, in reduced intestinal microcirculation (after 30 min P < 0.05, or better; after 60 min FHb infusion P < 0.05 for jejunum and colon). Circulating FHb levels correlated significantly with release of iLBP (Spearman r = 0.72, P = 0.0011). Epithelial cell injury of the villi was histologically observed after water and FHb infusions.
CONCLUSION: This study shows that circulating FHb leads to a reduction in intestinal microcirculatory blood flow with marked injury to intestinal epithelial cells. These data support the hypothesis that circulating FHb contributes to the development of intestinal injury.
- Citation: Hanssen SJ, Lubbers T, Hodin CM, Prinzen FW, Buurman WA, Jacobs MJ. Hemolysis results in impaired intestinal microcirculation and intestinal epithelial cell injury. World J Gastroenterol 2011; 17(2): 213-218
- URL: https://www.wjgnet.com/1007-9327/full/v17/i2/213.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i2.213
Gastrointestinal complications following cardiovascular surgery are feared, as these complications are associated with high patient morbidity and mortality rates[1-4]. The proposed pathophysiological mechanisms underlying intestinal complications include: (1) Ischemia/reperfusion and hypoperfusion injury as a result of redistribution of blood flow and increased oxygen demand; (2) Inflammatory mediated endothelial dysfunction and priming; and (3) Increased mesenteric vascular resistance[5-7].
It is widely accepted that cardiovascular surgery is associated with considerable injury to red blood cells resulting in hemolysis. The use of extracorporeal circulation, donor blood transfusion and cell salvage devices inevitably leads to increased circulating levels of the hemolytic product cell-free oxyhemoglobin (FHb)[8-10]. Such circulating FHb has been reported to scavenge endothelial nitric oxide (NO) in chronic hemolytic diseases, potentially perturbing microcirculatory blood flow which may result in organ injury and/or organ dysfunction[11,12]. The effect of hemolysis on intestinal microcirculation and gut wall integrity remains unclear. We hypothesized that intravascular FHb compromises intestinal blood flow and consequently induces intestinal epithelial cell injury.
The present study aimed to evaluate intestinal blood flow and intestinal epithelial cell injury due to elevated circulating FHb levels. An animal model was developed with FHb plasma levels similar to those found during cardiovascular surgery. The influence of circulating FHb on intestinal microcirculation was studied using fluorescent microspheres and intestinal injury was evaluated both biochemically and histopathologically.
The Animal Ethics Committee of the Maastricht University Medical Center approved the study. Male Sprague-Dawley rats, 450-500 g (Charles River Laboratories, Maastricht, The Netherlands) were housed under controlled conditions of temperature and humidity. Prior to the experiments, rats were fed standard rodent chow ad libitum and had free access to water.
To generate FHb-containing solution for infusion, heparinized blood was obtained from rats through aortic punction 1 d prior to intervention. Red blood cells were isolated by centrifugation (2750 ×g, 15 min at 4°C). Supernatant and buffy-coat were carefully discarded and the remaining red blood cells were washed thrice in fresh sterile saline (1:3 v/v). Hemolysis was induced by freeze-thaw cycles. To remove all red blood cell membranes the solution was ultra-centrifuged (20 000 ×g, 30 min at 4°C) and filtered (0.2 μm, Schleicher and Schuell, Dassel, Germany). Final FHb concentration was adjusted with sterile saline to 300 μmol/L. FHb concentration for infusion, as well as plasma values, were measured by derivative spectrophotometry[13].
After induction with 4% isoflurane, anesthesia was maintained at 2% during the whole study protocol. To calculate mean arterial pressure (MAP) a cannule (polyethylene tubing, PE-10) was placed in the left femoral artery and connected to an external pressure transducer (Uniflow®; Baxter, Utrecht, The Netherlands). Microspheres were infused via a cannule (PE-10, 11 cm) that was placed in the aortic arch via the right femoral artery. The left femoral vein was used to infuse saline, water or FHb.
Three groups (n = 6 per group) were included in the study. To induce intravascular hemolysis and yield circulating FHb, the first group received sterile pyrogene-free water infusion (prime 0.6 mL/100 g BW, continuous infusion of 2 mL/100 g BW per hour); the second group received FHb infusion (prime 0.65 mL/100 g BW, continuous infusion 1.3 mL/100 g BW per hour). The control group received saline infusion in the same volume as the water infusion group.
