Published online May 14, 2011. doi: 10.3748/wjg.v17.i18.2315
Revised: January 6, 2011
Accepted: January 13, 2011
Published online: May 14, 2011
AIM: To demonstrate that (-)-Epigallocatechin-3-gallate (EGCG) inhibits vascular endothelial growth factor (VEGF) expression and angiogenesis induced by interleukin-6 (IL-6) via suppressing signal transducer and activator of transcription 3 (Stat3) activity in gastric cancer.
METHODS: Human gastric cancer (AGS) cells were treated with IL-6 (50 ng/mL) and EGCG at different concentrations. VEGF, total Stat3 and activated Stat3 protein levels in the cell lyses were examined by Western blotting, VEGF protein level in the conditioned medium was measured by enzyme-linked immunosorbent assay, and the level of VEGF mRNA was evaluated by reverse transcription polymerase chain reaction (RT-PCR). Stat3 nuclear translocation was determined by Western blotting with nuclear extract, and Stat3-DNA binding activity was examined with Chromatin immunoprecipitation (ChIP) assay. IL-6 induced endothelial cell proliferation was measured with 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazoliumbromide assay, in vitro angiogenesis was determined with endothelial cell tube formation assay in Matrigel, and IL-6-induced angiogenesis in vitro was measured with Matrigel plug assay.
RESULTS: There was a basal expression and secretion of VEGF in AGS cells. After stimulation with IL-6, VEGF expression was apparently up-regulated and a 2.4-fold increase was observed. VEGF secretion in the conditioned medium was also increased by 2.8 folds. When treated with EGCG, VEGF expression and secretion were dose-dependently decreased. IL-6 also increased VEGF mRNA expression by 3.1 folds. EGCG treatment suppressed VEGF mRNA expression in a dose-dependent manner. EGCG dose-dependently inhibited Stat3 activation induced by IL-6, but did not change the total Stat3 expression. When treated with EGCG or AG490, VEGF expressions were reduced to the level or an even lower level in the tumor cells not stimulated with IL-6. However, PD98059 and LY294002 did not change VEGF expression induced by IL-6. EGCG inhibited Stat3 nucleus translocation, and Stat3-DNA binding activity was also markedly decreased by EGCG. Furthermore, EGCG inhibited IL-6 induced vascular endothelial cell proliferation and tube formation in vitro and angiogenesis in vitro.
CONCLUSION: EGCG inhibits IL-6-induced VEGF expression and angiogenesis via suppressing Stat3 activity in gastric cancer, which has provided a novel mechanistic insight into the anti-angiogenic activity of EGCG.
-
Citation: Zhu BH, Chen HY, Zhan WH, Wang CY, Cai SR, Wang Z, Zhang CH, He YL. (-)-Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6
via Stat3 in gastric cancer. World J Gastroenterol 2011; 17(18): 2315-2325 - URL: https://www.wjgnet.com/1007-9327/full/v17/i18/2315.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i18.2315
(-)-Epigallocatechin-3-gallate (EGCG), the most abundant and active component of green tea, has shown to have chemopreventive and chemotherapeutic properties for a variety of cancers[1,2]. Previous studies demonstrated that EGCG inhibited tumor growth by anti-angiogenesis, as well as by inhibiting proliferation and inducing apoptosis[3,4]. Angiogenesis is necessary for the growth and metastasis of solid tumors, and vascular endothelial growth factor (VEGF) is the most potent angiogenic factor. EGCG inhibited angiogenesis mainly by targeting VEGF signaling pathway[5-7]. Recent studies have shown that EGCG directly inhibits VEGF expression in multiple tumors[8-10]. We also demonstrated that EGCG reduced VEGF production in gastric cancer, and the inhibitory effect was at transcriptional level, suggesting that EGCG inhibited VEGF expression by reducing VEGF gene transcription[11]. However, the detailed molecular mechanism underlying the inhibitory effect of EGCG on VEGF expression is not fully understood.
VEGF expression associates with a variety of cytokines, growth factors, transcription factors, and oncoproteins, such as interleukin-6 (IL-6)[12,13]. Significantly, many of these molecules transmit signals through signal transducer and activator of transcription 3 (Stat3), a member of Janus-activated kinase (JAK)/STAT signaling pathway[12-15]. Activation by phosphorylation of tyrosine residue is required for the activity of Stat3, which is normally a transient and tightly regulated process. Once activated, Stat3 translocates into nucleus, binds to specific DNA promoter sequence and induces downstream gene expression[15]. Aberrant activation of Stat3 is found in a variety of tumors and contributes to oncogenesis by enhancing proliferation and preventing apoptosis[16,17]. Recent studies showed that abnormal Stat3 activation directly promoted VEGF expression and angiogenesis, and blockage of Stat3 activation inhibited these effects[18-20]. Abnormal Stat3 activation is also found in various gastric cancer cell lines and specimens, and associated with tumor status[21-23]. Phosphorylated Stat3 expression is significantly correlated with VEGF expression and microvessel density in gastric cancer, and is an independently prognostic factor of poor survival[22,23]. Blockade of Stat3 activation induced cell apoptosis and growth inhibition in gastric cancer[21,22]. Furthermore, gastric cancer cells transfected with dominant-negative Stat3 exhibits a decreased VEGF expression and less angiogenic phenotype[23], suggesting that blockade of Stat3 activation could inhibit VEGF expression and angiogenesis in gastric cancer. Our recent study showed that EGCG inhibited Stat3 activation and VEGF expression in gastric cancer[11]. Previous studies also demonstrated that EGCG inhibited activation of Stat3 in multiple tumor cells[9,24,25]. However, to our knowledge, whether EGCG inhibits VEGF expression and angiogenesis via Stat3 remains to elucidate.
An etiologic relation between high risk of gastric cancer and chronic gastritis with Helicobacter pylori has been firmly established[26]. Consequently, various cytokines have been implicated in the pathogenesis of gastric cancer. As a multifunctional cytokine, IL-6 has received particular attention. IL-6 promotes tumor growth and metastasis by up-regulating VEGF expression and VEGF-mediated angiogenesis, and is closely associated with disease status and outcome of gastric cancer[27,28]. Recent studies demonstrated that IL-6 induced VEGF expression and angiogenesis via Stat3 in multiple tumors[29-31] and gastric cancer[32]. Blocking Stat3 signaling pathway down-regulated VEGF promoter activity, and effectively abolished IL-6-induced VEGF expression and angiogenesis[33,34]. Therefore, this study was designed to demonstrate that EGCG inhibited IL-6-induced VEGF expression and angiogenesis via suppressing Stat3 activity in gastric cancer in an attempt to further understand the molecular mechanism underlying the anti-angiogenic activity of EGCG.
