Published online May 21, 2011. doi: 10.3748/wjg.v17.i19.2437
Revised: October 15, 2010
Accepted: October 22, 2010
Published online: May 21, 2011
AIM: To evaluate the concomitant effects of appendectomy and oral tolerance on colitis.
METHODS: Delayed-type hypersensitivity (DTH) was investigated at a 7-d interval after ovalbumin (OVA) administration and immunization under normal and colitis conditions in appendectomized or sham-operated mice. Pathological scores for the colon were graded after ingestion of colon-extracted protein (CEP) and induction of dextran sulfate sodium (DSS) colitis in appendectomized or sham-operated mice. Thereafter, Th1 and Th2 in Peyer’s patches and spleen lymphocytes were detected in CEP-treated and bovine serum albumin (BSA)-treated control mice.
RESULTS: In appendectomized mice, DTH was not inhibited at day 7 after OVA administration and at the initial phase of DSS colitis, whereas it was inhibited at day 14 and day 21. However, in sham-operated mice, it was inhibited during the whole procedure and the onset of DSS colitis. The protective role of CEP against DSS colitis was present in sham-operated mice, with predominant improvement of colonic pathological changes, while vanished in the appendectomized mice. A shift from Th1 to Th2 in Peyer’s patches resulted from a decrease of Th1 cells with the ingestion of CEP. Compared with BSA in the sham-operated group, no predominant changes were observed in the appendectomized mice.
CONCLUSION: Appendectomy interferes with the protective role of CEP in DSS colitis via a shift from Th2 to Th1 during oral tolerance induction.
- Citation: Yue M, Shen Z, Yu CH, Ye H, Ye YF, Li YM. Effects of appendectomy and oral tolerance on dextran sulfate sodium colitis. World J Gastroenterol 2011; 17(19): 2437-2445
- URL: https://www.wjgnet.com/1007-9327/full/v17/i19/2437.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i19.2437
Ulcerative colitis (UC) is one of the major inflammatory bowel diseases (IBDs), and is a chronic inflammatory disorder of the intestine. Multiple factors cause the initiation and pathogenesis of this disorder, including genetic polymorphisms, environmental factors, and immune responses[1]. Studies on mucosal immunological disturbance help greatly understand the course of UC, and establish better therapeutic strategies.
Oral tolerance is one of the major features in gut immunology, and refers to the active non-response to dietary antigens and commensal enteric bacteria or substances administered orally[2,3]. IBD results from environmental trigging agents that act on suspected hosts to cause uncontrolled immune reactions in the gut, and is considered to be a consequence of the breakdown of normal mucosal tolerance[4]. Oral tolerance has been confirmed to be a therapy for a variety of autoimmune diseases including IBD[5].
As a part of the intestine, the appendix has long been considered as a deteriorated organ, until epidemiological research revealed that patients who have had an appendectomy have a lower incidence of UC than those with an intact appendix[6]. Furthermore, appendectomy shows a protective effect on colitis in TCR-α-/- mice and trinitrobenzene sulfonic acid (TNBS)-induced murine colitis[7-9]. These results suggest that appendectomy is a protective factor against UC[10].
Although appendectomy and oral tolerance have been shown to have a potential role in intestine immunology, the combined effect of appendectomy and oral tolerance in the normal gut immune environment and in colitis is still unclear. In this study, the combined effect of appendectomy and oral tolerance was evaluated in mice.
Male BALB/c mice, 4-5 wk old, were purchased from Vital River (Beijing, China). Mice were kept under special pathogen-free conditions with free access to food and water, unless required by the experimental protocol. Every six mouse was allocated to the treatment or control group. The study protocol was approved by the Ethics Committee of Zhejiang University School of Medicine.
Ovalbumin (OVA; chicken egg albumin, grade V), complete Freund’s adjuvant (CFA), bovine serum albumin (BSA), phorbol myristate acetate (PMA) and ionomycin were purchased from Sigma-Aldrich (St Louis, MO, USA). Dextran sulfate sodium (DSS) was purchased from MP Biomedicals (USA). Antibodies including CD3ε, CD4, CD8α, CD69, interferon (IFN)-γ and interleukin (IL)-4, either conjugated with PE, FITC, PE-cy5 or APC, or their corresponding controls in flow cytometry were purchased from Becton Dickinson Biosciences (USA). RPMI 1640 was purchased from Invitrogen (USA) and Percoll from Amersham Bioscience (USA).
BALB/c mice were anesthetized with ketamine hydrochloride under sterile conditions. For all surgical interventions, mice underwent a standardized procedure, starting with a midline laparotomy, followed by mobilization and exteriorization of the appendix. In mice that were subjected to appendectomy, division of the appendix was performed between two ligatures that were placed proximal to the border of the cecum and appendix. The cecal stump was irrigated with saline. In all groups, the abdominal wall was closed in two layers, using a running suture technique. Mice were monitored during the immediate recovery phase after anesthesia, and were assessed daily throughout the recovery period of 1 wk.
Intragastric administration of 250 μg OVA or colon-extracted protein (CEP) for five times (both were controlled by BSA) was applied in each group, followed by subcutaneous injection of 200 μg OVA in 200 μL 50% CFA.
For delayed-type hypersensitivity (DTH) test, all mice were injected subcutaneously in the right rear footpad with 25 μL physiological saline that contained 100 μg OVA. After 24 h, the increase in footpad thickness was measured using a helicocaliper, and controlled by the normal left footpad that was treated with the same volume of PBS[11].
DSS colitis was induced by feeding 5% (w/v) DSS diluted in water for 7 d, during which, disease activity index was calculated daily according to body weight, stool consistency, and the occult blood test[12]. Mice were all sacrificed 7 d after treatment with DSS for pathological examination of the descending colon and flow cytometric detection.
Microscopic examination of the colon was performed in a blinded fashion on formalin-fixed tissue sections that were stained with hematoxylin and eosin, as previously described[13]. The sections were classified as 0-3 to evaluate the amount and depth of inflammation, and 0-4 to evaluate the amount of crypt damage or regeneration. These pathological changes were also quantified as the percentage of involvement by the disease process: 1, 1%-25%; 2, 26%-50%; 3, 51%-75%; and 4, 76%-100%. Each section was then scored for each feature separately by establishing the product of the grade for that feature and the percentage involvement (0-12 for inflammation and extent, and 0-16 for regeneration and crypt damage).