To evaluate the intestinal microcirculatory blood flow pre-infusion, and after 15, 30 and 60 min, fluorescent microspheres with different colors were used (yellow, lemon, orange or persimmon; diameter 15 μm, 1 × 106 microspheres/mL; Dye-Trak®, Triton Technology, San Diego, CA). Infusion of microspheres (0.25-0.3 mL) and calculation of organ blood flow were performed as described previously[14].
To evaluate enterocyte damage, the release of intestinal ileal lipid binding protein (iLBP) was measured. For iLBP assessment, arterial blood samples (600 μL) were collected before infusion and after 15, 30 and 60 min of infusion. ILBP was measured in plasma by an Enzyme Linked Immuno Sorbent Assay (ELISA, detection limit 1.28 ng/mL), kindly provided by Hycult biotechnology (Hbt, Uden, The Netherlands).
After sacrifice, ileum tissue samples were fixed in neutral buffered formaldehyde and embedded in paraffin wax. For morphological evaluation, deparaffinized 3 μm sections were either stained with hematoxylin and eosin or anti-iLBP, using a polyclonal rabbit anti-mouse iLBP (cross-reactive with rat, Hbt, Uden, The Netherlands) and a biotinylated polyclonal antibody swine anti-rabbit, a streptavidin-biotin system (Dako, Glostrup, Denmark), and visualized by applying 3-amino-9-ethylcarbazole (AEC, Sigma, St. Louis, MO). A Nikon eclipse E800 microscope with a Nikon digital camera DXM1200F was used to capture images.