Human gastric cancer (AGS) cells (Cell Bank of Sun Yet-San University, Guangzhou, China) were maintained in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum (FBS), (Gibco BRL, Gaithersburg, MD) and incubated at 37°C in a humidified incubator at 5% CO2. Human umbilical vein endothelial cells (HUVECs) were prepared from fresh human umbilical cord obtained from the Department of Obstetrics and Gynecology, First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China as described previously[11], and grown in human endothelial-serum free medium (Gibco BRL, Gaithersburg, MD) supplemented with 10% FBS, 100U penicillin, streptomycin and fungizone, and incubated at 37°C in a humidified incubator at 5% CO2. To maintain a uniform condition, all experiments were carried out between cell passages 4-6.
After serum starvation for 24 h, AGS cells (5 × 105 cells/well) seeded in 90 mm plates were stimulated with IL-6 (50 ng/mL, R&D systems, Minneapolis, Minn., USA) in the presence of EGCG (Sigma-Aldrich Chemical Co., St Louis, MO, USA) at concentrations indicated for another 24 h to determine the VEGF protein level or for 1 h to determine the Stat3 protein level. Total protein was extracted from the cell lysates with mammalian cell lysis kit (Bio Basic Inc., Ontario, Canada). Protein level was quantified with Bio-Rad protein assay kit (Bio-Rad Laboratories, Richmond, CA). Total protein (100 μg) was separated in 12% sodium dodecyl sulfate (SDS)-PAGE, and transferred onto PVDF membrane (Invitrogen, Carlsbad, CA, USA). The membrane was blocked with 5% skim milk and incubated at 4°C overnight with a rabbit polyclonal anti-VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), a rabbit polyclonal anti-Stat3 antibody (Santa Cruz), or a goat polyclonal anti-p-Stat3 antibody [Phospho-Stat3 (tyr-705); Santa Cruz]. After being washed with 0.1% Tween 20 in Tris-saline three times, the membrane was incubated with biotin-labeled anti-rabbit or anti-goat IgG for 1 h at room temperature with agitation. The probe proteins were detected using enhanced chemiluminescence system (Amersham International, Piscataway, NJ, USA). The same membrane was stripped and re-blotted with an antibody specific to β-actin (Santa Cruz). Protein expression levels were normalized by β-actin.
AGS cells were seeded in 90 mm plates at 5 × 105 cells per well and stimulated with IL-6 (50 ng/mL) for another 24 h in the presence of EGCG at concentrations indicated after serum starvation for 24 h. The conditioned media were harvested and centrifuged. VEGF concentrations in the supernatant were measured using VEGF enzyme-linked immunosorbent assay (ELISA) kit (R&D systems).
AGS cells (5 × 105 cells/well) were seeded in 90 mm plates. After serum starvation for 24 h, the cells were stimulated with IL-6 (50 ng/mL) for 12 h in the presence of EGCG at concentrations indicated. Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The levels of human VEGF mRNA were evaluated by reverse transcription polymerase chain reaction (RT-PCR). Reverse transcription was performed with 1 μg total RNA and 100 pmol random hexamers in a total volume of 20 μL to produce first-strand cDNA. PCR experiments were performed with 1 μL of the first-strand cDNA in a 50 μL reaction mixture. Human VEGF cDNA was amplified with specific primers (sense primer, 5'-TGCATTCACATTTGTTGTGC-3'; antisense primer, 5'-AGACCCTGGTGGACATCTTC-3'; a 200 bp product) and β-actin specific primers (sense primer, 5'-TCATCACCATTGGCAATGAG-3'; antisense primer, 5'-CACTGTGTTGGCGTACAGGT-3'; a 150 bp product). Amplification protocol was as follows: denaturation at 94°C for 1 min, annealing at 60°C (for β-actin, 55°C) for 1 min, and extension at 72°C for 1 min. All PCRs were linear up to 30 cycles. The PCR products were subjected to 2.5% agarose gel electrophoresis, stained with ethidium bromide and quantified by densitometry using the Image Master VDS system and associated software (Pfizer, NY, USA).
AGS cells were seeded into a 90 mm plate at 5 × 105 cells per well. After serum starvation for 24 h, the cells were stimulated with IL-6 (50 ng/mL) for another 24 h in the presence or absence of 50 μmol EGCG or signaling inhibitors: 20 μmol AG490 (Calbiochem, La Jolla, Calif., USA), 25 μmol PD98059 (Sigma) or 25 μmol LY294002 (Sigma). AG490 is a JAK2 inhibitor, PD98059 is a MAPK/ERK kinase (MEK) inhibitor, and LY294002 is a phosphatidylinositol-3-kinase (PI3K) inhibitor. Total proteins were extracted from the cell lysates and subjected to Western blotting analysis for VEGF expression.
AGS cells (5 × 105 cells/well) were seeded in 90 mm plates. After serum starvation for 24 h, the cells were stimulated with IL-6 (50 ng/mL) for 1 h in the presence or absence of 50 μmol EGCG. The cells were washed with cold phosphate buffered saline and collected with a policeman cell scraper. The cells were suspended in a hypotonic buffer (10 mmol HEPES, 2 mmol MgCl2, 10 mmol KCl, 0.1 mmol EDTA, 1 mmol DTT, 0.5 mmol phenylmethylsulfonyl fluoride and 0.5% Nonidet P-40) and incubated on ice for 10 min. The cell lysates were then centrifuged at 15 000 ×g for 5 min and the pellets were resuspended in a high salt buffer (50 mmol HEPES, 300 mmol NaCl, 50 mmol KCl, 0.1 mmol EDTA, 1 mmol DTT, 0.5 mmol phenylmethylsulfonyl fluoride and 10% glycerol), and then incubated with rotation for 30 min at 4°C. Lysates were centrifuged at 15 000 ×g at 4°C for 30 min. The supernatant was collected as a nuclear fraction and used for Stat3 nuclear translocation assay with an anti-p-Stat3 antibody, Phospho-Stat3 (tyr-705; Santa Cruz) by Western blotting.
AGS cells (1 × 107 cells) were seeded in 90 mm plates. After serum starvation for 24 h, the cells were stimulated with IL-6 (50 ng/mL) for 6 h in the presence or absence of 50 μmol EGCG. Chromatin immunoprecipitation (ChIP) assays were performed essentially as previously described[35]. Briefly, cells were cross-linked using 1% formaldehyde at room temperature for 10 min. After sonication, the soluble chromatin was diluted 10-fold with ChIP dilution buffer (0.01% SDS; 1.1% Triton X-100; 1.2 mmol EDTA; 16.7 mmol Tris-HCl, pH 8.1; 167 mmol NaCl), and precleared with Protein A beads blocked with 1% salmon sperm DNA and 1% BSA. The precleared chromatin solution was immunoprecipitated by anti-p-Stat3 antibody (Santa Cruz) overnight at 4°C with rotation. The immunoprecipitates were then pelleted, washed, and the antibody/protein/DNA complex was eluted off the beads by resuspending the pellets in 50 mmol NaHCO3 and 1% SDS for 30 min. Cross-linking was reversed, and protein and RNA were removed by adding 10 μg Proteinase K and 10 μg RNase A, followed by incubation at 42°C for 3 h. Purified DNA was subjected to PCR with primers for VEGF promoter as follows: forward: 5'-AGACTCCACAGTGCATACGTG-3' and reverse: 5'-AGTGTGTCCCTCTGACAATG-3', which amplify 235 bp fragments flanking the Stat3 binding element. The final products were subjected to 2.5% agarose gel electrophoresis, stained with ethidium bromide and quantified by densitometry.