CEP was prepared as described previously[14]. The colon was sampled at 7 d after DSS treatment, minced on ice, and centrifuged for 20 min at 15 000 ×g. Protein concentration was measured after supernatant collection and filtration for sterilization.
The experiment procedure is shown schematically in Figure 1. The combined effects of appendectomy and OVA-induced oral tolerance were tested under conditions of normal gut immune status and DSS colitis. Oral tolerance was produced in mice by repeated intragastric administration of OVA at day 7 after appendectomy or sham operation, followed by 200 g OVA injected subcutaneously on day 18. DTH assessment was performed on days 25, 32 and 39 (7, 14 and 21 d after OVA immunization). To observe the effect of appendectomy on OVA oral tolerance in colitis, on day 26, a group of mice was challenged with 5% DSS, and DTH assessment was performed at the beginning and end points.
To observe the combined effect of appendectomy and CEP on protection of colitis, another group of mice was challenged with 5% DSS on day 18, and pathological assessment and lymphocyte analysis were performed at the end points.
For lymphocyte isolation of SPL and PPL[15], spleen or Peyer’s patch was removed, minced, passed through a nylon strainer, and counted after staining with trypan blue. Th1 and Th2 cells were separated by IFN-γ and IL-4 staining. Lymphocytes were cultured at 37°C for 6 h in medium that contained ionomycin (500 ng/mL), PMA (5 ng/mL) and GolgiStop.
All data are presented as mean ± SD. Paired t test was used throughout the experiment, except that the statistical significance of the pathological differences was evaluated by non-parametric Wilcoxon rank sum test. P < 0.05 was defined as statistical significance.
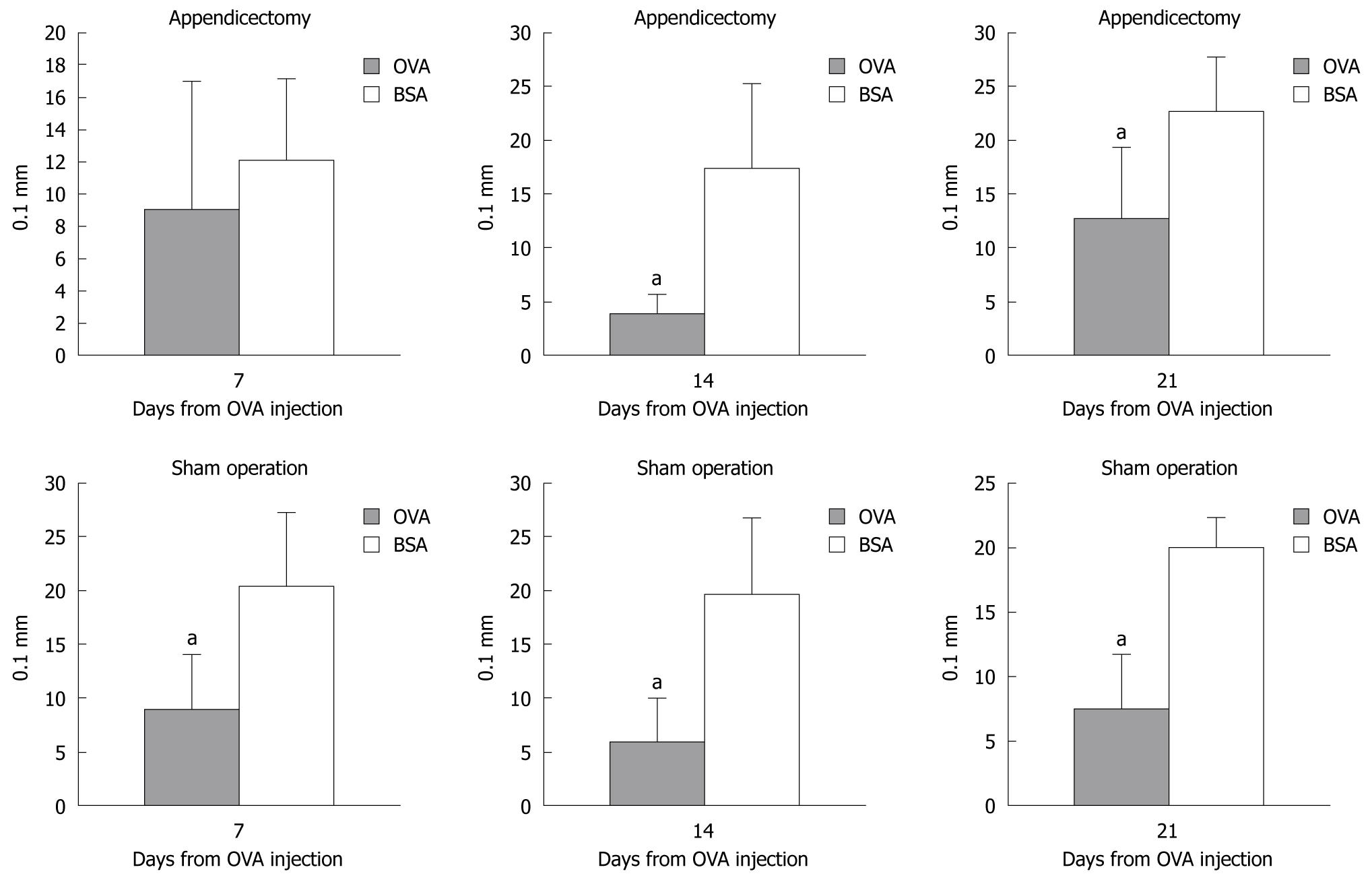
To assess the establishment of oral tolerance and verify the role of appendectomy, both the appendectomized and the sham-operated mice were injected subcutaneously with 200 g OVA. DTH was scored by measuring the thickness of the footpad at days 7, 14 and 21 after OVA injection (Figure 2).
Compared with the BSA-treated mice, the footpad thickness in the OVA-treated mice increased less significantly at days 14 and 21 in the appendectomized and sham-operated mice. However, on day 7, there was no difference in footpad thickness between the OVA- and BSA-treated appendectomized mice, but there was an obvious difference in the sham-operated mice. This suggests that appendectomy postponed the oral tolerance response.
