Published online May 21, 2011. doi: 10.3748/wjg.v17.i19.2397
Revised: January 13, 2011
Accepted: January 20, 2011
Published online: May 21, 2011
AIM: To investigate whether the function of hepatocytes co-cultured with bone marrow mesenchymal stem cells (MSCs) could be maintained in serum from acute-on-chronic liver failure (ACLF) patients.
METHODS: Hepatocyte supportive functions and cytotoxicity of sera from 18 patients with viral hepatitis B-induced ACLF and 18 healthy volunteers were evaluated for porcine hepatocytes co-cultured with MSCs and hepatocyte mono-layered culture, respectively. Chemokine profile was also examined for the normal serum and liver failure serum.
RESULTS: Hepatocyte growth factor (HGF) and Tumor necrosis factor; tumor necrosis factor (TNF)-α were remarkably elevated in response to ACLF while epidermal growth factor (EGF) and VEGF levels were significantly decreased. Liver failure serum samples induced a higher detachment rate, lower viability and decreased liver support functions in the homo-hepatocyte culture. Hepatocytes co-cultured with MSCs could tolerate the cytotoxicity of the serum from ACLF patients and had similar liver support functions compared with the hepatocytes cultured with healthy human serum in vitro. In addition, co-cultured hepatocytes maintained a proliferative capability despite of the insult from liver failure serum.
CONCLUSION: ACLF serum does not impair the cell morphology, viability, proliferation and overall metabolic capacities of hepatocyte co-cultured with MSCs in vitro.
-
Citation: Shi XL, Gu JY, Zhang Y, Han B, Xiao JQ, Yuan XW, Zhang N, Ding YT. Protective effects of ACLF sera on metabolic functions and proliferation of hepatocytes co-cultured with bone marrow MSCs
in vitro . World J Gastroenterol 2011; 17(19): 2397-2406 - URL: https://www.wjgnet.com/1007-9327/full/v17/i19/2397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i19.2397
Patients with fulminant hepatic failure (FHF) or acute-on-chronic liver failure (ACLF) represent a major challenge with a high mortality rate despite advances in critical care[1]. Orthotopic liver transplantation (OLT) is one of the choices for the treatment of acute liver failure and end-stage liver diseases although the donors are still insufficient[2]. In addition, OLT is not suitable for the rapidly deteriorating patients due to the irreversible brain damage caused by the cerebral edema and intracranial hypertension. Therefore, alternative therapies are needed to stabilize such patients until an organ is available for transplantation[3]. In the last few years, the isolated, viable and functional cells have been used as a promising therapeutic tool[3]. Applications of cell-based therapy in liver diseases include the development of extracorporeal liver support devices, commonly known as bioartificial livers (BAL), and hepatocyte transplantation, which would ideally serve as a bridge to transplantation or liver regeneration via providing temporary hepatic support[4,5].
To date, several designs for BAL and hepatocyte transplantation have been completed or are being assessed in animal models and human trials[6-8]. It is generally agreed that none of the cell-based therapy currently available can be used as a well defined and practical option in clinical settings, although convincing evidences have validated the usefulness of these modalities in some biochemical parameters and clinical manifestations[6-8]. This is primarily due to the accumulation of a wide range of putative toxic substances within the circulation of hepatic failure patients, which potentially diminish the efficacy of BAL devices or cell transplant[9-10]. In vitro studies have demonstrated that serum of patients with acute hepatic failure or FHF can inhibit DNA, RNA and protein synthesis and disrupt cellular integrity, ion transport and metabolic functions in hepatocytes[9-13]. Given that most of the studies concerning the effects of patients’ plasma or serum on liver cells use monolayer-cultured hepatocytes or transformed cell lines, and direct contact exists between the patient’s serum, plasma or blood and the exogenous hepatocytes in the current cell-based therapy, it is necessary to investigate whether three-dimensional (3-D) hepatocyte culture system could resist the cytotoxicity of circulating inhibitory factors in the serum or plasma of the liver failure patients[14-15].
In our previous studies, we reported a new-brand BAL configuration for the development of a 3-D porcine hepatocyte culture system by co-culturing with bone marrow mesenchymal stem cells (MSCs) in vitro[16-18]. Inductions of albumin production, urea synthesis and cytochrome P4503A1 activities were maximally achieved at a seeding ratio of 2/1 for hepatocytes versus MSCs[17]. Hepatocytes co-cultured with MSCs were largely accumulated in the G2-S phase, while less accumulated in the G0-G1 phase compared with the control[17]. In addition, high levels of diversified extracellular matrix (ECM) and soluble cytokines synthesis were confirmed within the distinct cells in hepatocyte homeostasis[17,18]. The aim of the present study was to investigate the in vitro effects of the sera from patients with viral hepatitis B-induced ACLF in China on the metabolic functions and proliferation of 3-D hepatocytes spheroids by co-culturing with bone marrow MSCs, which to a great extent approaches to the clinical reality when extracorporeal BAL and hepatocyte transplantation occur.
Three outbred white female pigs (15-20 kg) received humane care and all animal procedures were approved by the Animal Care Ethics Committee of Nanjing Drum Tower Hospital, and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes for Health (NIH Publication 85-23 revised 1996). All chemicals were of analytical grade and purchased from GIBCO (Grand Island, NY) unless otherwise stated.
Serum samples were obtained from 18 male patients with ACLF due to severe viral hepatitis B at the onset of plasmapheresis or plasma exchange. Diagnosis of these patients was based on the criteria of severe hepatitis described in the Viral Hepatitis Protection, and the Cure Guideline established by the Chinese Society of Infection and Hepatology. Informed consent was obtained from all the study subjects. A sample of blood was collected from the patients for measurement of biochemical parameters (Table 1), and normal serum was collected from 18 healthy volunteers.