AGS cells seeded in 90 mm plates at 5 × 105 cells per well were stimulated with 50 ng/mL IL-6 for another 24 h in the presence or absence of 50 μmol EGCG or 20 μmol AG490 after serum starvation for 24 h. The conditioned media were generated from the supernatants centrifuged using Amicron® Ultra-15 Centrifugal Filter Devices (Millipore Filter, Bedford, Mass., USA) at 4000 ×g for 15 min.
For proliferation assay, HUVECs were seeded in 96-well plates pre-coated with 1% gelatin at 5 × 103 cells per well and cultured with 100 μL conditioned media supplemented with 2% FBS in the presence or absence of 10 ng/mL VEGF neutralizing antibody. After cultured for 48 h, the viable cells were quantified by 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazoliumbromide assay.
For tube formation assay, Matrigel was thawed at 4°C in an ice-water bath. Matrigel (300 μL per well) was carefully added to a pre-chilled 24-well plate using a cold pipette and allowed to polymerize for 1h at 37°C. After polymerization, HUVECs (5 × 104 cells/well) in the conditioned media supplemented with 2% serum in the presence or absence of 10 μg/mL VEGF neutralizing antibody were layered on the top of the polymerized gel. Cells were incubated for 48 h at 37°C in a humidified incubator at 5% CO2, and the formed tubes were fixed with 10% buffered neutral formalin, stained with Diff-Quick Solution II (Baster, McGraw Park, IL), and photographed (100 ×). For quantification of tube formation, the total length of tubes formed in a unit area was measured using national institute of health image program.
Matrigel plug assay was performed as described previously with some modifications[36]. Briefly, 6-8-wk female BALB/c nude mice, weighing 18-22 g (Experimental Animal Center of Sun Yet-san University, Guangzhou, China) were subcutaneously injected with 0.5 mL of a liquid mixture composed of Matrigel (350 μL, 10mg/mL) and the conditioned media (150 μL) prepared as described above with or without 10 μg/mL of VEGF neutralizing antibody near the abdominal midline. The Matrigel was quickly polymerized in vivo to form a single and solid gel plug, which allowed the angiogenic factors to release and stimulate angiogenesis. One week later, the Matrigel plugs were harvested, weighed, and then minced and digested in 5 mL Drabkin reagent (Drabkin reagent kit 525; Sigma) for hemoglobin content measurement. Final hemoglobin concentration was calculated from a standard calibration curve.
All data were presented as means ± SE. Statistical significance was calculated using unpaired Student’s t test. A P value less than 0.05 was considered statistically significant. All analyses were performed using SPSS version 13.0 (SPSS Inc, USA).
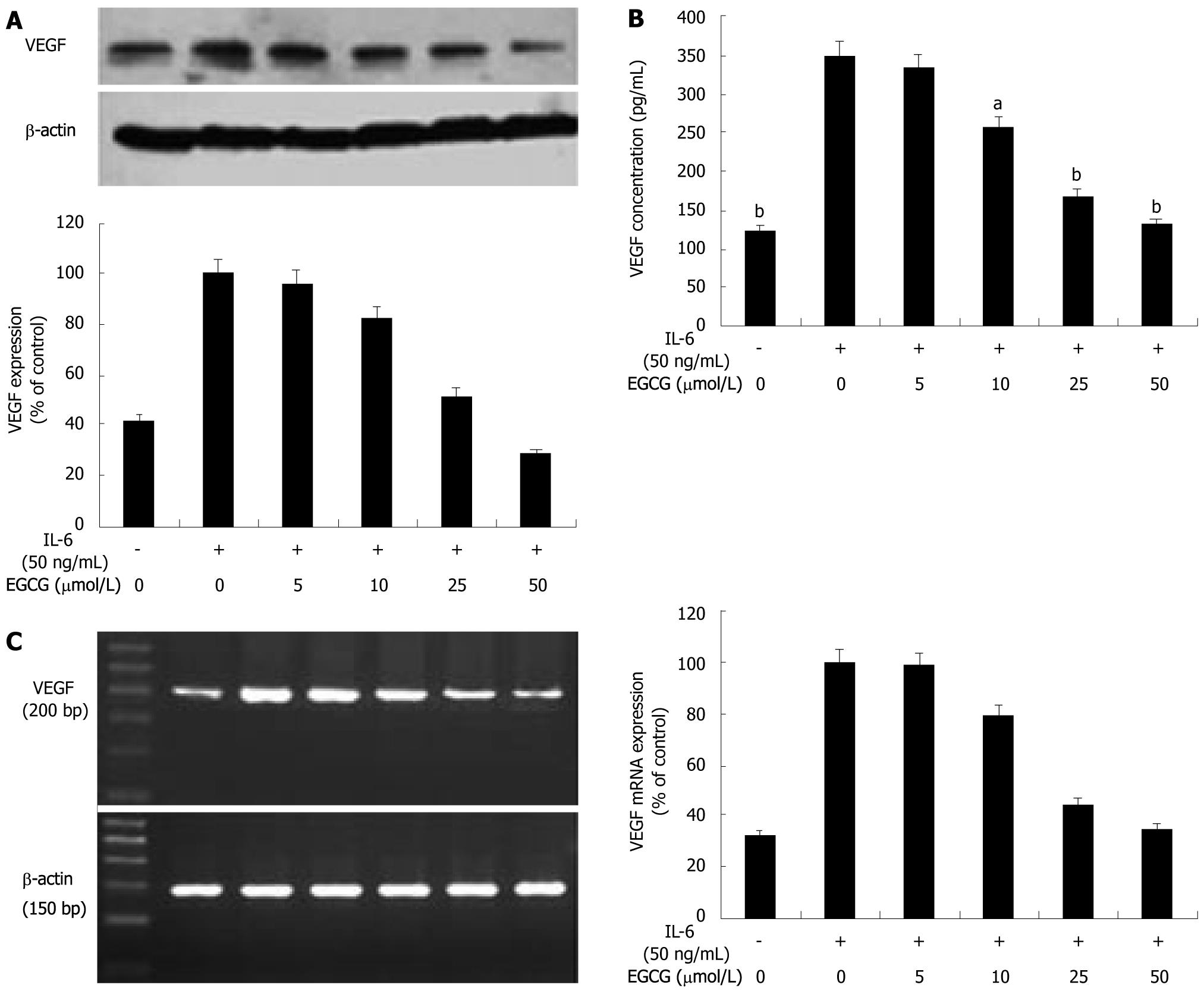
To assess the effect of EGCG on VEGF expression induced by IL-6 in human gastric cancer, we first examined VEGF expression induced by IL-6 in AGS cells treated with EGCG. AGS cells were treated with EGCG at different concentrations and stimulated with IL-6 (50 ng/mL) for 24 h. VEGF protein levels in tumor cell lysates were analyzed by Western blotting. As shown in Figure 1A, there was a basal expression of VEGF in AGS cells. After stimulation with IL-6, VEGF expression was apparently up-regulated and a 2.4-fold increase was observed. When treated with EGCG, VEGF expressions were dose-dependently decreased. This inhibitory effect was not due to the toxic effect of IL-6, because IL-6 at the concentration less than 100 ng/mL did not cause growth inhibition of AGS cells within 24 h (data not shown).