To test for differences in plasma levels of FHb and iLBP, two-way analysis of variance with Bonferroni post-tests was used. To test for differences between changes in microcirculatory blood flow, two-tailed unpaired t-test was used. To evaluate an association between plasma FHb and plasmatic iLBP release, Spearman correlation analysis for nonparametric data was used on area under the curve (AUC) between FHb and iLBP for each individual subject of every group.
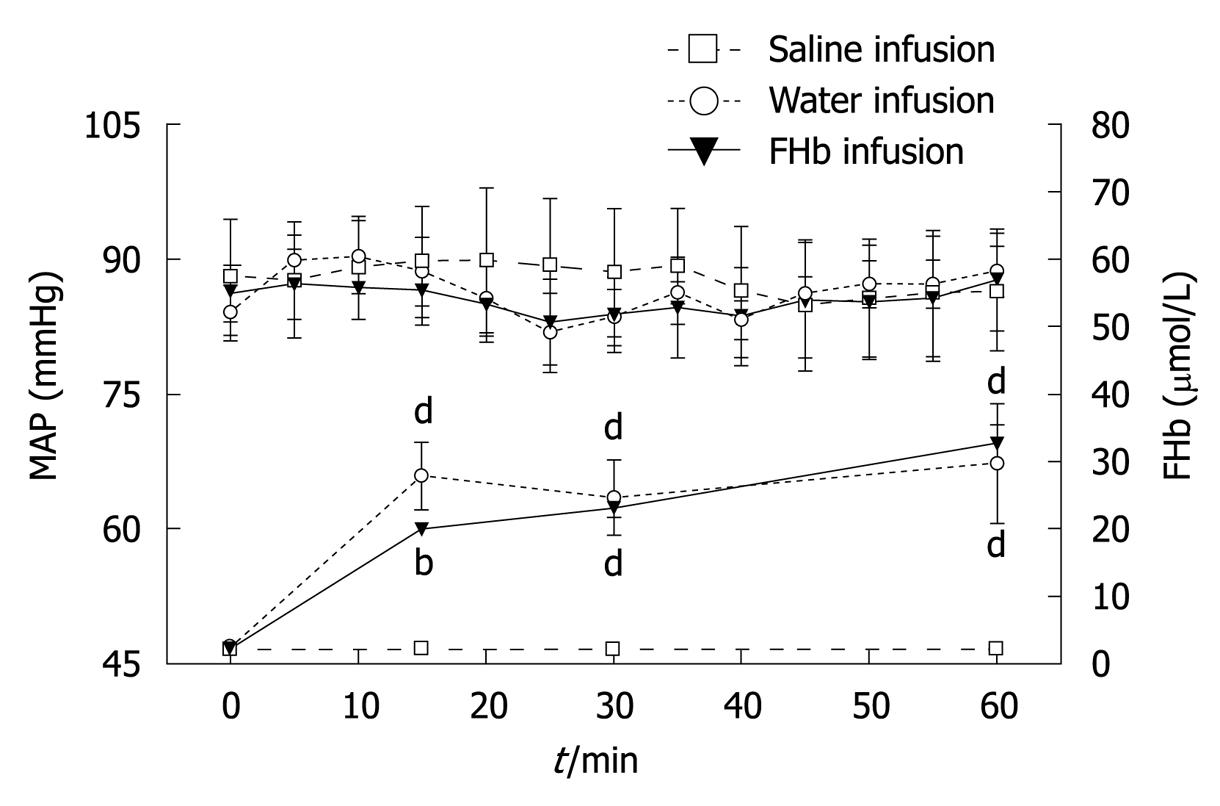
Plasma levels of FHb before intervention were comparable between all groups (Figure 1). Water and FHb infusions resulted in significantly elevated plasma levels of FHb [peak values 29.6 (8.9) μmol/L and 32.6 (2.7) μmol/L, respectively, P < 0.001]. These levels are comparable to those found in patients during cardiovascular surgery in our University Medical Center, whereas infusion of saline did not result in elevated plasma FHb levels. Infusion of either solution did not lead to changes in MAP.
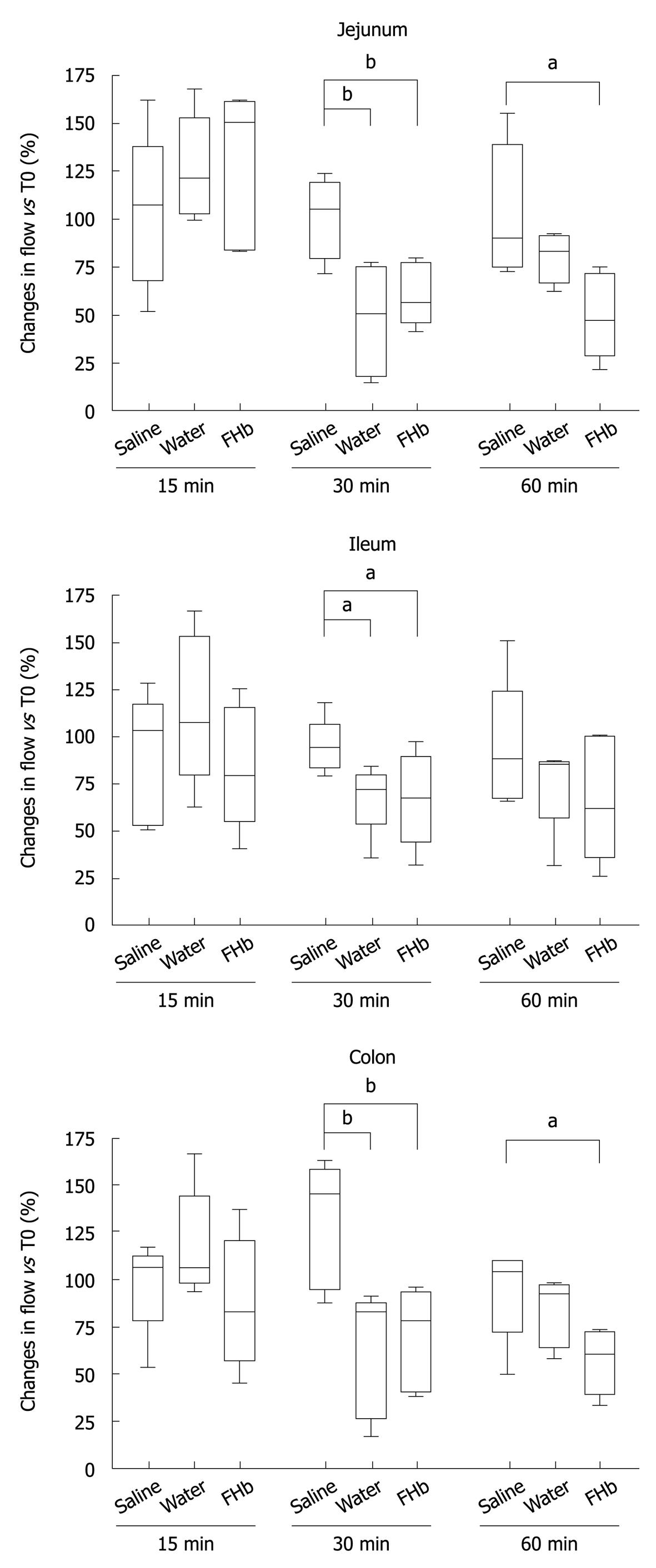
The microcirculation was evaluated in the jejunum, ileum and colon (Figure 2). Whereas after 15 min of infusion no differences in blood flow occurred, at 30 min a significant decrease in microcirculatory blood flow of the jejunum, ileum and colon was seen, when compared to the saline group (P < 0.05 or better). After 60 min, the jejunal and colonic microcirculation was still significantly reduced in the FHb infusion group (jejunum and colon: FHb vs saline infusion, P < 0.05). These data indicate a deleterious effect of FHb on intestinal microcirculatory blood flow.
