Published online May 21, 2011. doi: 10.3748/wjg.v17.i19.2446
Revised: February 8, 2011
Accepted: February 15, 2011
Published online: May 21, 2011
Small bowel tumors and Crohn’s disease are common causes of small bowel obstruction. Early stage neoplasms can easily be mistaken for Crohn’s disease. Therefore, thorough work-ups including imaging studies and endoscopic evaluation with biopsies are critical for accurate diagnosis. Here we report a case of an otherwise healthy female with progressive onset of multiple, recurrent obstructive symptoms secondary to terminal ileal narrowing who was referred for management of steroid-dependent Crohn’s disease. After thorough evaluation, the diagnosis was revised to myeloid granulocytic sarcoma involving the terminal ileum. In this case, a delay in diagnosis can be detrimental for prognosis, as myeloid granulocytic sarcoma is highly predictive of underlying acute myeloid leukemia and needs urgent referral for chemotherapy and/or resection.
- Citation: Kwan LY, Targan SR, Shih DQ. A case of steroid-dependent myeloid granulocytic sarcoma masquerading as Crohn’s disease. World J Gastroenterol 2011; 17(19): 2446-2449
- URL: https://www.wjgnet.com/1007-9327/full/v17/i19/2446.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i19.2446
Small bowel tumors are uncommon and represent 1%-2% of all gastrointestinal (GI) neoplasms[1-3]. The most common locations are in order: (1) ileum; (2) duodenum; and (3) jejunum. Two large population studies reviewing small intestinal cancers reported the most common histologic types and respective subtypes were: (1) neuroendocrine (39%)/carcinoid (90%); (2) carcinoma (31%)/adenocarcinoma (69%); (3) lymphoma (18%)/large B cell (50%); and (4) sarcoma (10%)/GI stromal tumor (57%)[4,5]. Myeloid sarcoma of the small intestine is unique and rare, with only 3 of 10 945 cases reported in a recent SEER (Surveillance, Epidemiology and End Results) population study[5]. Patients often present with abdominal pain, obstruction, GI bleeding, or an abdominal mass. For patients with underlying Crohn’s disease, these symptoms can often mimic an active flare. Here we report a case of a steroid-dependent patient who was referred to our tertiary disease center. After endoscopy and workup, a rare case of myeloid granulocytic sarcoma of the ileum was diagnosed in an otherwise healthy, non-leukemic patient.
A 39-year-old female with no significant past medical history, presented with a 5-mo history of nausea, vomiting, non-bloody water diarrhea, and right lower quadrant crampy abdominal pain, resulting in multiple, recurrent small bowel obstructions. Her initial hospitalization and diagnostic workup gave a preliminary diagnosis of Crohn’s disease based on computerized tomography (CT) findings of a thick walled terminal ileum. She responded immediately to bowel rest and steroid therapy, and was then discharged home on a 6-wk taper regimen, and started on mesalamine (Pentasa) 4 g/d. However, she had recurrent obstructive symptoms when weaned below 10 mg prednisone daily resulting in another Emergency Department visit and a repeat course of oral prednisone. In the subsequent 2 mo, she was hospitalized twice more for recurrent small bowel obstruction, each time after attempts to wean off oral steroid therapy. She remained steroid-dependent despite mesalamine (Pentasa) 4 g/d and a trial of budesonide 9 mg/d. Previous NSAID use was limited to one regular strength ibuprofen per day for menstrual cramps. She associated symptoms of nausea, vomiting, bloating, and oral ulcers with increased stress.
Laboratory tests were unremarkable including inflammatory markers (erythrocyte sedimentation rate 9, C-reactive protein < 0.07) and inflammatory bowel disease (IBD)-7 serology panel, ASCA (anti-S. cerevisiae antibody) IgA < 12 EU/mL, ASCA IgG < 12 EU/mL, anti-OMPC (outer membrane protein C of E. Coli) IgA 3.9 EU/mL, anti-CBir1 3.8, and pANCA (perinuclear anti-neutrophil cytoplasmic antibody) < 12.1. Initial colonoscopy and ileoscopy were unremarkable with normal biopsies. Wireless capsule enteroscopy showed inflammation in the proximal ileum.