VEGF secretion is a crucial step for tumor-induced angiogenesis, so we further evaluated the effect of EGCG on VEGF secretion induced by IL-6. VEGF protein levels in the conditioned medium were measured by ELISA. IL-6 also induced VEGF secretion in AGS cells and a 2.8-fold increase was observed. Consistent with the result of Western blotting analysis, the secreted proteins of VEGF induced by IL-6 in the conditioned media were reduced by EGCG in a dose-dependent manner (Figure 1B). These data provided direct evidence that EGCG inhibited VEGF production induced by IL-6 in gastric cancer cells.
To determine whether the inhibitory effect of EGCG on IL-6-induced VEGF expression was at transcriptional level in gastric cancer, we examined VEGF mRNA expression in AGS cells by RT-PCR. We found that IL-6 induced VEGF mRNA expression in AGS cells. When stimulated with IL-6 (50 ng/mL) for 12 h, a 3.1-fold increase in VEGF mRNA expression was observed. Treated with EGCG, VEGF mRNA expressions were dose-dependently reduced (Figure 1C). These findings suggested that EGCG inhibited VEGF expression induced by IL-6 in gastric cancer cells at transcriptional level.
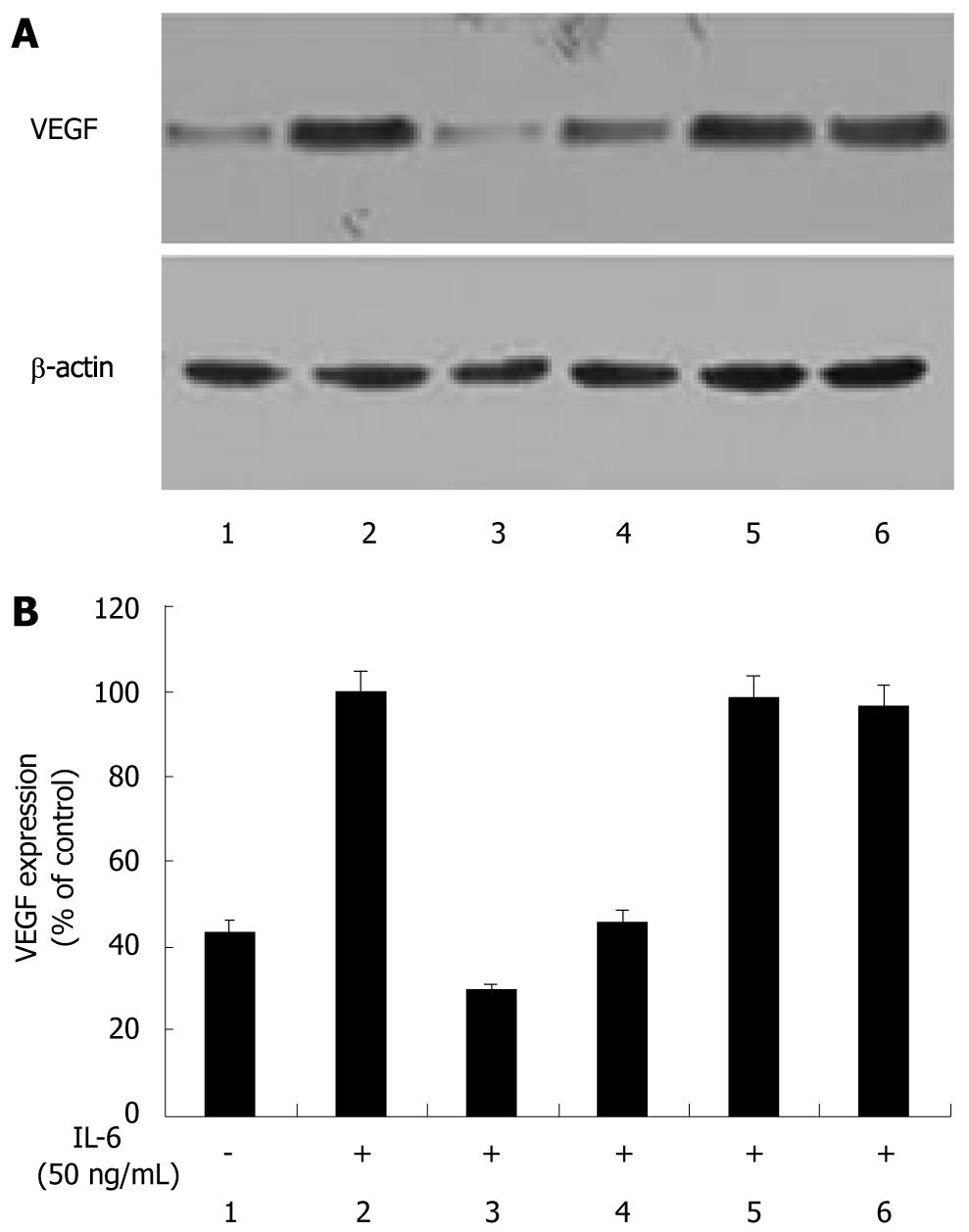
IL-6 is known to signal through Stat3, MAPK and PI3K in gastric cancer[32]. To elucidate the signaling pathway that EGCG inhibited VEGF expression in gastric cancer, we tested the effect of several signaling pathway inhibitors, including JAK/STAT, MAPK and PI3K signaling pathway. IL-6 markedly increased VEGF expression in AGS cells. When treated with 50 μmol EGCG or 20 μmol AG490, VEGF expressions were reduced to near the basal level or even lower. However, the other two groups treated with PD98059 or LY294002 still exhibited enhanced VEGF expression, approximating the level of that stimulated with IL-6 (Figure 2). Because PI3K, MEK/ERK, and Stat3 activities could all be suppressed by EGCG, we further analyzed the combined effect of EGCG and the specific inhibitors on IL-6-induced VEGF expression. We found that treatment with 50 μmol EGCG or 20 μmol AG490 alone apparently inhibited IL-6-induced VEGF expression. However, EGCG treatment combined with AG490 did not suppress VEGF expression, and EGCG or AG490 treatment combined with PD98059 or LY294002 did not suppress VEGF expression either (data not shown). These data suggested that EGCG reduced VEGF expression induced by IL-6 via Stat3 signaling pathway in gastric cancer.