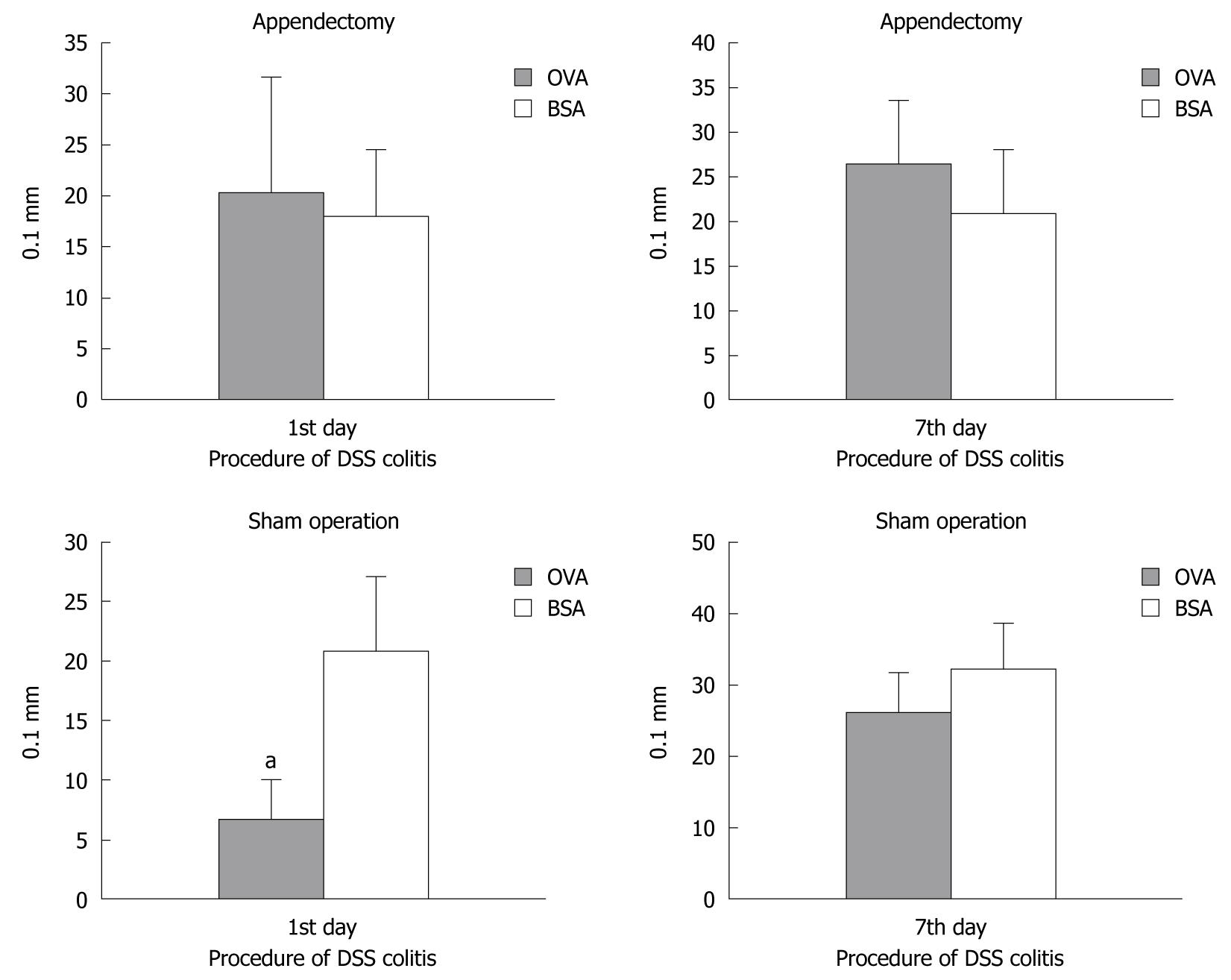
To evaluate whether appendectomy had an impact on oral tolerance in DSS-induced colitis, appendectomized and sham-operated mice were treated with 5% DSS at day 26 after OVA or BSA ingestion and OVA injection. The immune response was assessed by DTH at days 1 and 7 after DSS treatment (Figure 3).
In the appendectomized mice, DTH assessment showed no difference between OVA and BSA ingestion. In the group of sham-operated mice, although the OVA-ingested mice showed slighter immunoresponse than that of BSA-ingested mice on the first day, we could not observe the difference of immunoresponse between OVA- and BSA-ingested mice group. This was consistent with previous results and suggested that appendectomy did not enhance the oral tolerance protection in colitis. Furthermore, OVA treatment showed no protective effect against colitis both in the appendectomized and the sham-operated mice after 7 d, and the index of footpad thickness showed no significant difference. Taken together, these results suggested that OVA-induced oral tolerance was only present in sham-operated mice, and appendectomy suppressed oral tolerance in DSS-induced colitis.