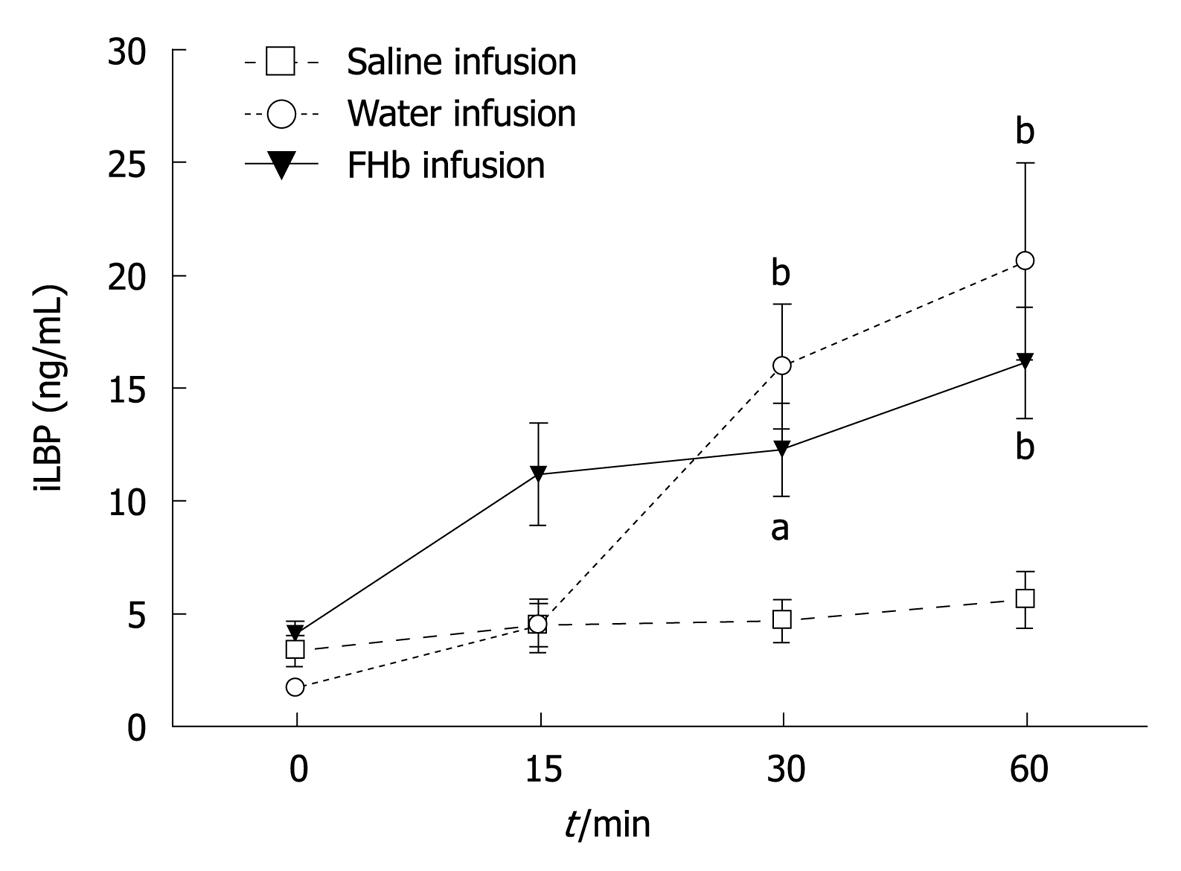
Release of iLBP: iLBP levels before infusion were comparable in all groups (Figure 3). Interestingly, at the time of reduced microcirculatory blood flow, at 30 and 60 min, plasma iLBP levels were significantly elevated in both the water and FHb infusion groups (peak values 20.6 (4.4) ng/mL and 16.1 (2.5) ng/mL, respectively). Moreover, the AUC for plasma FHb levels correlated significantly with the AUC for iLBP release (r = 0.72, P = 0.0011), indicating a strong association between hemolysis and intestinal injury.