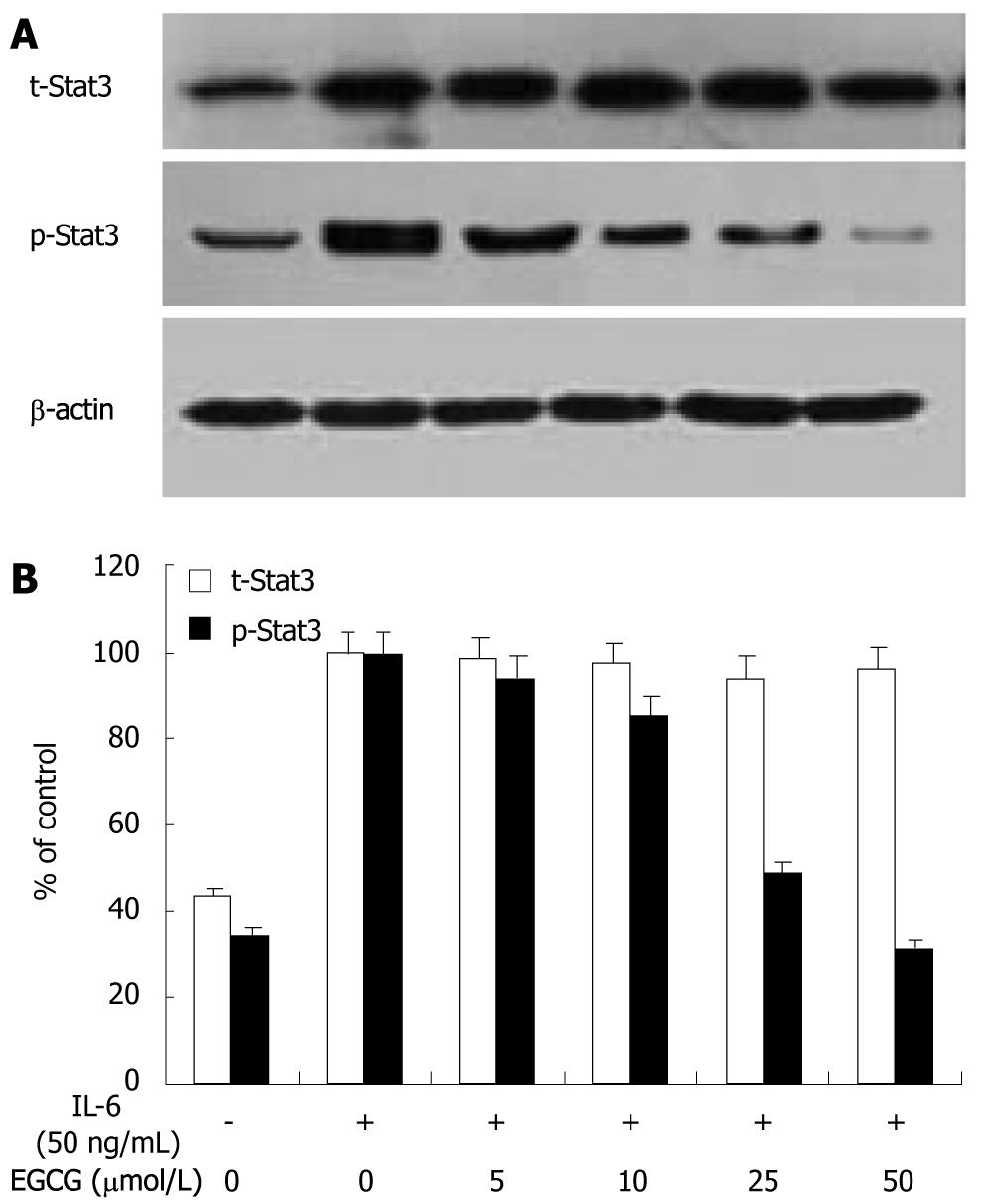
We previously demonstrated that EGCG inhibited Stat3 activation without changing Stat3 expression in gastric cancer[11]. In this study, we further assessed the effect of EGCG on Stat3 expression and activation induced by IL-6 in gastric cancer. AGS cells were treated with or without EGCG at different concentrations and stimulated with 50 ng/mL IL-6 for 1 h. Total Stat3 and phospho-Stat3 protein levels were examined using Western blotting with anti-Stat3 antibody to detect total Stat3 protein expression, and with anti-p-Stat3 antibody (specific for tyr-705) to detect phospho-Stat3, respectively. As shown in Figure 3, Stat3 was constitutively activated in AGS cells. When stimulated with IL-6 (50 ng/mL) for 1 h, phosphorylation of Stat3 at tyrosine 705 increased by 2.9 folds and the total Stat3 expression increased by 2.3 folds. EGCG treatment inhibited IL-6-induced activation of Stat3 in a dose-dependant manner, without affecting total Stat3 expression. These findings suggested that EGCG reduced IL-6-induced VEGF expression in gastric cancer by inhibiting Stat3 activation instead of Stat3 expression.
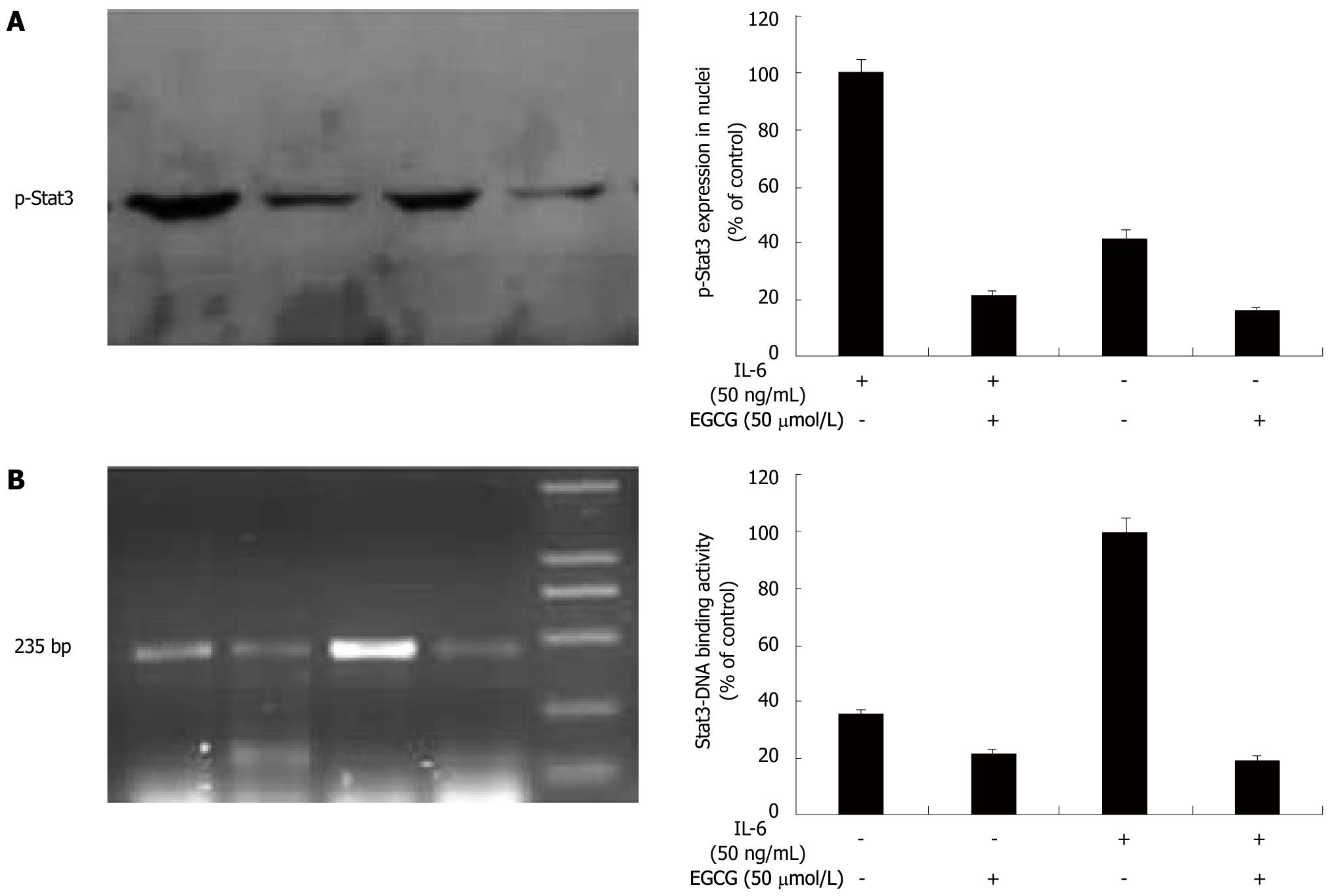
Our results showed that EGCG inhibited Stat3 activation and reduced VEGF expression at transcriptional level in gastric cancer. Activated Stat3 acts as a transcription activator, and is capable of translocating into nucleus, binding to the Stat3 consensus sequence in VEGF promoter region, thereby up-regulating VEGF expression[15]. To clarify whether EGCG affected Stat3 nuclear translocation and DNA binding activity, we first performed Western blotting with extraction of nuclear protein to visualize the nuclear translocation of phospho-Stat3 after IL-6 stimulation. As shown in Figure 4A, before IL-6 stimulation, less phospho-Stat3 was localized in the nucleus. Once stimulated with IL-6 for 1 h, phospho-Stat3 was apparently increased and translocated into the nucleus. After EGCG treatment, phospho-Stat3 in the nucleus was markedly decreased.