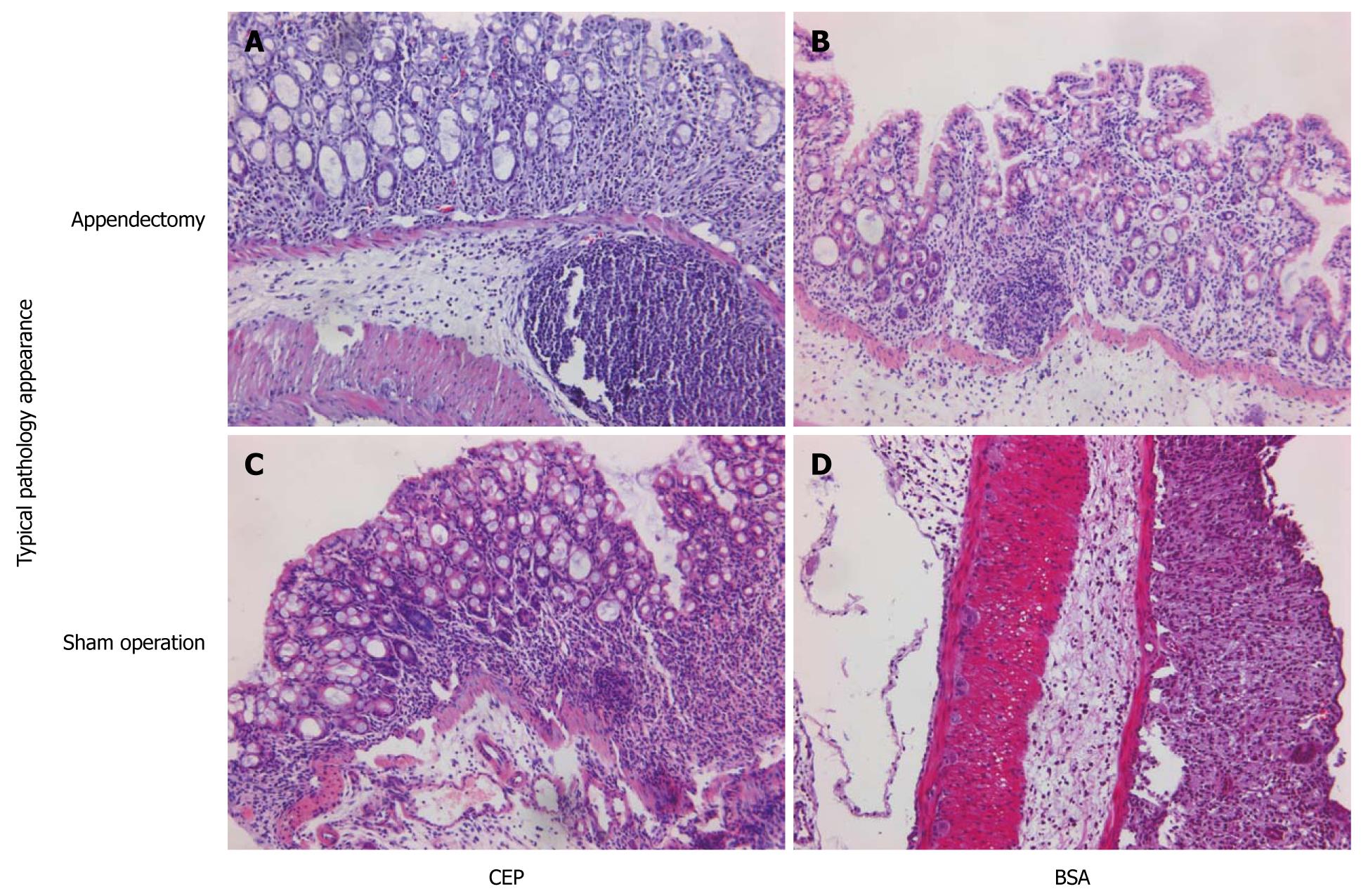
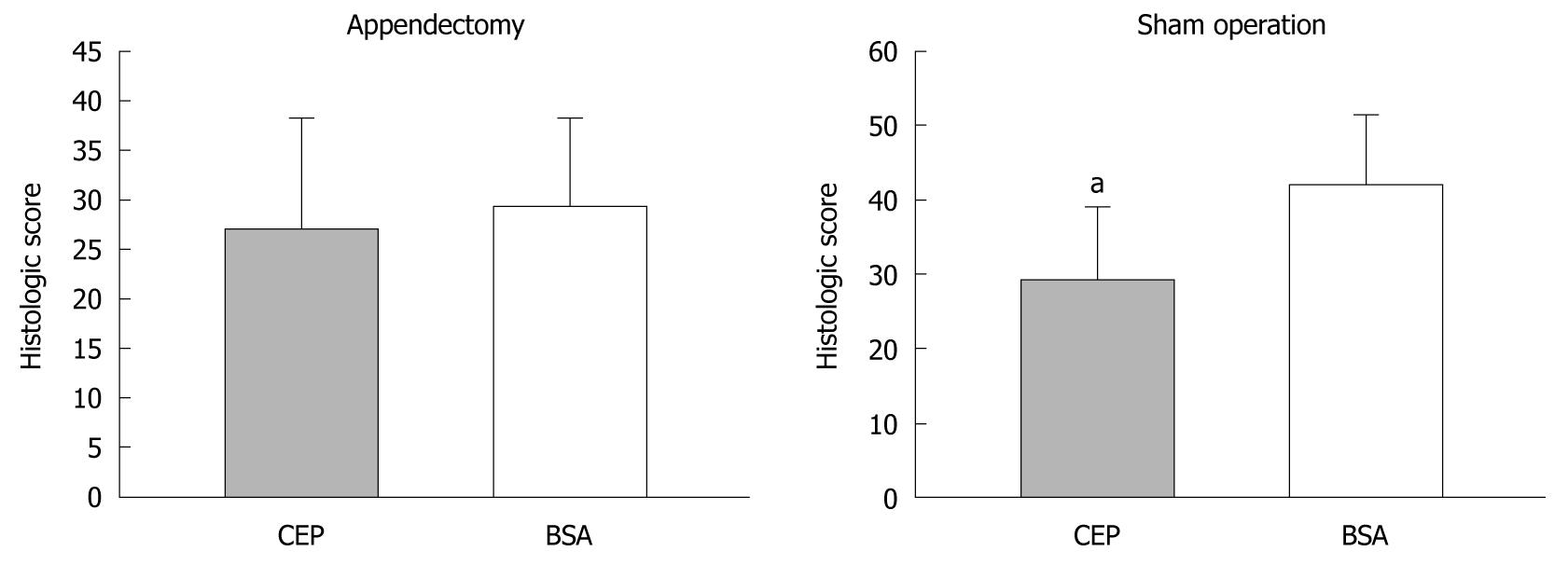
It is possible that the non-protection of OVA-induced oral tolerance to DSS colitis resulted from the fact that OVA is not a specific oral tolerance antigen to DSS colitis. Therefore, we replaced OVA with CEP in the appendectomized and the sham-operated mice on day 8. After repeated intragastric administration of CEP or BSA, for five times on day 10, the mice were challenged with 5% DSS. Murine DSS colitis was characterized by destruction of the basic structure of colonic villi and almost total cast of epithelium, with the disappearance of crypts and massive infiltration of lymphocytes. In the appendectomized mice, CEP- and BSA-treated groups showed similar histological features, with massive lymphocytes infiltration, loss of goblet cells, crypt damage, mucosal ulceration, and accompanying submucosal edema (Figures 4 and 5). In the sham-operated mice, the CEP-treated group showed less amelioration of colonic inflammation compared with the BSA-treated control group. Pathological score showed that, in sham-operated mice, ingestion of CEP resulted in mild pathological changes in the descending colon, with a significantly lower pathological score compared with the BSA controls. However, in the appendectomized mice, no significant difference was observed between the CEP- and BSA-treated groups. Our results suggested that appendectomy eliminated the protective role of CEP against DSS colitis.