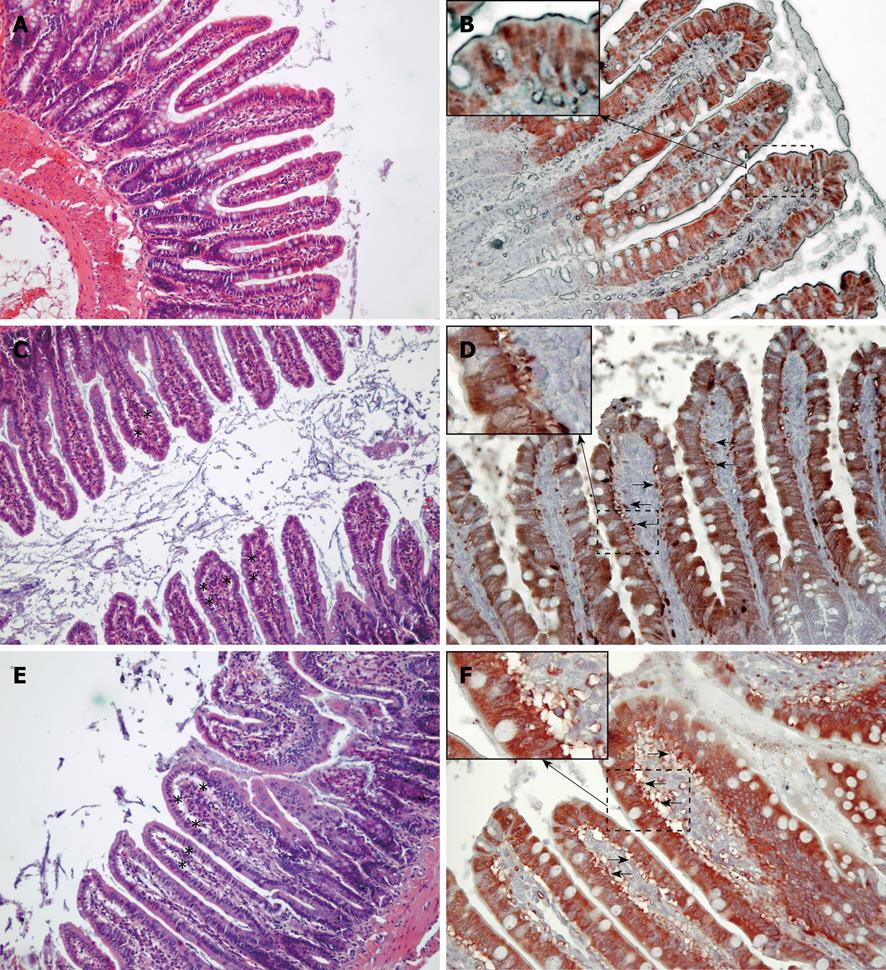
Histological analysis (Figure 4) showed subepithelial spaces and injury at the tip of the villi in both the water infusion and FHb infusion groups, but not in the saline infusion group. Immunohistochemical staining of the ileum showed subepithelial spaces positive for iLBP in both the water and FHb infusion groups, indicating that iLBP had leaked from epithelial cells. No staining was observed in control sections (data not shown). These data indicate epithelial cell injury of the gut.
Taken together, these data show that elevated plasma levels of circulating FHb, as occurs during intravascular hemolysis, decrease intestinal microcirculation and result in intestinal injury, as demonstrated by the release of iLBP and confirmed immunohistochemically.