To further evaluate the Stat3-DNA binding activity, ChIP assay was performed. Immunoprecipitation was conducted with an anti-p-Stat3 antibody followed by PCR using oligonucleotide primers that amplified a 235 bp region spanning Stat3 binding site in VEGF promoter. IL-6 apparently increased this band in AGS cells, suggesting that IL-6 up-regulated VEGF expression by promoting Stat3 binding to VEGF promoter and activating VEGF transcription. When treated with EGCG, Stat3-DNA binding activity was markedly decreased (Figure 4B). Taken together, these data provided direct evidence that EGCG down-regulated VEGF expression induced by IL-6 through inhibiting Stat3 translocating into nucleus and binding to VEGF promoter in gastric cancer.
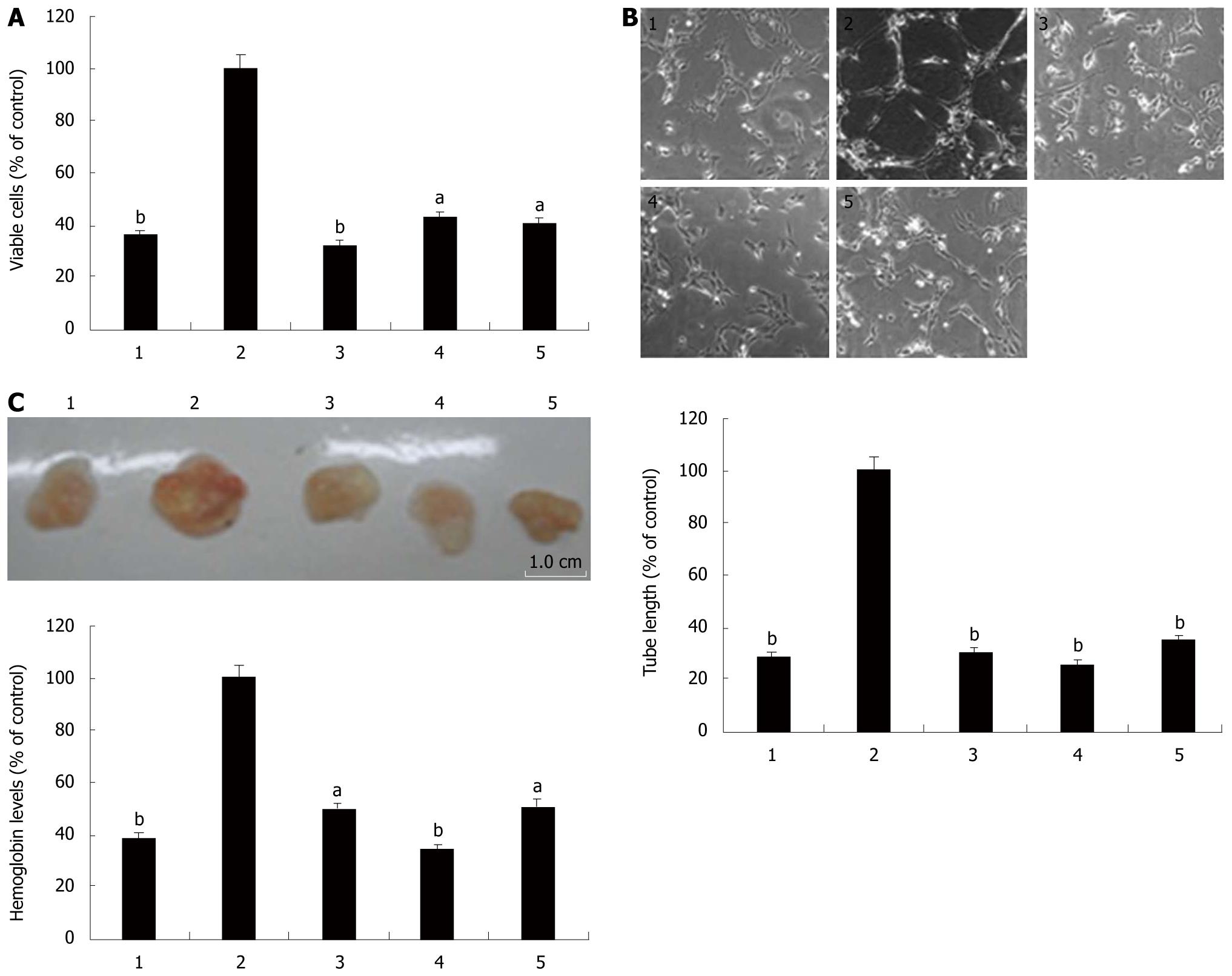
We further evaluated the effect of EGCG on IL-6-induced angiogenesis in vitro by assessing proliferation and tube formation of HUVECs cultured in the conditioned media. As shown in Figure 5A and B, the conditional media stimulated with IL-6 promoted HUVECs proliferation and tube formation when compared with the non-stimulated cell culture media. VEGF neutralizing antibody effectively blocked the enhancement of proliferation and tube formation of HUVECs cultured in the IL-6-stimulated conditional media, suggesting that IL-6 increased the angiogenic ability of AGS cells through up-regulating VEGF induction. Treatment with EGCG or AG490 also abrogated the effect of IL-6 on HUVECs cell proliferation and tube formation. These data further confirmed that EGCG inhibited angiogenesis induced by IL-6 through targeting Stat3/VEGF signaling pathway.