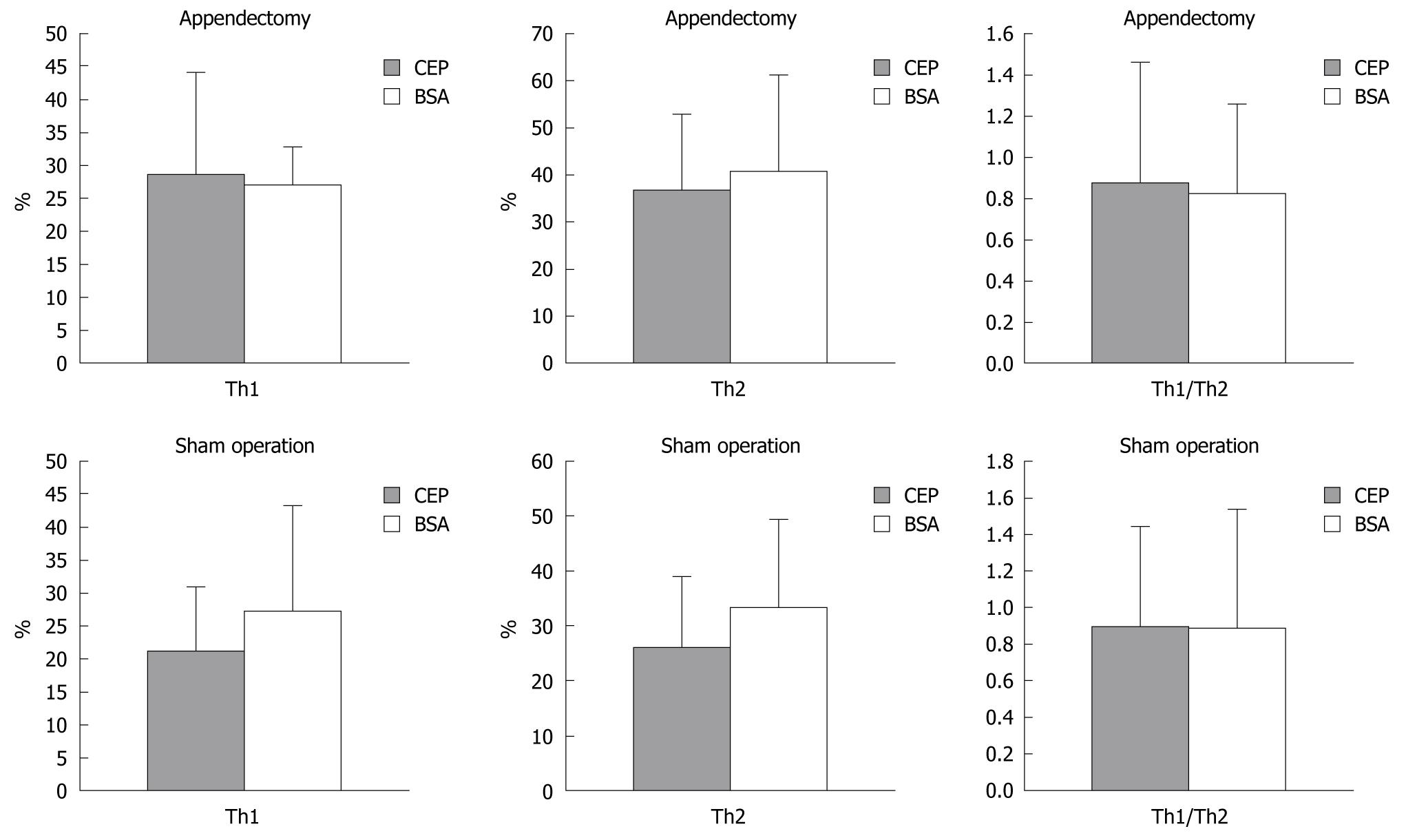
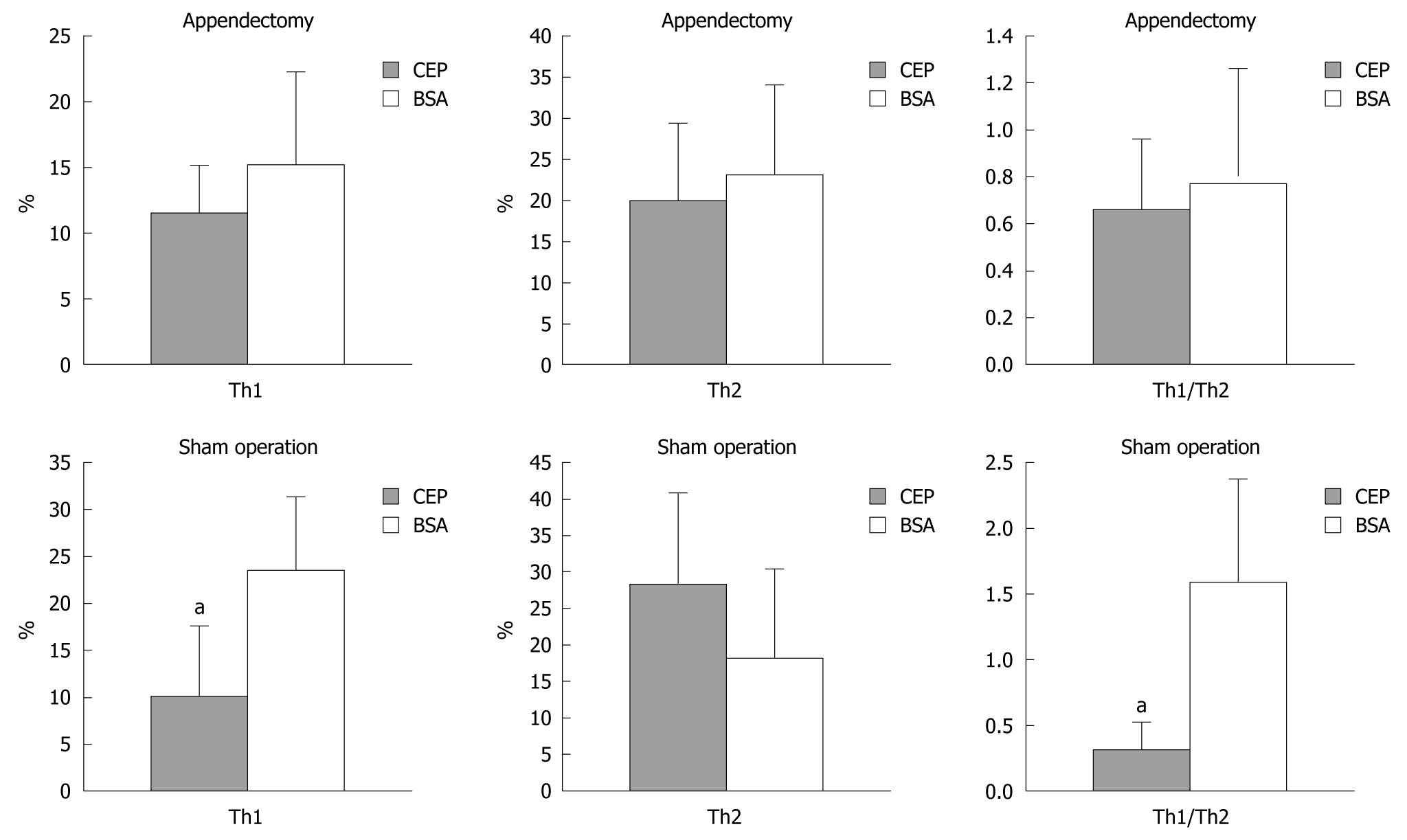
We investigated the possible role of Th1 and Th2 in the difference of CEP protection against DSS colitis between the appendectomized the sham-operated mice (Figures 6 and 7). Th1 and Th2 of PPL and SPL were isolated at the end of colitis and stained with antibodies to IFN-γ and IL-4. The ratio of Th1/Th2 in SPL showed no significant difference between appendectomized and sham-operated mice. However, in PPL, Th1 decreased predominantly after ingestion of CEP in the sham-operated mice compared with BSA control mice, which resulted in the same trend change in Th1/Th2 ratio. Meanwhile, this situation was not observed in Th2 cells. With regard to the appendectomized mice, the number of Th1 and Th2 and their ratio did not change significantly.
UC is a chronic relapsing inflammatory disorder of the large intestine. Results from experimental and clinical studies have indicated that there are multifactors in initiation and pathogenesis of this disorder, involving interactions among genetic, environmental, and immune factors[16]. Studies on mucosal immunological disturbance help greatly to understand the course of UC and might be useful to establish better therapeutic strategies. Among the immunotherapeutic options for UC, there are two phenomena that differ from other anti-cytokine strategies. One is the relationship between appendectomy and UC, and the other is the application of oral tolerance.
Appendicular lymphoid tissue, together with Peyer’s patches, intestinal intraepithelial and lamina propria lymphocytes, and mesenteric lymph nodes, has been considered as one component of the gut-associated lymphatic tissue (GALT). Several studies have suggested an inverse association between appendectomy and UC[17,18]. UC with prior appendectomy is associated with a milder disease, as defined by the lesser requirement for immuno-suppression or colectomy for severe colitis[19]. A meta-analysis has reported a strong and consistent inverse association between appendectomy and the development of UC[20]. Further support for the involvement of the appendix in IBD is provided by the observation that appendectomy prevents the development of spontaneous experimental colitis in T-cell receptor α mutant mice[7]. However, things seem to be more complicated in humans. Histopathological findings that show the skip lesion of inflammation in the appendix in UC patients suggest that inflammation at one site in the large intestine of predisposed individuals might trigger UC at distant sites. Okazaki et al[21] have reported a case with a positive response of UC to appendectomy, with improved colonoscopic and microscopic findings. Several studies have shown inadequate and obscure effects on UC, including a cohort study in Denmark that has proved that appendectomy has no beneficial effect on admission rates in patients with UC[22]. If we disregard those studies with poor or inadequately described methods, the evidence still supports an inverse association between appendectomy, particularly at a young age, and later risk of UC[17]. All the problems might come from the fact that the specific immunological changes after appendectomy remain poorly defined.