| Case No. | Tbil (μ mol/L) | Dbil (μ mol/L) | ALB (g/L) | ALT (U/L) | AST (U/L) | CHE (U/L) | ALP (U/L) | LDH (U/L) | γ-GT (U/L) |
| 1 | 306 | 168 | 34 | 218 | 342 | 3449 | 152 | 218 | 69 |
| 2 | 489 | 278 | 32 | 96 | 113 | 2896 | 136 | 288 | 74 |
| 3 | 456 | 281 | 36 | 202 | 315 | 1813 | 223 | 278 | 86 |
| 4 | 889 | 483 | 39 | 156 | 155 | 2224 | 195 | 279 | 49 |
| 5 | 400 | 202 | 33 | 65 | 104 | 3002 | 170 | 202 | 34 |
| 6 | 342 | 179 | 31 | 184 | 237 | 2408 | 138 | 197 | 42 |
| 7 | 456 | 263 | 33 | 261 | 158 | 1565 | 148 | 222 | 58 |
| 8 | 324 | 205 | 28 | 139 | 178 | 2672 | 125 | 234 | 36 |
| 9 | 591 | 366 | 32 | 87 | 101 | 2606 | 189 | 246 | 96 |
| 10 | 569 | 286 | 38 | 93 | 67 | 4737 | 121 | 266 | 104 |
| 11 | 425 | 268 | 43 | 187 | 108 | 5732 | 131 | 138 | 109 |
| 12 | 396 | 199 | 32 | 56 | 138 | 2783 | 138 | 277 | 38 |
| 13 | 499 | 313 | 34 | 29 | 98 | 4157 | 294 | 288 | 47 |
| 14 | 558 | 298 | 31 | 412 | 219 | 2025 | 104 | 245 | 99 |
| 15 | 239 | 151 | 28 | 235 | 236 | 1655 | 136 | 452 | 189 |
| 16 | 199 | 103 | 40 | 2343 | 2773 | 3858 | 147 | 1154 | 173 |
| 17 | 319 | 167 | 33 | 144 | 153 | 1698 | 116 | 276 | 93 |
| 18 | 301 | 222 | 33 | 64 | 95 | 3317 | 99 | 150 | 111 |
Chemokines in human serum were quantified using the following commercially available enzyme-linked immunosorbent assay (ELISA) kits in accordance with the manufacturer’s instructions: hepatocyte growth factor (HGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and tumor necrosis factor-α (TNF-α) (Biosource International, Camarillo, CA).
Porcine MSCs were isolated by bone marrow aspirates from the iliac crest of pigs as described previously[16-18]. The surface marker of the cultured MSCs was determined by fluorescein isothiocyanate (FITC)-labeled monoclonal antibody against CD45 (Antigenix America, Huntington Station, NY) and phycoerythrin (PE)-conjugated antibodies against CD29 (VMRD, Pullman, WA), CD44 and CD90 (Becton Dickinson) using a FACScan (Becton Dickinson, San Diego, CA). Isotypic antibodies served as controls.
Primary hepatocytes were harvested by a two-step in situ collagenase perfusion technique[16-18]. The viability of the isolated hepatocytes determined by Trypan blue exclusion was higher than 95%. The percentage of nonparenchymal cells, as judged by their size (< 10 μm in diameter) and morphology (nonpolygonal or stellate), was less than 1%, which was also verified by immunocytochemical analysis of albumin and cytokeratin 18.
To culture the pure primary hepatocytes, serum free DMEM-LG was removed 4 h after seeding 4 × 105 cells per well onto the 6-well culture plates (Corning Incorporated, Corning, NY), and 10% fetal bovine serum (FBS) fresh medium with 1 mm dexamethasone, 5 mg/mL insulin, 5 mg/mL transferrin, 5 mg/mL selenium, 100 IU/mL penicillin and 100 mg/mL streptomycin were added and exchanged every day thereafter. In heterotypic culture system, hepatocytes (4 × 105) were co-cultured with MSCs (2 × 105) during passages 3-5 in the 6-well culture plates.
The culture medium was replaced with fresh FBS-free medium containing pooled normal serum or ACLF serum at concentrations ranging from 10% to 100% for 24 h. To closely simulate BAL intervention and exclude the direct contact between porcine cells and immunoglobulin as well as complements derived from patients, human serum was co-cultured using a 35-mm Millicell culture insert (Millipore, Bedford, MA). Cell morphology, albumin expression levels, and cell cycles in different groups were measured to determine the optimal concentration of human serum for culturing hepatocytes. All the medium samples were subsequently collected and stored at -80°C for biochemical analysis, which represented the porcine hepatocyte-specific support functions on human serum.
For cell cycle analysis, MSCs pre-labeled with 10 μm solution of carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 10 min at 37°C were incubated in fresh medium for 30 min prior to co-culture with hepatocytes. Subsequently, cells of homo-culture and co-culture subgroups were stained with the CycleTEST PLUS DNA reagent kit (Becton Dickinson) according to the manufacturer’s instructions, respectively. The cell cycle profiles of samples were analyzed by FACScan (Becton Dickinson).
The concentrations of acetylcholine (CHE), glutamine (Gln), superoxide dismutase (SOD) and glucose (Glu) in medium samples were measured by commercial kits (Jiancheng Bioengineering, Nanjing, China) according to the manufacturer’s instructions. Secreted albumin in the culture medium was quantified by ELISA using purified goat anti-albumin and horseradish peroxidase-conjugated antibodies (Bethyl Laboratories, Montgomery, TX).
Both hepatocyte homo-culture and co-culture groups with the determined optimal concentration of serum from normal population or liver failure patients were thereafter replaced with fresh DMEM-LG supplemented with 10% FBS and re-incubated for another 24 h.
Transmission electron microscopy (TEM) analysis was performed according to the standard protocol.
A live/dead assay was performed using calcein-AM and SYTOX Orange dye (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Hoechst 33258 (Sigma-Aldrich, St. Louis, MO) staining was used for visualization of nuclei. Cells were visualized under a fluorescence microscope (Zeiss, Jena, Germany).
Lactate dehydrogenase (LDH) activities in culture media were measured with a kit (Jiancheng Bioengineering, Nanjing, China) in accordance with the manufacturer’s instructions.
To separate hepatocyte from co-cultures, CD44-CD45- cell population was sorted out using a fluorescence-activated cell sorting (FACS) caliber flow cytometer (Becton Dickinson). Briefly, cell suspension was simultaneously incubated with FITC-conjugated monoclonal antibody against CD45 and PE-conjugated antibody against CD44 at room temperature for 15 min. CD45 negative cells were gated and then analyzed for red fluorescence. Hepatocytes and MSCs alone served as controls, respectively.
Western blotting was performed using anti-PCNA monoclonal primary antibody (Chemicon, Melbourne, Australia) and HRP-conjugated secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA). Enhanced chemiluminescence reagents (Pierce Biotechnology, Rockford, IL) were used for visualization.
Means of three independent experiments were reported. The two-tailed unpaired Student’s t test or one-way analysis of variance was used to evaluate the statistical differences.