The effect of EGCG on angiogenesis induced by IL-6 in vivo was assessed using Matrigel plug assay. Matrigel plugs were harvested on the 7th d and examined by measuring the density of hemoglobin, as an indicator of vascularization. Hemoglobin concentrations were determined and normalized to represent the vascular densities in the plug. Grossly, the Matrigel plugs embedded with the conditioned media from IL-6-stimulated AGS cells developed substantial vasculature, as compared with the non-stimulated media. However, plugs that contained the conditioned media treated with VEGF neutralizing antibody, EGCG and AG490 exhibited considerably less vascularization (Figure 5C). Collectively, Matrigel plug assay demonstrated that IL-6 markedly potentiated AGS cells to enhance angiogenesis in vivo by inducing VEGF production, and EGCG abolished the pro-angiogenesis ability of AGS cells induced by IL-6 through inhibiting VEGF induction via Stat3.
Both VEGF over-expression and Stat3 over-activation occur at high frequency in human tumors[12-23], and IL-6 has shown to induce VEGF expression and angiogenesis by promoting Stat3 activity[29-31]. In gastric cancer, IL-6 induces VEGF expression via Stat3 signaling pathway[32], and is associated with tumor angiogenesis and disease status[27,28]. Previous studies have indicated that EGCG inhibits VEGF expression and Stat3 activation in multiple cancers[9,11,24,25]. However, whether EGCG inhibits VEGF expression and angiogenesis via Stat3 remains to be elucidated. In this study, we demonstrated that EGCG inhibited IL-6-induced VEGF expression and angiogenesis in gastric cancer via suppressing Stat3 activity.
EGCG has shown to inhibit VEGF expression and angiogenesis in a variety of tumors[8-11], and is most effective in inhibiting VEGF expression and angiogenesis among the four main catechins of green tea[7]. However, the exact mechanism for the inhibitory effect of EGCG on VEGF expression and angiogenesis is not well understood. IL-6 is reported to induce VEGF expression and tumor vasculature in gastric cancer[27,28]. To evaluate the effect of EGCG on VEGF expression induced by IL-6 in gastric cancer, we examined VEGF expression in AGS cells treated with EGCG and IL-6. As shown in Figure 1A and B, after stimulated with 50 ng/mL of IL-6, a 2.4-fold increase of VEGF protein in tumor cells and a 2.8-fold increase in the conditioned media were observed. When treated with EGCG, VEGF expression and secretion were decreased in a dose-dependent manner. EGCG also dose-dependently inhibited VEGF mRNA expression in AGS cells (Figure 1C). Here we demonstrated that EGCG inhibited IL-6-induced VEGF expression in gastric cancer cells, and this inhibitory effect was at transcriptional level.
The important role of VEGF in angiogenesis has been well established. Therefore, we further evaluated the effect of EGCG on IL-6-induced angiogenesis both in vitro and in vivo. As shown in Figure 5, the conditioned media promoted vascular endothelial cell proliferation and tube formation in vitro and vascularization of Matrigel plugs in vivo. VEGF neutralizing antibody effectively blocked these effects, confirming that IL-6-induced angiogenesis is VEGF-dependent. EGCG treatment also abrogated IL-6-induced angiogenesis in vitro and in vivo. These findings suggested that EGCG inhibited IL-6-induced angiogenesis via down-regulation of VEGF production in gastric cancer.
Several mechanisms have been proposed for the inhibitory effect of EGCG on VEGF expression[3-11]. In the present study, we found that EGCG inhibited IL-6-induced VEGF expression in AGS cells. IL-6 might act through several classic protein kinase cascades, such as MAPK, PI3K and Stat3[31,32]. To elucidate the signaling pathway that EGCG inhibited VEGF expression induced by IL-6 in AGS cells, we tested the effect of several signaling pathway inhibitors, and found that PD98059 and LY294002 did not affect IL-6-induced VEGF expression, suggesting that IL-6 did not induce VEGF expression in AGS cells through MAPK or PI3K signaling pathway, which was consistent with a previous study[32]. In contrast, EGCG and AG490 effectively inhibited VEGF expression induced by IL-6. In addition, EGCG and AG490 also effectively inhibited IL-6-induced vascular endothelial cell growth and tube formation in vitro and vascularization of Matrigel plug in vivo, indicating that EGCG inhibited IL-6-induced VEGF expression and angiogenesis via Stat3 signaling pathway. A previous study also demonstrated that Stat3 pathway was predominantly involved in the signaling of IL-6 stimulation in VEGF expression in gastric cancer[32]. These data suggested that EGCG inhibited IL-6-induced VEGF expression and angiogenesis via Stat3 signaling pathway in gastric cancer, and provided a novel mechanistic insight into the effect of EGCG on VEGF expression.
Activation by tyrosine phosphorylation is an indispensable prerequisite for the activity of Stat3. Compared with normal cells and tissues, abnormally activated Stat3 has been detected in a wide variety of human cancer cells and tissues, and associate with VEGF expression and tumor angiogenesis[14-23]. Previous studies have shown that EGCG inhibits activation of Stat3 in various cancer cells[9,24,25]. Our study also demonstrated that EGCG inhibited activation of Stat3 and VEGF expression in gastric cancer[11]. In this study, we found that Stat3 was constitutively activated in AGC cells. IL-6 induced a remarkable increase in Stat3 expression and activation. When treated with EGCG, Stat3 activation was inhibited in a dose-dependent manner, but the total Stat3 expression remained unchanged when compared with the control. EGCG treatment did not affect Stat3 mRNA expression, either (data not shown). These findings suggested that EGCG reduced VEGF expression in gastric cancer by suppressing Stat3 activation.
Once activated, Stat3 translocates into the nucleus, binds to specific DNA promoter sequence and induces downstream gene expression[15]. Stat3-binding site in VEGF promoter has been identified, providing evidence that VEGF is a direct target gene of Stat3. The activated Stat3 acts as a transcriptional activator and is capable of binding directly to the Stat3 consensus sequence in VEGF promoter region, thereby promoting the VEGF expression[18-20]. Previous studies showed that IL-6 up-regulated VEGF expression by promoting Stat3 binding to VEGF promoter[29-31]. Furthermore, blockage of Stat3 activation was associated with a decline in Stat3-DNA binding activity and VEGF mRNA expression in gastric cancer[32]. In this study, we found that IL-6 stimulation apparently increased Stat3 translocation into nucleus and Stat3-DNA binding activity. When treated with EGCG, Stat3 nuclear translocation and Stat3-DNA binding activity was markedly decreased. Masuda et al[9] also found that inhibition of Stat3 by EGCG significantly decreased VEGF promoter activity. Taken together, these data provided direct evidence that EGCG down-regulated VEGF expression induced by IL-6 via suppressing Stat3 activation, nuclear translocation and Stat3-DNA binding activity.