Oral tolerance is one of the main features in GALT. It can be described as a state of specific immunological unresponsiveness to antigens that are capable of inducing a protective or injurious immune response under other circumstances. Neurath et al[23] have reported that induction of oral tolerance could be beneficial to mural TNBS colitis, via the mediation of transforming growth factor β, suppressor lymphocytes, or suppression of humoral immunity[24,25]. Kraus et al[26] have confirmed a defect in oral tolerance in patients with IBD, with keyhole limpet hemocyanin, which implies that disturbance of gut tolerance is present in IBD. Good results have been obtained from a double-blind clinical trial for the treatment of Crohn’s disease by oral administration of Alequel, a mixture of autologous CEP, although the patients included might not be sufficient to achieve statistically significant results, but they still showed a good tendency[27].
Thus, appendectomy and oral tolerance were both involved in the disturbance of gut immunity in IBD. Their relationship and corresponding mechanism has not been clearly defined. Our study assessed the contribution of the appendix during oral tolerance induction by OVA in the normal or inflammatory gut environment. Our results clearly demonstrated that appendectomy postponed the occurrence of oral tolerance under normal and inflammatory conditions. At the same time, the protective role of CEP against mural DSS colitis could be inhibited when appendectomy was performed.
Concerning the effect of appendectomy on the traditional oral tolerance model induced by OVA feeding, our data from the normal gut immune status are in agreement with those from DSS colitis. The appendiceal lymphoid tissue is predominantly composed of CD4+ T-helper cells and B lymphocytes. Although the exact function of the appendix is undetermined, it is potentially involved in antigen sampling from the gut lumen and induces mucosal immunological responses as a part of the GALT, together with Peyer’s patches. Therefore, it is possible that the suppressive effect on oral tolerance is transient in normal mice, as indicated by the fact that DTH reactions were re-inhibited after 2-3 wk in the appendectomized mice. At the onset of DSS colitis, the inhibition of oral tolerance is still predominant, with no significant inhibition of DTH reaction in the appendectomized mice. No difference was noticed at the end of colitis in IBD, because no inhibition of DTH with the defect in oral tolerance was observed[26]. Clearly, the appendix is involved in oral tolerance, at least at the early inductive stage, but to maintain oral tolerance, it seems not to be as important as Peyer’s patches, which have a similar histological composition and may have compensative effects.