HGF and tumor necrosis factor (TNF)-α level of liver failure serum (5253.70 ± 1876.35 and 168.86 ± 38.69 pg/mL) (mean ± SD) was significantly higher than that of healthy human serum (469.47 ± 134.87 and 66.48 ± 28.56 pg/mL) (P = 0.002 and 0.001). In contrast, other two growth factors, EGF and VEGF, in the serum of ACLF patients (74.63 ± 18.36 pg/mL and undetectable) were lower than those in the normal serum (282.56 ± 41.55 pg/mL and 99.27 ± 19.42 pg/mL) (P = 0.000) (Table 2).
| NS group (n = 18) | FS group (n=18) | P value | |
| HGF (pg/mL) | 469.47 ± 134.87 | 5253.70 ± 1876.35 | 0.002 |
| EGF (pg/mL) | 282.56 ± 41.55 | 74.63 ± 18.36 | 0 |
| VEGF (pg/mL) | 99.27 ± 19.42 | ND | 0 |
| TNF-α | 66.48 ± 28.56 | 168.86 ± 38.69 | 0.001 |
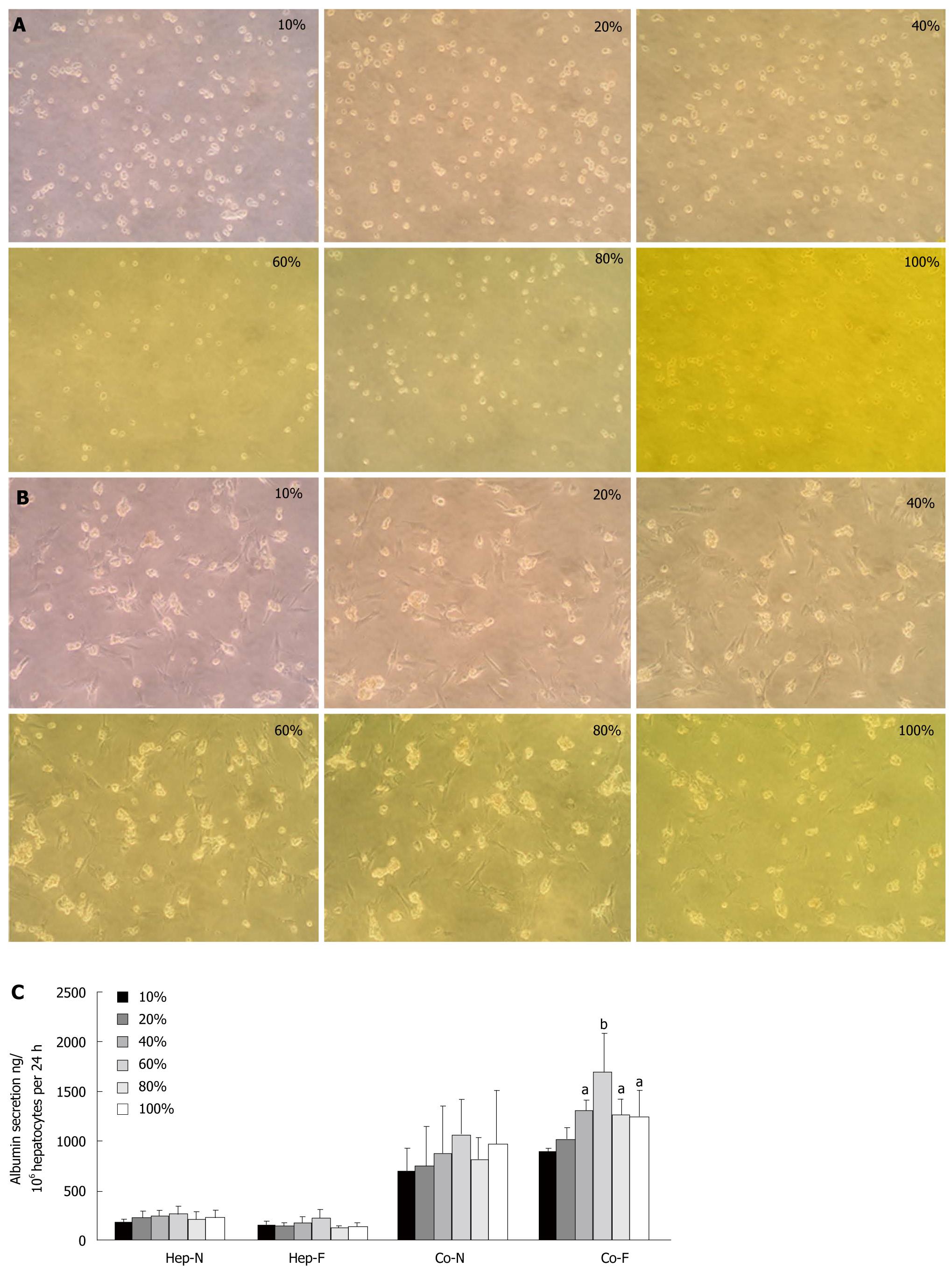
The morphology of hepatocyte homo-culture underwent a dramatic change (rounding and detachment) after 24 h of culture in the medium containing 10% liver failure serum. With increasing serum concentrations in the culture medium, less cell sheet exposure to 100% liver failure serum could constitute a confluent monolayer and the membranes of the hepatocytes became indistinct (Figure 1A). On the other hand, hepatocytes co-cultured with MSCs maintained anchored spherical multi-cellular aggregates and displayed minor difference when incubated with different concentrations of liver failure serum (Figure 1B), indicating that 3-D hepatocyte spheroids co-cultured with MSCs were not affected by liver failure serum upon 24 h incubation. There was no significant difference of albumin secretion between homo-hepatocytes cultured by various concentrations of liver failure serum (Hep-F) and those cultured by normal serum (Hep-N) (Figure 1C). As for the co-culture system, the albumin in the medium of both liver failure serum (Co-F) and normal serum (Co-N) groups were significantly higher than those of Hep-F and Hep-N (P < 0.01). When the concentration of serum reached or surpassed 40%, albumin in the medium of Co-F group was significantly higher than that in the Co-N group (P < 0.05) (Figure 1C). The largest amount of albumin secretion was observed in the co-cultured hepatocytes with 60% of serum (P < 0.01) (Figure 1C). The ratio of G2-S phase cells in Hep-F group ranged from 7.81% to 23.43%, while larger populations of CFSE-negative cells (hepatocytes) were accumulated in G2-S phase in response to MSCs, peaking in the 60% serum subgroup with 32.08% (Table 3). Taken together, 60% liver failure serum in culture medium was used for the following investigations.