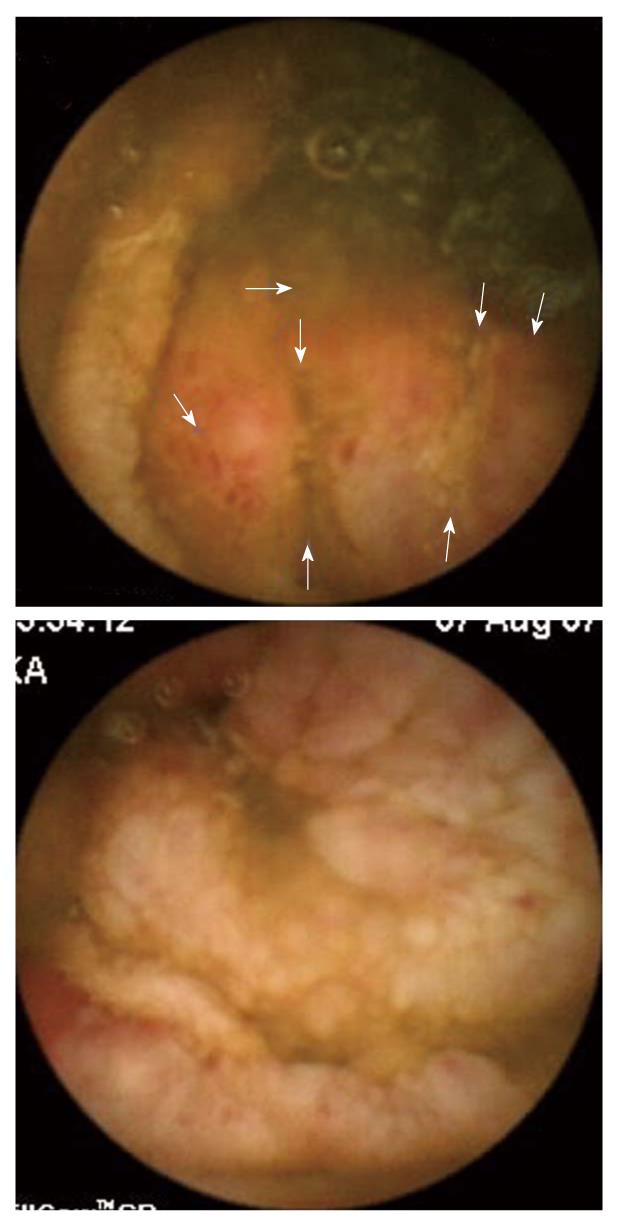
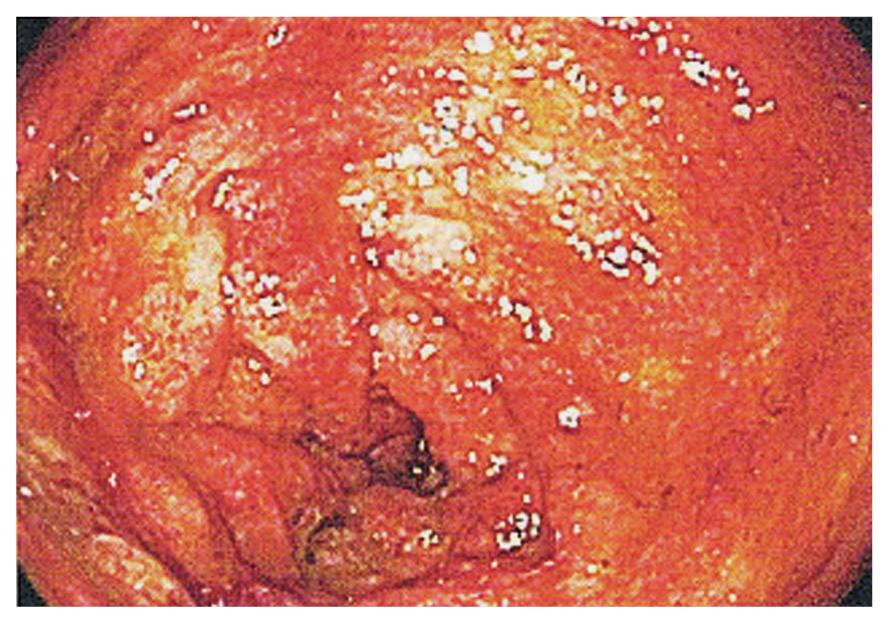
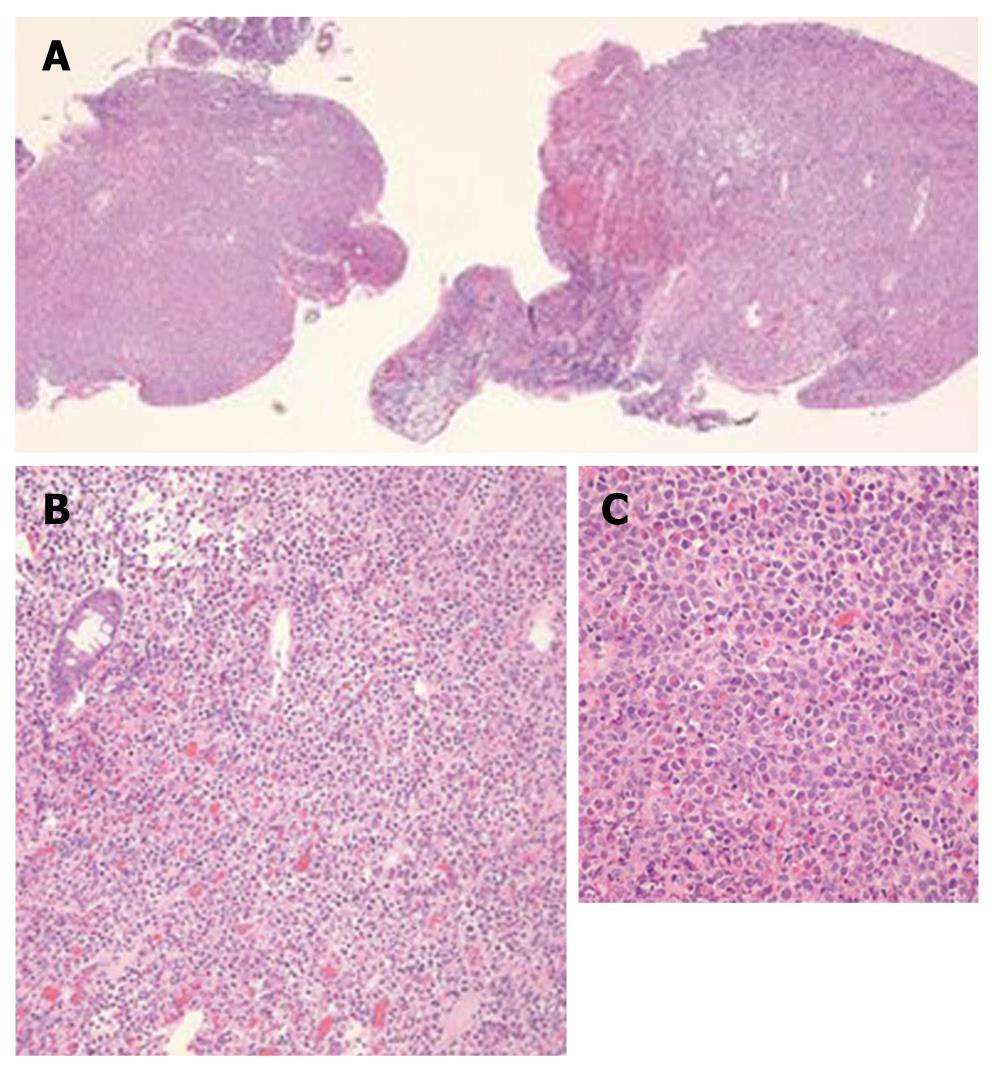
The patient was referred to our IBD center for management of steroid-dependent Crohn’s disease. We reviewed her wireless capsule study and noted additional findings that included ulcerated lumpy mucosa from the distal jejunum to proximal ileum (Figure 1), suggestive of neoplastic changes. Colonoscopy using a pediatric colonoscope showed normal colonic mucosa and ileocecal valve, but there was erythematous, ulcerated, lumpy mucosa for at least 20 cm into the terminal ileum (Figure 2). We could not advance the pediatric colonoscope further due to ileal narrowing. Ileal biopsies showed an extramedullary myeloid cell tumor (granulocytic sarcoma) with immunologic characterization consistent with myeloid lineage (myeloperoxidase positive) with co-expression of CD34, lysozyme, CD15 and CD68. Biopsies were negative for B-cell markers (CD20, CD79a, PAX-5) and T-cell markers (CD2, CD3, CD4, CD5, CD7, and CD8) (Figure 3). Other work-ups included positron emission tomography/CT that showed increased activity of the thickened bowel in the mid-anterior right quadrant, normal bone marrow aspirate, normal peripheral blood smear and flow cytometry, and fluorescent in situ hybridization (FISH) cytogenetics were negative for inv(16) and t(8;21) gene fusion. She underwent standard chemotherapy for acute myeloid leukemia (AML) followed by resection of the ileum and is currently disease free for 2 years.