For the clinical application of oral tolerance in IBD, the effectiveness of oral administration of CEP has been demonstrated in a series of rodent experiments[14,28,29]. TNBS colitis, which features Th1 oversensitivity, is the main model involved in the proof of protection from oral tolerance. The fact that it functions well in DSS colitis implies that oral tolerance could be an innovative strategy for immunotherapy of IBD. While as the appendix may be involved, the protective role of oral tolerance could be indistinctive in the appendectomized mice which shows the possibility of limited application in patients with appendix removed. Therefore, if oral tolerance is to work effectively, whether the appendix remains intact should be confirmed, as well as the dose to ensure a better response. Although we demonstrated the correlation between appendectomy and oral tolerance in UC, the mechanisms that mediate the protection afforded by appendectomy or oral tolerance, and their relationship, are still under investigation.
Mucosal immune dysfunction has been frequently proposed as a key factor in the development of IBD. Studies in animals or humans with IBD imply an abnormal intestinal mucosal barrier function, overproduction of Th1 or Th2 cytokines, and uncontrolled activation of CD4+ T cells by components of colonic bacteria as contributing factors to the pathogenesis of UC[30]. The Th1/Th2 paradigm has been shown to be crucial for oral tolerance to maintain the immune homeostasis in the gut, and at the same time, its disequilibrium correlates with occurrence of IBD[31,32]. Another strong proof of the participation of the Th1/Th2 paradigm in IBD is a series of studies on the effect of worm infection, which can induce a predominant Th2 immune response. Epidemiological research has demonstrated a high prevalence rate of IBD and low worm infection rate in developed countries, while the opposite results have been obtained in developing countries[33]. Worm infection can even be therapeutic for colitis in IL-10 knockout mice or TNBS colitis with a deviation in the Th1/Th2 balance[34,35]. The question remains whether Th1/Th2 is involved in the effect of the appendix on oral tolerance induced by CEP administration in DSS colitis.
A variety of experimental models, including the DSS model, have been developed to investigate mechanisms involved in the pathogenesis of IBD[36]. Oral administration of DSS to mice can cause acute or chronic colitis with similar histopathology as human UC, and has proven to be valuable for the assessment of cause and effect relationships between specific treatment and colitis. However, the specific pathogenic mechanisms that underlie DSS colitis remain elusive. DSS-mediated colonic injury is thought to result from mucosal damage, followed by the upregulation of Th1 and Th2 cytokines, as well as other pro-inflammatory mediators[37]. We investigated the Th1/Th2 ratio and the secretion of IFN-γ and IL-4 from SPL and PPL in appendectomized or control mice after CEP ingestion and DSS colitis. The deviation from Th1 to Th2 disappeared, together with improvement of colonic pathology in PPL caused by CEP induction in the appendectomized mice, whereas in sham-operated mice, there was a predominant Th1 to Th2 deviation that resulted from Th1 inhibition caused by CEP ingestion compared with BSA. This implies a crucial role for the appendix in the development of mucosal oral tolerance, and this could be via the deviation of Th1/Th2 in PPL. With regard to the complexity of the immune network in GALT, our results suggest that the appendix is involved in the initial phase of the immune response, which is complied with the histologic composition. The continued changes remain to be further investigated.
In summary, the appendix gives us an approach to investigate gastrointestinal immunology. We studied the effect of appendectomy on CEP-induced tolerance to DSS colitis and its relationship with the Th1/Th2 paradigm. Our results imply that the appendix plays a role in maintenance of oral tolerance induced by CEP in DSS colitis, and this function could be related to the deviation from Th1 to Th2 in PPL. These results provide a new insight into the pathogenesis of IBD, and perhaps more efficient use of appendectomy or oral tolerance as a therapeutic tool.
Oral tolerance refers to the active non-response to dietary antigens and commensal enteric bacteria or substances administered orally. Inflammatory bowel disease (IBD) results from environmental trigging agents acting on suspected hosts to cause uncontrolled immune reactions in the gut. Oral tolerance has been validated to be therapeutic in a variety of autoimmune diseases including IBD. Appendectomy showed a protective effect against colitis. However, the combined effect of appendectomy and oral tolerance in the normal gut immune environment and colitis is still unclear.
Studies on mucosal immunology disturbance help greatly to understand the course of ulcerative colitis, and may be useful to establish better therapeutic strategies.
The combined effect of appendectomy and oral tolerance was evaluated by challenge in dextran sulfate sodium (DSS) colitis with oral tolerance induction, with or without appendectomy.
The result is novel and offers great potential for understanding the role of the appendix in the maintenance of immune homeostasis during oral tolerance in the gut. Appendectomy interferes with the protective role of colonic extracted protein against DSS colitis, via a shift from Th2 to Th1 during oral tolerance induction. The appendix might play a role in the maintenance of immune homeostasis during oral tolerance in the gut.
Oral tolerance: oral tolerance is one of the major features in gut immunology, and refers to the active non-response to dietary antigens and commensal enteric bacteria or substances administered orally. It has been confirmed to be a therapy for a variety of autoimmune diseases including IBD.
This study investigated the effect of appendectomy on oral tolerance protection against DSS-induced murine colitis. The results are novel and offer great potential for understanding the role of the appendix in the maintenance of immune homeostasis during oral tolerance in the gut. The study was performed in a detailed and convincing manner. The results are interesting.