| Concentration of serum % | ||||||
| 10 | 20 | 40 | 60 | 80 | 100 | |
| G2-S phase in Hep-F group | 9.26 | 13.47 | 18.66 | 23.43 | 13.99 | 7.81 |
| G2-S phase in Co-F group | 16.02 | 23.28 | 28.25 | 32.08 | 31.24 | 9.94 |
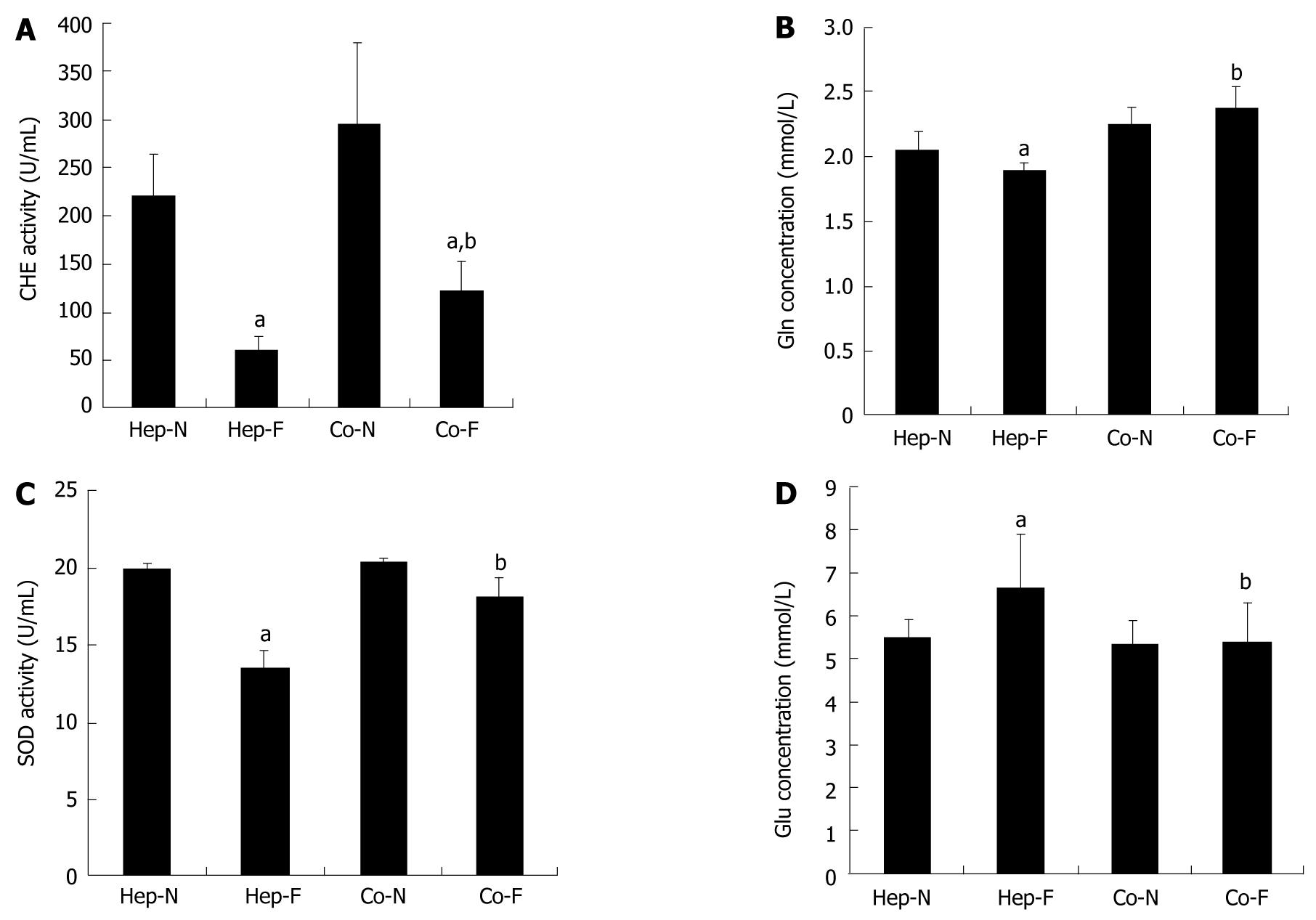
To investigate the hepatocyte’s supportive functions when incubated with liver failure serum, the metabolic capacity of medium samples from the 60% serum subgroups upon the first 24 h incubation was determined. As shown in Figure 2A, CHE activity in Hep-F and Co-F groups was significantly lower than that in the Hep-N and Co-N groups, respectively (P = 0.000) (Figure 2A). Fortunately, CHE activity in Co-F was 2-folds that in Hep-F (123.90 ± 28.15 U/mL vs 60.53 ± 13.97 U/mL, P = 0.043). There was no significant difference of Gln synthesis between Co-N and Co-F groups (P = 0.083) (Figure 2B). Gln synthesis in Hep-F group was significantly lower than that in Hep-N group (P = 0.033) (Figure 2B). Gln concentration in Co-F group was significantly higher than in Hep-F group (P = 0.000) (Figure 2B). SOD activity in Hep-F group was significantly lower than that in Hep-N group (P = 0.000) (Figure 2C). There was no significant difference in the SOD activity between the Co-F group and Co-N group (P = 0.088) (Figure 2C). Furthermore, SOD activity in Co-F group was significantly increased compared with that in Hep-F group (18.15 ± 1.25 U/mL vs 13.49 ± 1.17 U/mL, P = 0.001) (Figure 2C). The concentration of Glu in the medium indirectly represented the Glu consumption by hepatocytes. The results showed that Glu in Hep-F group was significantly higher than that in the Hep-N group (P = 0.041) (Figure 2D), suggesting that liver failure serum delayed the glycometabolism of homo-hepatocytes. There was no significant difference of Glu between Co-F and Co-N groups (P = 0.926) (Figure 2D), suggesting that liver failure serum does not affect the glycometabolism of hepatocytes co-cultured with MSCs. Importantly, Glu concentration in Co-F group was significantly lower than that in Hep-F group (5.42 ± 0.91 vs 6.60 ± 1.28 mmol/L, P = 0.026), indicating that Glu consumption rate was improved in response to MSCs treatment.