Increasing evidences have suggested that cytokine/Stat3 signaling pathway plays an important role in tumor development and progression. Stat3 represents a point of convergence for these cytokine signaling pathways and has become a novel promising molecular target for intervention in cancer treatment. Great efforts have been made to disrupt Stat3 signaling pathway for inhibiting angiogenesis and tumor growth[37]. However, the toxicity with these approaches might be increased because multiple down-stream targets are affected. Recently, pharmacological approaches to Stat3 inhibition aim to identify natural products as inhibitors of Stat3 signaling pathway[38,39]. In this study, we demonstrated that EGCG inhibited IL-6-induced VEGF expression and angiogenesis in gastric cancer by the suppression of Stat3 activation, nuclear translocation and Stat3-DNA binding activity. As a natural, low cost and non-toxic product, EGCG has drawn special attention, and may become a promising inhibitor of Stat3 and an angiogenic inhibitor.
(-)-Epigallocatechin-3-gallate (EGCG), the most abundant and active component of green tea, has shown to have chemopreventive and chemotherapeutic properties for a variety of cancers, especially gastrointestinal cancers. Anti-angiogenic activity is one of its main effects against cancer. However, the detailed molecular mechanism is not fully understood.
Angiogenesis is necessary for solid tumor growth and metastasis. Vascular endothelial growth factor (VEGF) is the most potent angiogenic factor, and EGCG has shown to inhibit angiogenesis and tumor growth via suppressing VEGF expression. However, the molecular mechanism remains unclear.
Increasing evidences suggest that cytokine/Stat3 signaling pathway plays an important role in tumor development and progression. Signal transducer and activator of transcription 3 (Stat3) represents a point of convergence for cytokine signaling pathways and has become a novel promising molecular target for intervention in cancer therapy. In this study, the authors demonstrate that EGCG inhibited IL-6-induced VEGF expression and angiogenesis via suppressing Stat3 activity in gastric cancer, and provided further evidence of the molecular mechanism underlying the anti-angiogenic activity of EGCG.
By understanding how EGCG inhibits IL-6-induced VEGF expression and angiogenesis via suppressing Stat3 activity, this study may represent a future strategy for therapeutic intervention in the treatment of gastric cancer.
Stat3 represents a point of convergence for cytokine signaling pathways. IL-6 induces VEGF expression and angiogenesis via Stat3 in multiple tumors. Non-surprisingly, EGCG down-regulates VEGF expression induced by IL-6 via suppressing Stat3 activation, nuclear translocation and Stat3-DNA binding activity.
The authors for the first time examined how EGCG inhibits IL-6-induced VEGF expression and angiogenesis via suppressing Stat3 activity. It provided direct evidence that EGCG down-regulated VEGF expression induced by IL-6 via suppressing Stat3 activation, nuclear translocation and Stat3-DNA binding activity. The results may represent a new molecular mechanism of anti-angiogenic activity of EGCG, and identified a new natural product as an inhibitor of Stat3 signaling pathway.
Peer reviewer: Itaru Endo, MD, PhD, Professor and Chairman, Department of Gastroenterological Surgery, Yokohama City University, 3-9 Fukuura, Kanazawa-ku, Yokohama 2360004, Japan
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429-439. |
| 2. | Khan N, Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269-280. |
| 3. | Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440-452. |
| 4. | Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumour growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001;84:844-850. |
| 5. | Shirakami Y, Shimizu M, Adachi S, Sakai H, Nakagawa T, Yasuda Y, Tsurumi H, Hara Y, Moriwaki H. (-)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009;100:1957-1962. |
| 6. | Rodriguez SK, Guo W, Liu L, Band MA, Paulson EK, Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int J Cancer. 2006;118:1635-1644. |
| 7. | Kondo T, Ohta T, Igura K, Hara Y, Kaji K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002;180:139-144. |
| 8. | Zhang Q, Tang X, Lu Q, Zhang Z, Rao J, Le AD. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol Cancer Ther. 2006;5:1227-1238. |
| 9. | Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350-359. |
| 10. | Sartippour MR, Shao ZM, Heber D, Beatty P, Zhang L, Liu C, Ellis L, Liu W, Go VL, Brooks MN. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J Nutr. 2002;132:2307-2311. |
| 11. | Zhu BH, Zhan WH, Li ZR, Wang Z, He YL, Peng JS, Cai SR, Ma JP, Zhang CH. (-)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13:1162-1169. |
| 12. | Roskoski R Jr. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179-213. |
| 13. | Xie K, Wei D, Shi Q, Huang S. Constitutive and inducible expression and regulation of vascular endothelial growth factor. Cytokine Growth Factor Rev. 2004;15:297-324. |
| 14. | Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001-7010. |
| 15. | Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81-83. |
| 16. | Burke WM, Jin X, Lin HJ, Huang M, Liu R, Reynolds RK, Lin J. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925-7934. |
| 17. | Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659-6666. |
| 18. | Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28:185-200. |
| 19. | Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319-329. |
| 20. | Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000-2008. |
| 21. | Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921-4929. |
| 22. | Choi JH, Ahn MJ, Park CK, Han HX, Kwon SJ, Lee YY, Kim IS. Phospho-Stat3 expression and correlation with VEGF, p53, and Bcl-2 in gastric carcinoma using tissue microarray. APMIS. 2006;114:619-625. |
| 23. | Gong W, Wang L, Yao JC, Ajani JA, Wei D, Aldape KD, Xie K, Sawaya R, Huang S. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386-1393. |
| 24. | Masuda M, Suzui M, Weinstein IB. Effects of epigallocatechin-3-gallate on growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220-4229. |
| 25. | Masuda M, Suzui M, Lim JT, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of HER-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9:3486-3491. |
| 26. | Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979-2990. |
| 27. | Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, Kim SG, Kim SH, Jang JS, Kim MC. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155. |
| 28. | Liao WC, Lin JT, Wu CY, Huang SP, Lin MT, Wu AS, Huang YJ, Wu MS. Serum interleukin-6 level but not genotype predicts survival after resection in stages II and III gastric carcinoma. Clin Cancer Res. 2008;14:428-434. |
| 29. | Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115:202-213. |
| 30. | Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517-1527. |
| 31. | Yang L, Wang L, Lin HK, Kan PY, Xie S, Tsai MY, Wang PH, Chen YT, Chang C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Commun. 2003;305:462-469. |
| 32. | Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517-527. |
| 33. | Kim NH, Lee MY, Park SJ, Choi JS, Oh MK, Kim IS. Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology. 2007;122:607-614. |
| 34. | Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, Sethi G, Aggarwal BB. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024-3032. |
| 35. | Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296-1298. |
| 36. | Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992;67:519-528. |
| 37. | Jing N, Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601-607. |
| 38. | Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270-1279. |