Peer reviewers: Xiang Chen, MD, Department of Biochemistry, Molecular Biology and Biophysics, University of Minnesota, 6-155 Jackson Hall, 321 Church Street SE, Minneapolis, MN 55455, United States; Sanaa Ahmed Ali, Assistant professor, Department of Therapeutic Chemistry, National Research Centre, El Behooth St., Dokki, Giza, 12622 Cairo, Egypt
S- Editor Sun H L- Editor Ma JY E- Editor Zheng XM
| 1. | Vatn MH. Natural history and complications of IBD. Curr Gastroenterol Rep. 2009;11:481-487. |
| 2. | Wang J, Toes RE. Mechanisms of oral tolerance revisited. Arthritis Res Ther. 2008;10:108. |
| 3. | Sonier B, Patrick C, Ajjikuttira P, Scott FW. Intestinal immune regulation as a potential diet-modifiable feature of gut inflammation and autoimmunity. Int Rev Immunol. 2009;28:414-445. |
| 4. | Blumberg RS. Inflammation in the intestinal tract: pathogenesis and treatment. Dig Dis. 2009;27:455-464. |
| 5. | Hyun JG, Barrett TA. Oral tolerance therapy in inflammatory bowel disease. Am J Gastroenterol. 2006;101:569-571. |
| 6. | Judge T, Lichtenstein GR. Is the appendix a vestigial organ? Its role in ulcerative colitis. Gastroenterology. 2001;121:730-732. |
| 7. | Mizoguchi A, Mizoguchi E, Chiba C, Bhan AK. Role of appendix in the development of inflammatory bowel disease in TCR-alpha mutant mice. J Exp Med. 1996;184:707-715. |
| 9. | Eckmann L. Animal models of inflammatory bowel disease: lessons from enteric infections. Ann N Y Acad Sci. 2006;1072:28-38. |
| 10. | Rutgeerts P, D'Haens G, Hiele M, Geboes K, Vantrappen G. Appendectomy protects against ulcerative colitis. Gastroenterology. 1994;106:1251-1253. |
| 11. | Eaton AD, Xu D, Garside P. Administration of exogenous interleukin-18 and interleukin-12 prevents the induction of oral tolerance. Immunology. 2003;108:196-203. |
| 12. | Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694-702. |
| 13. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. |
| 14. | Dasgupta A, Kesari KV, Ramaswamy KK, Amenta PS, Das KM. Oral administration of unmodified colonic but not small intestinal antigens protects rats from hapten-induced colitis. Clin Exp Immunol. 2001;125:41-47. |
| 15. | López MC, Márquez MG, Slobodianik NH, Roux ME. Oral tolerance to dextrin mediated by specific suppressor T-cells induced in the intestinal intraepithelium and their systemic migration. Lymphology. 2003;36:26-38. |
| 16. | Braus NA, Elliott DE. Advances in the pathogenesis and treatment of IBD. Clin Immunol. 2009;132:1-9. |
| 17. | Bolin TD, Wong S, Crouch R, Engelman JL, Riordan SM. Appendicectomy as a therapy for ulcerative proctitis. Am J Gastroenterol. 2009;104:2476-2482. |
| 18. | Timmer A, Obermeier F. Reduced risk of ulcerative colitis after appendicectomy. BMJ. 2009;338:b225. |
| 19. | Florin TH, Pandeya N, Radford-Smith GL. Epidemiology of appendicectomy in primary sclerosing cholangitis and ulcerative colitis: its influence on the clinical behaviour of these diseases. Gut. 2004;53:973-979. |
| 20. | Koutroubakis IE, Vlachonikolis IG. Appendectomy and the development of ulcerative colitis: results of a metaanalysis of published case-control studies. Am J Gastroenterol. 2000;95:171-176. |
| 21. | Okazaki K, Onodera H, Watanabe N, Nakase H, Uose S, Matsushita M, Kawanami C, Imamura M, Chiba T. A patient with improvement of ulcerative colitis after appendectomy. Gastroenterology. 2000;119:502-506. |
| 22. | Halfvarson J, Jess T, Magnuson A, Montgomery SM, Orholm M, Tysk C, Binder V, Järnerot G. Environmental factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm Bowel Dis. 2006;12:925-933. |
| 23. | Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-beta-mediated oral tolerance. J Exp Med. 1996;183:2605-2616. |
| 24. | Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174-2185. |
| 25. | Ilan Y, Weksler-Zangen S, Ben-Horin S, Diment J, Sauter B, Rabbani E, Engelhardt D, Chowdhury NR, Chowdhury JR, Goldin E. Treatment of experimental colitis by oral tolerance induction: a central role for suppressor lymphocytes. Am J Gastroenterol. 2000;95:966-973. |
| 26. | Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771-1778. |
| 27. | Margalit M, Israeli E, Shibolet O, Zigmond E, Klein A, Hemed N, Donegan JJ, Rabbani E, Goldin E, Ilan Y. A double-blind clinical trial for treatment of Crohn's disease by oral administration of Alequel, a mixture of autologous colon-extracted proteins: a patient-tailored approach. Am J Gastroenterol. 2006;101:561-568. |
| 28. | Dasgupta A, Ramaswamy K, Giraldo J, Taniguchi M, Amenta PS, Das KM. Colon epithelial cellular protein induces oral tolerance in the experimental model of colitis by trinitrobenzene sulfonic acid. J Lab Clin Med. 2001;138:257-269. |
| 29. | Gotsman I, Shlomai A, Alper R, Rabbani E, Engelhardt D, Ilan Y. Amelioration of immune-mediated experimental colitis: tolerance induction in the presence of preexisting immunity and surrogate antigen bystander effect. J Pharmacol Exp Ther. 2001;297:926-932. |
| 30. | Nakase H, Mikami S, Chiba T. Alteration of CXCR4 expression and Th1/Th2 balance of peripheral CD4-positive T cells can be a biomarker for leukocytapheresis therapy for patients with refractory ulcerative colitis. Inflamm Bowel Dis. 2009;15:963-964. |
| 31. | Dohi T, Fujihashi K, Koga T, Shirai Y, Kawamura YI, Ejima C, Kato R, Saitoh K, McGhee JR. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology. 2003;124:672-682. |
| 32. | Dohi T, Fujihashi K, Rennert PD, Iwatani K, Kiyono H, McGhee JR. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med. 1999;189:1169-1180. |
| 33. | Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490-494. |
| 34. | Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690-2698. |
| 35. | Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF Jr, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385-G391. |
| 36. | Mähler M, Bristol IJ, Sundberg JP, Churchill GA, Birkenmeier EH, Elson CO, Leiter EH. Genetic analysis of susceptibility to dextran sulfate sodium-induced colitis in mice. Genomics. 1999;55:147-156. |
| 37. | Ueno Y, Tanaka S, Sumii M, Miyake S, Tazuma S, Taniguchi M, Yamamura T, Chayama K. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of valpha14 natural killer T cells in mice. Inflamm Bowel Dis. 2005;11:35-41. |