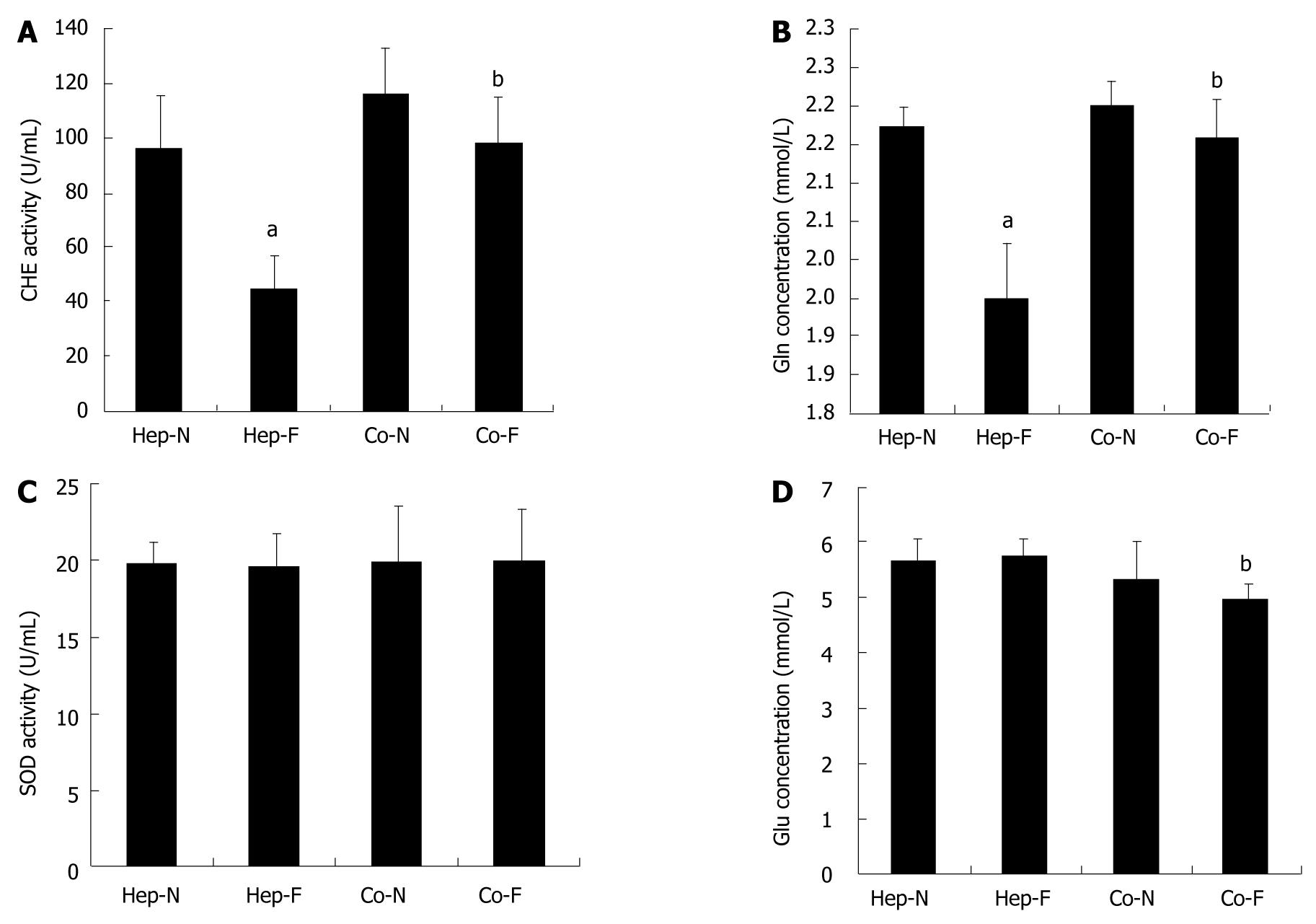
We further determined the effect on the hepatocyte metabolic functions after the second 24 h incubation with 10% FBS instead of human serum, which was defined as the post-incubation influence of liver failure serum on hepatocytes. As shown in Figure 3A, B and D, the pattern of CHE activity, Gln concentration and Glu content in the medium were similar to the effect of the first 24 h incubation with liver failure serum. Surprisingly, there was no significant difference of SOD activity in each group when human serum was changed into FBS (P > 0.05) (Figure 3C).
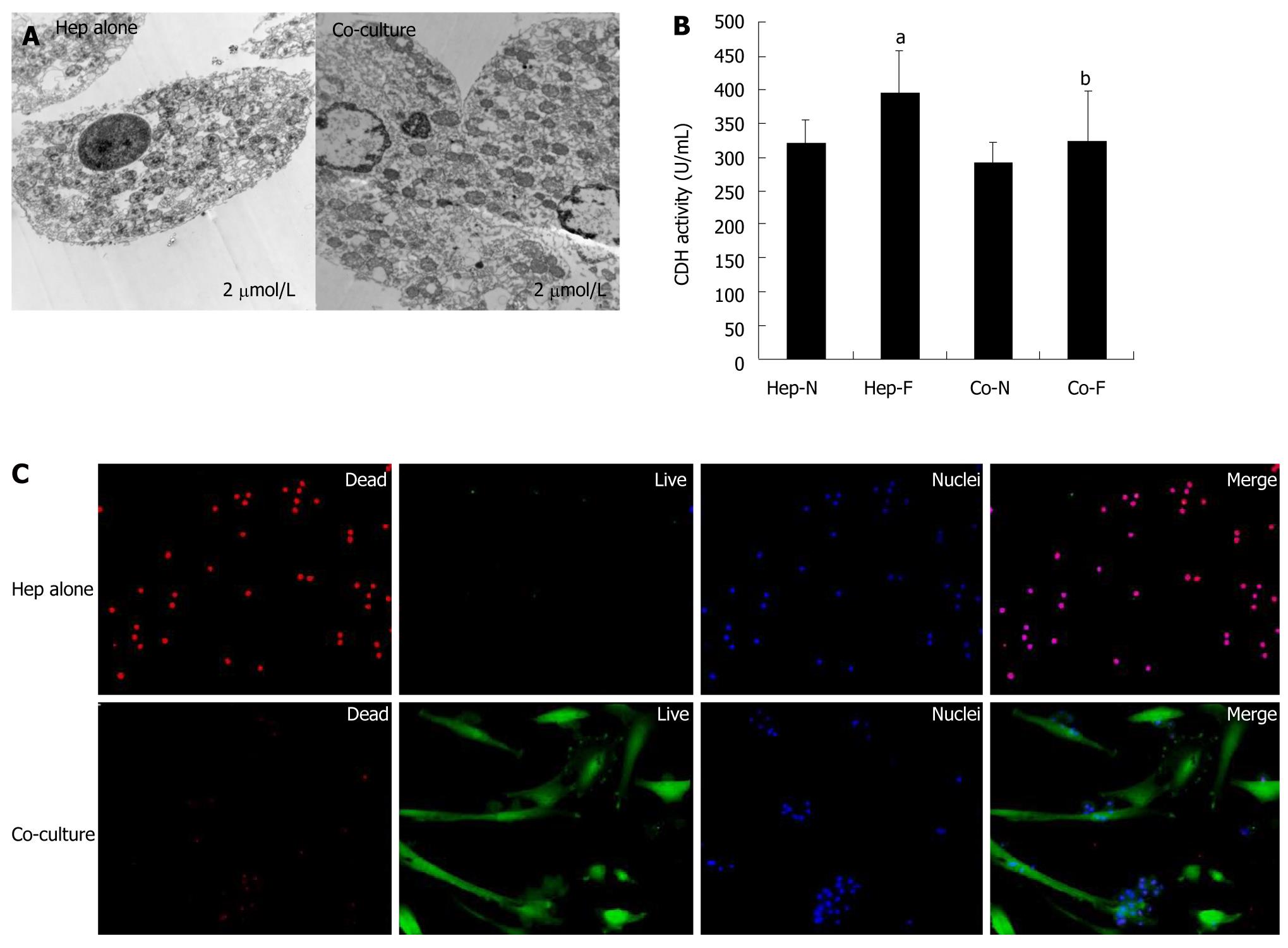
TEM, dead/live assay and LDH leakage were used to determine the cytotoxic effects of liver failure serum on the homo-hepatocytes (Hep-F) and co-cultured hepatocytes (Co-F). Figure 4A displays quite a few occurrences of nuclear pyknosis, dilated endoplasmic reticulum, mitochondrial hydropic degeneration and large peroxisomes in the cytoplasm of hepatocytes in Hep-F group. On the contrary, hepatocytes co-cultured with MSCs maintained distinct membranes and cell ultrastructure, which was similar to that of hepatocytes in vivo, irrespective of presence or absence of liver failure serum (Figure 4A). Hepatocyte viability assay revealed that few hepatocytes were labeled with nonviable cell marker SYTOX (red nuclei) in co-culture spheroids and a large population of cells, including hepatocytes and MSCs, was stained with viable cell marker calcein-AM (green cytoplasm) (Figure 4B, lower panel). In contrast, almost all hepatocytes scattering in full field showed a lower viability in Hep-F group (Figure 4B, upper panel). In addition, LDH activity in Hep-F group was significantly higher than that in Hep-N group (P = 0.034) (Figure 4C). In contrast, there was no significant difference in the LDH activity between Co-N and Co-F groups (P = 0.340) (Figure 4C), indicating that co-cultured hepatocytes did not response to liver failure serum. Finally, LDH activity in Co-F group was significantly decreased compared with that in Hep-F group (P = 0.036) (Figure 4C).