Clinical presentation of a small bowel neoplasm can be nonspecific and often mimics active Crohn’s disease. Here we demonstrate a case of a presumed Crohn’s flare concealing an underlying small bowel malignancy. We also report a rare case of a sporadic isolated extramedullary myeloid cell tumor of the terminal ileum.
Myeloid sarcoma occurs in about 2 per 1 000 000 adults and 0.7 per 1 000 000 children[6]. Myeloid granulocytic sarcoma can present as an isolated tumor (67%) or as multiple lesions involving a variety of anatomic locations: skin, bone/spine, lymph nodes[7,8]. The GI tract was reported to be involved in 4 of 61 (7%) cases[9] with the small intestine being the most frequent site (10%-11%)[7,9]. In a series of 20 cases of granulocytic sarcoma of the small intestine, 17 were localized; 11 of 17 involved the ileum, 12 were in non-leukemic patients, and one occurred during a myeloid leukemia blast crisis. Eight of 12 aleukemic cases progressed to AML within a mean of 10.8 mo[8]. Another report found 90% of aleukemic patients with granulocytic sarcoma progressed to AML within 10.5-11 mo[10].
Isolated myeloid infiltrative small bowel tumors are often mistaken for non-Hodgkin lymphoma or diffuse large B cell lymphoma[11]. Other considerations which must be ruled out include lymphoproliferative disorders and poorly differentiated carcinoma in adults, and melanoma, neuroblastoma, rhabdosarcoma, Ewing sarcoma, and medulloblastoma, in children[12]. Thus, accurate diagnosis of granulocytic sarcoma by histochemical and immunoperoxidase staining, cytogenetic, FISH, or flow cytometry is critical[8].
Cytogenetic abnormalities often reported in aleukemic cases of isolated GI tract granulocytic sarcoma are inversion of p16 and/or t(8; 21) fusion gene[8]. Here we report a case of isolated granulocytic sarcoma of the ileum in an aleukemic patient without inv(16) or t(8; 21) and with no clinical manifestations of a leukemic disorder at diagnosis.
New diagnosis of granulocytic sarcoma in a non-leukemic patient often predicts onset of AML and a potential blast crisis within 1 year. In a case series, 4 of 7 sporadic intestinal granulocytic sarcoma developed AML within an average of 8 mo (range, 4-21 mo)[13]. Another report found a median time of 9 mo for isolated granulocytic sarcoma to develop AML with median survival of 22 mo[14].
The prognosis of isolated myeloid sarcoma is variable but is better if diagnosed early[8,15,16]. The prognosis is poor once there is infiltration of the GI tract with leukemic cells[17]. Systemic chemotherapy, especially AML type induction chemotherapy can delay the time to develop AML and prolong the survival period[7,9,14,18,19]. Yamauchi et al[7] showed a longer non-leukemic period after diagnosis of granulocytic sarcoma (median 12 mo) in patients treated with systemic chemotherapy (median 3-6 mo). In a series of 74 cases of primary granulocytic sarcoma without transformation to AML within 1 mo of diagnosis, 58% who received chemotherapy were disease-free for up to 11 mo, and 19% for up to 2 years compared with 5% who did not receive chemotherapy[8]. Surgical intervention or radiotherapy has not been proven to influence survival but may be indicated for symptomatic complications of tumors such as obstruction or perforation[14]. Nevertheless, a management plan with the goal of complete eradication and remission of leukemia and its respective potential needs to be made on an individualized basis.