Our animal model was designed after a canine model developed by Minneci et al[11] to study the effects of water infusion-induced intravascular hemolysis on NO bioavailability and hemodynamic variables serving as a model for chronic hemolysis, such as occurs in sickle-cell anemia. Our infusion protocol was adjusted in order to attain plasma FHb levels comparable to those found during cardiovascular surgery in our university medical center (Figure 1)[8]. The observed decreased microcirculatory blood flow at 30 min (Figure 2) suggests a rapid exhaustion of FHb scavenger proteins (haptoglobin, CD 163)[15]. The reduction in microcirculatory blood flow was less pronounced after 60 min in both the water and FHb infusion groups. This might be explained by the gradual upregulation of heme-catabolizing enzymes (HO-1, biliverdin reductase)[16,17] or enhanced NO-generating systems (NOS I, II and III)[18,19], through either enhancement of FHb clearance or counteraction of the deleterious effects of FHb, respectively.
Intestinal damage, assessed by intestinal fatty acid-binding proteins such as iLBP (Figure 3), has been reported previously in cardiovascular surgical patients[2,5]. To the authors’ best knowledge, so far no studies have been performed to evaluate a possible correlation between FHb and gastrointestinal injury in clinical or experimental studies. Recently, we have shown that intestinal epithelial cell injury occurring during and after major cardiovascular surgery is associated with a systemic inflammatory response[20]. It is tempting to speculate that FHb plays a role in the development of intestinal and also renal injury in such patients, where levels of circulating FHb are found comparable or even higher than those reached in this study[20,21]. Acute gastrointestinal complications after cardiovascular surgery are known to result in a complicated postoperative period and are associated with high mortality rates. In a current study, we show that the concentrations of plasma FHb and urine N-acetyl glucosaminidase (NAG) levels increase during extracorporeal bypass-assisted cardiovascular surgery, which indicates that hemolysis and tubular epithelial injury have occurred. Interestingly, we have found that the total release of plasma FHb is independently correlated with urine NAG, which in turn is independently associated with the postoperative increase in serum creatinine[21] used as a marker for acute kidney injury.
Taken together, our data suggest that circulating FHb is not only a health risk in chronic diseases such as sickle-cell anemia, thalassemia and malaria, but also may be a cause for concern in patients subject to extracorporeal circulation, blood transfusion and cell salvage devices resulting in substantial acute hemolysis. Therefore, circulating FHb levels should be closely monitored in clinical practice when treating these patients, who are at risk of developing gastrointestinal complications. Future studies are needed to develop means to counteract the deleterious effects of circulating FHb.
Gastrointestinal complications after cardiovascular surgery are feared, while their pathophysiology is often unclear. Cardiovascular surgery is associated with considerable injury to red blood cells, resulting in hemolysis with concomitant release of cell-free hemoglobin. Recently, studies in patients experiencing elevated levels of cell-free hemoglobin have shown a disturbed microcirculatory blood flow.
Hemolysis is not uncommon during cardiovascular surgery, resulting in elevated circulating levels of cell-free hemoglobin. The effect of hemolysis on intestinal microcirculation and gut wall integrity is unclear, so far. In this study, the authors demonstrate that hemolysis decreases intestinal microcirculatory blood flow, and that hemolysis, in particular the release of cell-free hemoglobin, is associated with intestinal damage.
Recent studies have emphasized the deleterious effects of hemolysis on microcirculatory blood flow and possible induction of organ injury. Moreover, the release of cell-free hemoglobin appears to play a pivotal role through its interference with vascular endothelial nitric oxide. This is the first study to show a decreased intestinal microcirculatory blood flow during elevated levels of circulating cell-free hemoglobin, resulting in intestinal damage. Moreover, a strong correlation between elevated circulating cell-free hemoglobin and intestinal injury is revealed.
By identifying hemolysis, and in particular the release of cell-free hemoglobin, as a potential initiator of intestinal damage, future studies can now address its specific role in the pathophysiology of gastrointestinal complications in the clinical setting of cardiovascular surgery. Furthermore, studies evaluating therapy options focussed on counteracting the deleterious effects of cell-free hemoglobin are needed.
Intravascular hemolysis results in elevated levels of circulating cell-free hemoglobin. Due to its nitric oxide scavenging properties (nitric oxide being the most potent endothelial vasodilator known), the intravascular release of cell-free hemoglobin consequently might result in decreased tissue microcirculation and cause tissue damage. In this study, intestinal injury was measured by evaluating release of ileal lipid binding protein, a protein expressed mainly in the ileum, in mature enterocytes only.