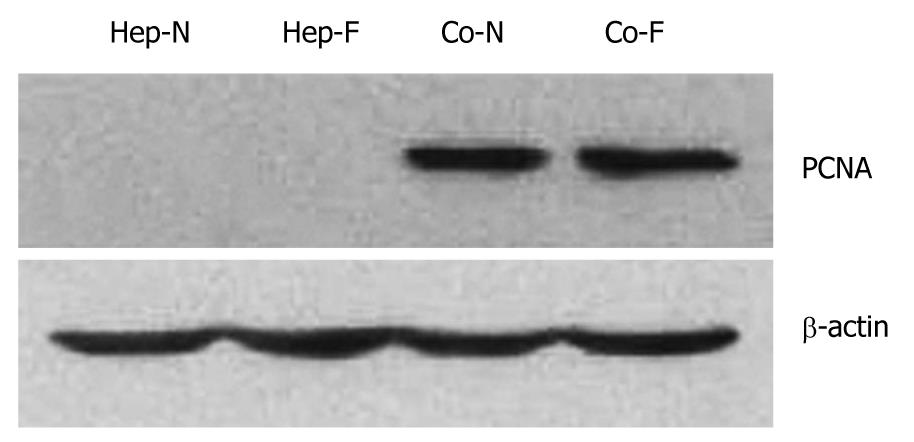
Proliferating cell nuclear antigen (PCNA) level is one of the markers of cell proliferation. The PCNA of hepatocyte in Hep-N and Hep-F groups was not detected by Western blot analysis (Figure 5). A significant amount of PCNA was detected in the hepatocytes of Co-F and Co-N groups (Figure 5). Statistical analysis showed that there was no significant difference in the PCNA level between Co-N group and Co-F group (P > 0.05).
Of the estimated 112 million persons with chronic hepatitis B in China, 15%-40% will eventually develop liver complications including liver cirrhosis, chronic hepatic failure and hepatocellular carcinoma[19]. Ideal treatment is still not available for severe viral hepatitis. Chronic severe hepatitis could cause ACLF with a mortality rate of >70% if liver transplantation cannot be performed[20]. Therefore, more and more attention has been paid to delaying the progression of ACLF by using BAL devices or hepatocyte transplantation in China and other Asian countries[21-23]. Porcine hepatocytes have been serving as the most ideal cell source for both preclinical and clinical liver support due to their physiological similarities with human hepatocytes and their availability[24-25].
A wide range of endogenous toxic metabolites as well as cytokines accumulate in the circulation of patients with liver failure[9-10]. Serum from acute liver failure or FHF patients inhibited the growth, proliferation and metabolic functions of various hepatocytes, e.g, primary hepatocytes[11], Hep G2 cells[9,10] and HHY41 cells[12] by interfering RNA, DNA and protein synthesis and inducing apoptosis. However, recent studies argued that serum from adult patients with acute liver failure did not have detrimental effects on the overall cell metabolic activity, protein synthesis, and cytochrome P450 activity in the freshly isolated human hepatocytes[13]. One of the reasons for interpreting the controversial results of these studies is that several hepatotrophic substances, e.g., cytokines and growth factors are elevated in the sera of patients with liver failure[26,27]. Therefore, the effect of liver failure serum on the liver or hepatocytes is the net result of the balance between circulating stimulatory molecules and inhibitory compounds.
To date, the greatest challenge to the restoration of well-functioning liver cells in vitro lies in maintaining the short-term viability and facilitating the rapid phenotypic de-differentiation of primary hepatocytes in standard mono-layered culture[28]. The maintenance of cell phenotype is dependent on the cellular physiological microenvironment in vivo, which consists of multiple signals including paracrine cytokines and neighboring cells[29,30]. Recently, hepatocytes are co-cultured with a range of different cell types in vitro to mimic the liver-like microenvironment[31]. Our previous studies provided the first in vitro 3-D co-culture system in which porcine hepatocytes were co-cultured with bone marrow MSCs. The morphology and functionality of the hepatocytes in this co-culturing system were well maintained and were superior to the hepatocyte homo-culture. In addition, the role of soluble factors derived from MSCs was confirmed as a vital component to facilitate hepatocyte homeostasis[16-18]. Therefore, studies are needed to determine whether such a robust co-culture system could maintain an ideal liver support functions in vitro and avoid the cytotoxicity of liver failure serum.
The pool of 18 liver failure serum samples induced a higher detachment rate, lower viability and decreased liver support functions of mono-layered hepatocytes, which is consistent with the main findings described previously[9,10]. In addition, presence of the post-incubation effect of liver failure serum suggested that some of the metabolic functions such as CHE activity and Gln concentration were still maintained at a lower level in hepatocyte homo-culture compared with the control group. In contrast, the newly developed 3-D hepatocyte co-culture system could tolerate the cytotoxicity of the serum from ACLF patients and have a similar performance to that incubated with healthy human serum in vitro. These results demonstrated that co-culture system displayed a better advantage over the pure liver cells in hepatocyte-based therapy for end-stage liver diseases. Moreover, hepatocytes co-cultured with MSCs achieved a higher proliferation capacity despite of the presence of the liver failure serum.