In summary, intestinal granulocytic sarcoma is rare but should be part of the differential diagnosis for any sporadic small bowel mass of unknown origin, especially in the non-leukemic patient. An early and accurate diagnosis predicts the best outcome and survival. Granulocytic sarcoma is often a precursor to a diagnosis of AML needing urgent systemic chemotherapy and/or any combination of surgical resection, radiotherapy, or stem cell transplantation. Incomplete workup and presumptive diagnosis of Crohn’s disease for the initial manifestation of an ileal narrowing or obstruction can easily delay the diagnosis of malignant neoplasms which require urgent intervention and treatment.
Peer reviewer: Sharad Karandikar, Consultant General and Colorectal Surgeon, Department of General Surgery, Birmingham Heartlands Hospital, Birmingham, B95SS, United Kingdom
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Minardi AJ Jr, Zibari GB, Aultman DF, McMillan RW, McDonald JC. Small-bowel tumors. J Am Coll Surg. 1998;186:664-668. |
| 2. | Ito H, Perez A, Brooks DC, Osteen RT, Zinner MJ, Moore FD Jr, Ashley SW, Whang EE. Surgical treatment of small bowel cancer: a 20-year single institution experience. J Gastrointest Surg. 2003;7:925-930. |
| 3. | Feldstein RC, Sood S, Katz S. Small bowel adenocarcinoma in Crohn's disease. Inflamm Bowel Dis. 2008;14:1154-1157. |
| 4. | Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, Longo WE. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg. 2007;142:229-235. |
| 5. | Qubaiah O, Devesa SS, Platz CE, Huycke MM, Dores GM. Small intestinal cancer: a population-based study of incidence and survival patterns in the United States, 1992 to 2006. Cancer Epidemiol Biomarkers Prev. 2010;19:1908-1918. |
| 6. | Breccia M, Mandelli F, Petti MC, D'Andrea M, Pescarmona E, Pileri SA, Carmosino I, Russo E, De Fabritiis P, Alimena G. Clinico-pathological characteristics of myeloid sarcoma at diagnosis and during follow-up: report of 12 cases from a single institution. Leuk Res. 2004;28:1165-1169. |
| 7. | Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002;94:1739-1746. |
| 8. | Kohl SK, Aoun P. Granulocytic sarcoma of the small intestine. Arch Pathol Lab Med. 2006;130:1570-1574. |
| 9. | Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, Bennett JM. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981;48:1426-1437. |
| 10. | Hutchison RE, Kurec AS, Davey FR. Granulocytic sarcoma. Clin Lab Med. 1990;10:889-901. |
| 11. | Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340-350. |
| 12. | Alexiev BA, Wang W, Ning Y, Chumsri S, Gojo I, Rodgers WH, Stass SA, Zhao XF. Myeloid sarcomas: a histologic, immunohistochemical, and cytogenetic study. Diagn Pathol. 2007;2:42. |
| 13. | Corpechot C, Lémann M, Brocheriou I, Mariette X, Bonnet J, Daniel MT, Bertheau P, Lavergne A, Modigliani R. Granulocytic sarcoma of the jejunum: a rare cause of small bowel obstruction. Am J Gastroenterol. 1998;93:2586-2588. |
| 14. | Imrie KR, Kovacs MJ, Selby D, Lipton J, Patterson BJ, Pantalony D, Poldre P, Ngan BY, Keating A. Isolated chloroma: the effect of early antileukemic therapy. Ann Intern Med. 1995;123:351-353. |
| 16. | Martinelli G, Vianelli N, De Vivo A, Ricci P, Remiddi C, Testoni N, Visani G, Baravelli S, Farabegoli P, Tura S. Granulocytic sarcomas: clinical, diagnostic and therapeutical aspects. Leuk Lymphoma. 1997;24:349-353. |
| 17. | Antic D, Elezovic I, Bogdanovic A, Vukovic NS, Pavlovic A, Jovanovic MP, Jakovic L, Kraguljac N. Isolated myeloid sarcoma of the gastrointestinal tract. Intern Med. 2010;49:853-856. |
| 18. | Wong SW, Lai CK, Lee KF, Lai PB. Granulocytic sarcoma of the small bowel causing intestinal obstruction. Hong Kong Med J. 2005;11:204-206. |