The study examined the effects of free hemoglobin and H2O infusion on artery pressure, intestinal blood flow, and ileal lipid binding protein release in rats, and found that both factors can induce intestinal epithelial injury. The topic is interesting, study is well designed and carefully carried out, manuscript is well written, and results are convincing.
Peer reviewer: Dr. Jianyuan Chai, PhD, MS, BS, Assistant Professor, Research (09-151), VA Long Beach Healthcare System, 5901 E. 7th St, Long Beach, CA 90822, United States
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Halm MA. Acute gastrointestinal complications after cardiac surgery. Am J Crit Care. 1996;5:109-118; quiz 119-120. |
| 2. | Welborn MB, Oldenburg HS, Hess PJ, Huber TS, Martin TD, Rauwerda JA, Wesdorp RI, Espat NJ, Copeland EM 3rd, Moldawer LL. The relationship between visceral ischemia, proinflammatory cytokines, and organ injury in patients undergoing thoracoabdominal aortic aneurysm repair. Crit Care Med. 2000;28:3191-3197. |
| 3. | Achouh PE, Madsen K, Miller CC 3rd, Estrera AL, Azizzadeh A, Dhareshwar J, Porat E, Safi HJ. Gastrointestinal complications after descending thoracic and thoracoabdominal aortic repairs: a 14-year experience. J Vasc Surg. 2006;44:442-446. |
| 4. | Filsoufi F, Rahmanian PB, Castillo JG, Scurlock C, Legnani PE, Adams DH. Predictors and outcome of gastrointestinal complications in patients undergoing cardiac surgery. Ann Surg. 2007;246:323-329. |
| 5. | Morariu AM, Loef BG, Aarts LP, Rietman GW, Rakhorst G, van Oeveren W, Epema AH. Dexamethasone: benefit and prejudice for patients undergoing on-pump coronary artery bypass grafting: a study on myocardial, pulmonary, renal, intestinal, and hepatic injury. Chest. 2005;128:2677-2687. |
| 6. | Tao W, Zwischenberger JB, Nguyen TT, Vertrees RA, McDaniel LB, Nutt LK, Herndon DN, Kramer GC. Gut mucosal ischemia during normothermic cardiopulmonary bypass results from blood flow redistribution and increased oxygen demand. J Thorac Cardiovasc Surg. 1995;110:819-828. |
| 7. | Ohri SK, Velissaris T. Gastrointestinal dysfunction following cardiac surgery. Perfusion. 2006;21:215-223. |
| 8. | Fransen EJ, Ganushchak YM, Vijay V, de Jong DS, Buurman WA, Maessen JG. Evaluation of a new condensed extra-corporeal circuit for cardiac surgery: a prospective randomized clinical pilot study. Perfusion. 2005;20:91-99. |
| 9. | Nishiyama T, Hanaoka K. Free hemoglobin concentrations in patients receiving massive blood transfusion during emergency surgery for trauma. Can J Anaesth. 2000;47:881-885. |
| 10. | Serrick CJ, Scholz M, Melo A, Singh O, Noel D. Quality of red blood cells using autotransfusion devices: a comparative analysis. J Extra Corpor Technol. 2003;35:28-34. |
| 11. | Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409-3417. |
| 12. | Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653-1662. |
| 13. | Cruz-Landeira A, Bal MJ, Quintela , López-Rivadulla M. Determination of methemoglobin and total hemoglobin in toxicological studies by derivative spectrophotometry. J Anal Toxicol. 2002;26:67-72. |
| 14. | Prinzen FW, Bassingthwaighte JB. Blood flow distributions by microsphere deposition methods. Cardiovasc Res. 2000;45:13-21. |
| 15. | Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373-380. |
| 16. | Yamazaki H, Ohta K, Tsukiji H, Toma T, Hashida Y, Ishizaki A, Saito T, Arai S, Koizumi S, Yachie A. Corticosteroid enhances heme oxygenase-1 production by circulating monocytes by up-regulating hemoglobin scavenger receptor and amplifying the receptor-mediated uptake of hemoglobin-haptoglobin complex. Biochem Biophys Res Commun. 2007;358:506-512. |
| 17. | Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767-1812. |
| 18. | Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med. 2006;41:1771-1780. |
| 19. | Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088-3098. |
| 20. | Hanssen SJ, Derikx JP, Vermeulen Windsant IC, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008;248:117-125. |
| 21. | Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, Altintas S, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913-920. |