Recent studies have shown that VEGF, also known as the vascular permeability factor, plays an essential role in liver regeneration[32-35]. It has been shown that expression of VEGF in both hepatocytes and non-parenchymal cells are increased during liver regeneration induced by partial hepatectomy[33,35]. Furthermore, exogenous VEGF administration after partial hepatectomy facilitates the proliferative activity in the injured liver[32,34]. In contrast, neutralization of VEGF significantly inhibits the proliferative activity in the liver during regeneration after partial hepatectomy[32]. Therefore, VEGF is not only an angiogenic factor but also plays a role in the survival of liver. On the other hand, the serum VEGF levels were increased in the patients with acute hepatitis compared with controls and other types of liver disease such as chronic hepatitis, fulminant hepatitis and cirrhosis[36]. In addition, serum VEGF in survivors of fulminant hepatitis was significantly increased in the recovery phase compared with the levels on admission. In the liver cirrhosis patients, serum VEGF levels were significantly lower than those of the control group, suggesting that serum VEGF level may be associated with hepatocyte regeneration grade[36]. More recently, Namisaki and coworkers reported that VEGF treatment could significantly reduce the mortality rate of acute severe liver injury and on-going acute hepatic failure in rats[37]. Thus, VEGF levels in serum may represent a predictor of clinical outcome for patients with hepatic failure[38]. In the present study, we compared the expression levels of some key chemokines related to liver regeneration in the liver failure serum with those in the healthy human serum. Our results revealed that HGF and TNF-α were remarkably elevated in response to ACLF while EGF and VEGF levels were significantly decreased, which may partially account for the inhibitory effect of liver failure serum on hepatocyte mono-layered culture rather than stimulatory role. Since multipotent MSCs are a stable source of VEGF-producing cells in vivo and in vitro[39,40], have the ability to trans-differentiate into hepatocyte-like cells under certain circumstances[41], and are immunosuppressive by modulating the immune function of the major cell populations involved in alloantigen recognition and elimination[42], our co-culture system may be a promising candidate in future cell-based therapy.
In conclusion, 3-D spheroid culture system by co-culturing primary hepatocytes with bone marrow MSCs can tolerate the cytotoxicity of ACLF serum and has a better performance in maintaining the function of hepatocytes. Hence, primary hepatocytes co-cultured with MSCs may be suitable for the application in clinical practice of BAL and hepatocyte transplantation.
Bioartificial livers (BAL) and hepatocyte transplantation are the cell-based therapies which served as a bridge to transplantation or liver regeneration via providing temporary hepatic support. However, none of the cell-based therapy currently available can be used as a well defined and practical option in clinical settings. This is primarily due to the accumulation of a wide range of putative toxic substances within the circulation of hepatic failure patients.
Serum from acute liver failure or fulminant hepatic failure (FHF) patients inhibited the growth, proliferation and metabolic functions of various hepatocytes. The authors reported a new-brand BAL configuration for the development of a three-dimensional (3-D) porcine hepatocyte culture system by co-culturing with bone marrow mesenchymal stem cells (MSCs) in vitro. So it is necessary to investigate whether 3-D hepatocyte culture system could resist the cytotoxicity of circulating inhibitory factors in the serum or plasma from liver failure patients.
Although most of the studies concerning the effects of patients’ plasma or serum on liver cells use monolayer-cultured hepatocytes or transformed cell lines, and direct contact exists between the patient’s serum, plasma, or blood and the exogenous hepatocytes in the current cell-based therapy, 3-D hepatocyte culture system has not been investigated. This study focused on the in vitro effects of sera from patients with viral hepatitis B-induced acute-on-chronic liver failure (ACLF) in China on the metabolic functions and proliferation of 3-D hepatocytes spheroids by co-culturing with bone marrow MSCs.
The study found that 3-D spheroid culture system by co-culturing primary hepatocytes with bone marrow MSCs can tolerate the cytotoxicity of ACLF serum and has a better performance in maintaining the function of hepatocytes. It could be applied in clinical practice of BAL and hepatocyte transplantation.
Three-dimensional (3-D) hepatocyte culture system is a culture system of porcine hepatocytes and bone marrow MSCs in a co-culture manner in vitro. Co-culture of hepatocytes with non-parenchymal cells is beneficial for optimizing cell functions via heterotypic interactions.
This is an interesting study aimed to investigate whether the function of hepatocytes co-cultured with bone marrow mesenchymal stem cells could be maintained in the presence of serum from patients with acute-on-chronic liver failure.
Peer reviewer: Atsushi Nakajima, Professor, Division of Gastroenterology, Yokohama City University Graduate School of Medicine, 3-9 Fuku-ura, Kanazawa-ku, Yokohama 236-0004, Japan
S- Editor Sun H L- Editor MaJY E- Editor Ma WH
| 1. | Riordan SM, Williams R. Perspectives on liver failure: past and future. Semin Liver Dis. 2008;28:137-141. |
| 2. | Liou IW, Larson AM. Role of liver transplantation in acute liver failure. Semin Liver Dis. 2008;28:201-209. |
| 3. | Sgroi A, Serre-Beinier V, Morel P, Bühler L. What clinical alternatives to whole liver transplantation? Current status of artificial devices and hepatocyte transplantation. Transplantation. 2009;87:457-466. |
| 4. | Kobayashi N. Life support of artificial liver: development of a bioartificial liver to treat liver failure. J Hepatobiliary Pancreat Surg. 2009;16:113-117. |
| 5. | Pietrosi G, Vizzini GB, Gruttadauria S, Gridelli B. Clinical applications of hepatocyte transplantation. World J Gastroenterol. 2009;15:2074-2077. |
| 6. | Poyck PP, van Wijk AC, van der Hoeven TV, de Waart DR, Chamuleau RA, van Gulik TM, Oude Elferink RP, Hoekstra R. Evaluation of a new immortalized human fetal liver cell line (cBAL111) for application in bioartificial liver. J Hepatol. 2008;48:266-275. |
| 7. | Glanemann M, Gaebelein G, Nussler N, Hao L, Kronbach Z, Shi B, Neuhaus P, Nussler AK. Transplantation of monocyte-derived hepatocyte-like cells (NeoHeps) improves survival in a model of acute liver failure. Ann Surg. 2009;249:149-154. |
| 8. | Donato MT, Lahoz A, Montero S, Bonora A, Pareja E, Mir J, Castell JV, Gómez-Lechón MJ. Functional assessment of the quality of human hepatocyte preparations for cell transplantation. Cell Transplant. 2008;17:1211-1219. |
| 9. | Newsome PN, Tsiaoussis J, Masson S, Buttery R, Livingston C, Ansell I, Ross JA, Sethi T, Hayes PC, Plevris JN. Serum from patients with fulminant hepatic failure causes hepatocyte detachment and apoptosis by a beta(1)-integrin pathway. Hepatology. 2004;40:636-645. |
| 10. | Saich R, Selden C, Rees M, Hodgson H. Characterization of pro-apoptotic effect of liver failure plasma on primary human hepatocytes and its modulation by molecular adsorbent recirculation system therapy. Artif Organs. 2007;31:732-742. |
| 11. | Abrahamse SL, van de Kerkhove MP, Sosef MN, Hartman R, Chamuleau RA, van Gulik TM. Treatment of acute liver failure in pigs reduces hepatocyte function in a bioartificial liver support system. Int J Artif Organs. 2002;25:966-974. |
| 12. | Ito Y, Eguchi S, Kamohara Y, Inuo H, Yamanouchi K, Okudaira S, Yanaga K, Furui J, Kanematsu T. Influence of serum from rats with fulminant hepatic failure on hepatocytes in a bioartificial liver system. Int J Artif Organs. 2004;27:303-310. |
| 13. | Mitry RR, Bansal S, Hughes RD, Mieli-Vergani G, Dhawan A. In vitro effects of sera from children with acute liver failure on metabolic and synthetic activity of cryopreserved human hepatocytes. J Pediatr Gastroenterol Nutr. 2009;48:604-607. |
| 14. | Yamashita Y, Shimada M, Tsujita E, Shirabe K, Ijima H, Nakazawa K, Sakiyama R, Fukuda J, Funatsu K, Sugimachi K. High metabolic function of primary human and porcine hepatocytes in a polyurethane foam/spheroid culture system in plasma from patients with fulminant hepatic failure. Cell Transplant. 2002;11:379-384. |
| 15. | Nagaki M, Naito T, Ohnishi H, Akaike T, Muto Y, Moriwaki H. Effects of plasma from patients with fulminant hepatic failure on function of primary rat hepatocytes in three-dimensional culture. Liver Int. 2005;25:1010-1017. |
| 16. | Gu J, Shi X, Zhang Y, Chu X, Hang H, Ding Y. Establishment of a three-dimensional co-culture system by porcine hepatocytes and bone marrow mesenchymal stem cells in vitro. Hepatol Res. 2009;39:398-407. |
| 17. | Gu J, Shi X, Zhang Y, Ding Y. Heterotypic interactions in the preservation of morphology and functionality of porcine hepatocytes by bone marrow mesenchymal stem cells in vitro. J Cell Physiol. 2009;219:100-108. |
| 18. | Gu J, Shi X, Chu X, Zhang Y, Ding Y. Contribution of bone marrow mesenchymal stem cells to porcine hepatocyte culture in vitro. Biochem Cell Biol. 2009;87:595-604. |
| 19. | Yuan Y, Iloeje U, Li H, Hay J, Yao GB. Economic implications of entecavir treatment in suppressing viral replication in chronic hepatitis B (CHB) patients in China from a perspective of the Chinese Social Security program. Value Health. 2008;11 Suppl 1:S11-S22. |
| 20. | Cai CJ, Chen HA, Lu MQ, Chen GH. Model for end-stage liver disease-sodium predicts prognosis in patients with chronic severe hepatitis B. Chin Med J (Engl). 2008;121:2065-2069. |
| 21. | Riediger C, Berberat PO, Sauer P, Gotthardt D, Weiss KH, Mehrabi A, Merle U, Stremmel W, Encke J. Prophylaxis and treatment of recurrent viral hepatitis after liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii37-viii46. |
| 22. | Kjaergard LL, Liu J, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for acute and acute-on-chronic liver failure: a systematic review. JAMA. 2003;289:217-222. |
| 23. | Liu J, Kjaergard LL, Als-Nielsen B, Gluud C. Artificial and bioartificial support systems for liver failure: a Cochrane Hepato-Biliary Group Protocol. Liver. 2002;22:433-438. |
| 24. | Poyck PP, Mareels G, Hoekstra R, van Wijk AC, van der Hoeven TV, van Gulik TM, Verdonck PR, Chamuleau RA. Enhanced oxygen availability improves liver-specific functions of the AMC bioartificial liver. Artif Organs. 2008;32:116-126. |
| 25. | Sauer IM, Kardassis D, Zeillinger K, Pascher A, Gruenwald A, Pless G, Irgang M, Kraemer M, Puhl G, Frank J. Clinical extracorporeal hybrid liver support--phase I study with primary porcine liver cells. Xenotransplantation. 2003;10:460-469. |
| 26. | Barreiros AP, Sprinzl M, Rosset S, Höhler T, Otto G, Theobald M, Galle PR, Strand D, Strand S. EGF and HGF levels are increased during active HBV infection and enhance survival signaling through extracellular matrix interactions in primary human hepatocytes. Int J Cancer. 2009;124:120-129. |
| 27. | Ogata A, Kitano M, Yamanaka J, Yamasaki T, Hashimoto N, Iwasaki T, Hamano T, Fujimoto J, Kakishita E. Interleukin 18 and hepatocyte growth factor in fulminant hepatic failure of adult onset Still’s disease. J Rheumatol. 2003;30:1093-1096. |
| 28. | Zeisberg M, Kramer K, Sindhi N, Sarkar P, Upton M, Kalluri R. De-differentiation of primary human hepatocytes depends on the compositio n of specialized liver basement membrane. Mol Cell Biochem. 2006;283:181-189. |
| 29. | Price JA, Caldwell J, Hewitt NJ. The effect of EGF and the comitogen, norepinephrine, on the proliferative responses of fresh and cryopreserved rat and mouse hepatocytes. Cryobiology. 2006;53:182-193. |
| 30. | Zinchenko YS, Coger RN. Engineering micropatterned surfaces for the coculture of hepatocytes and Kupffer cells. J Biomed Mater Res A. 2005;75:242-248. |
| 31. | Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883-1900. |
| 32. | Bockhorn M, Goralski M, Prokofiev D, Dammann P, Grünewald P, Trippler M, Biglarnia A, Kamler M, Niehues EM, Frilling A. VEGF is important for early liver regeneration after partial hepatectomy. J Surg Res. 2007;138:291-299. |
| 33. | Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, Ito H, Nakagawa K, Yoshidome H, Kataoka M, Nakajima N. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001;34:683-689. |
| 34. | Assy N, Spira G, Paizi M, Shenkar L, Kraizer Y, Cohen T, Neufeld G, Dabbah B, Enat R, Baruch Y. Effect of vascular endothelial growth factor on hepatic regenerative activity following partial hepatectomy in rats. J Hepatol. 1999;30:911-915. |
| 35. | Mochida S, Ishikawa K, Inao M, Shibuya M, Fujiwara K. Increased expressions of vascular endothelial growth factor and its receptors, flt-1 and KDR/flk-1, in regenerating rat liver. Biochem Biophys Res Commun. 1996;226:176-179. |
| 36. | Akiyoshi F, Sata M, Suzuki H, Uchimura Y, Mitsuyama K, Matsuo K, Tanikawa K. Serum vascular endothelial growth factor levels in various liver diseases. Dig Dis Sci. 1998;43:41-45. |
| 37. | Namisaki T, Yoshiji H, Kojima H, Yoshii J, Ikenaka Y, Noguchi R, Sakurai S, Yanase K, Kitade M, Yamazaki M. Salvage effect of the vascular endothelial growth factor on chemically induced acute severe liver injury in rats. J Hepatol. 2006;44:568-575. |
| 38. | Auth MK. Are hepatic growth factors predictors of clinical outcome in fulminant hepatic failure? J Pediatr Gastroenterol Nutr. 2007;44:168-170. |
| 39. | Kagiwada H, Yashiki T, Ohshima A, Tadokoro M, Nagaya N, Ohgushi H. Human mesenchymal stem cells as a stable source of VEGF-producing cells. J Tissue Eng Regen Med. 2008;2:184-189. |
| 40. | Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32:270-279. |
| 41. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. |
| 42. | Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz). 2008;56:1-8. |