Published online May 7, 2011. doi: 10.3748/wjg.v17.i17.2191
Revised: September 25, 2010
Accepted: October 2, 2010
Published online: May 7, 2011
AIM: To assess the peripheral T lymphocyte subsets in chronic hepatitis B virus (HBV) infection, and their dynamics in response to adefovir dipivoxil monotherapy.
METHODS: Proportions and absolute counts of peripheral natural killer cells, B cells, CD8+, CD4+, CD8+CD38+, CD8+CD28+ and CD4+CD28+ T cells were determined using three-color flow cytometry in chronic hepatitis B patients (n = 35), HBV carriers (n = 25) and healthy controls (n = 35). Adefovir dipivoxil was initiated in 17 chronic hepatitis B patients who were regularly followed for 72 wk, during which period the T cell subsets and serum viral load were measured at each follow-up point.
RESULTS: The peripheral CD4+ T cell counts and CD8+ T cell counts decreased in chronic HBV infection. In chronic hepatitis B patients, proportions of CD8+CD38+ T cells were 62.0% ± 14.7%, much higher than those of HBV carriers and healthy controls. In the 13 hepatitis B patients who were treated and responded to adefovir dipivoxil, proportions of CD8+CD38+ T cells decreased from 53.9% ± 18.4% pre-therapy to 20.1% ± 11.3% by week 72 (P < 0.001), concomitant with viral load decline (HBV DNA fell from 7.31 to 3 log copies/mL). CD8+ T cell counts also underwent an average increase of 218 cells/μL by the end of 72-wk treatment. In those who failed the therapy, the CD8+CD38+ T cell population had more fluctuations.
CONCLUSION: CD8+ T cells abnormally activated in chronic HBV infection can be partially reversed by antiviral therapy. HBV-associated immune activation may be a crucial part of the pathogenesis and a promising target of treatment.
- Citation: Cao W, Qiu ZF, Li TS. Parallel decline of CD8+CD38+ lymphocytes and viremia in treated hepatitis B patients. World J Gastroenterol 2011; 17(17): 2191-2198
- URL: https://www.wjgnet.com/1007-9327/full/v17/i17/2191.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i17.2191
Hepatitis B virus (HBV) infection is a global public health concern. Despite the availability of effective vaccines since the 1980s, approximately one third of the world’s population has serological evidence of past or present infection with HBV[1]. In China, over 8% of the whole population is chronically infected. Although these patients can remain asymptomatic for years, chronic hepatitis B (CHB) is a progressive disease that can result in cirrhosis or hepatocellular carcinoma, and related death.
Hepatitis B virus is a noncytopathic virus. Clinical outcomes of HBV infection largely depend on the quality and strength of the host’s immune response. Studies have revealed that T cellular immune responses are essential for disease pathogenesis[2,3], and have identified CD8+ T lymphocytes as the main cellular subset responsible for viral control[4,5]. Compared with acute self-limiting infection, lack of vigorous and multispecific T cell response in chronic HBV infection has been observed, which leads to the failure of viral clearance and the progression of disease[6,7]. The composition of peripheral T cell subpopulations, on the other hand, serves as a valuable index for evaluating T immune status in chronic HBV infection. Impaired balance of peripheral T subpopulations has been reported at various stages of chronic HBV infections, associated with HBV replication levels, and can be partially restored after antiviral therapy[8-11]. However, results from previous studies are controversial as regards the exact changes taking place during chronic HBV infection, and few have gone so far as to investigate the dynamics of more specific T cell subpopulations (e.g., CD38+/CD28+ activated T cell subpopulation or other functional subpopulations), or their changes upon antiviral treatment.
CD38, a marker of T cell activation, has shown elevated level of expression with many acute or chronic infections[12,13]. With some infections, a correction of CD38 expression level could also be observed soon after effective treatment, e.g. human immunodeficiency virus (HIV), Epstein-Barr virus (EBV) infections[14]. Whether it is the same case with chronic HBV infection deserves more exploration. Adefovir dipivoxil (ADV), a synthetic nucleotide analogue of adenosine monophosphate, has been proven to provide biochemical, virological and histological improvement at the 10 mg oral dose daily[15-17]. Its potency at inhibiting HBV replication raises the possibility that ADV may help to improve and restore the T cell profile, including CD38+ expression. The present investigation intended to give an initial assessment of the peripheral T lymphocyte subpopulations at different clinical stages of chronic HBV infection, and evaluate dynamics of these subpopulations with ADV monotherapy in treatment-naive chronic hepatitis B patients, especially those of CD8+CD38+ T lymphocytes.
A total of 60 patients were enrolled from March to November 2007 at the Outpatient Hepatitis Clinic of Peking Union Medical College Hospital (PUMCH), Beijing, China. Among them, 35 patients displayed clinical, biochemical and virological evidence of HBe-positive chronic hepatitis B [hepatitis B surface antigen (HBsAg) positive, hepatitis B e antigen (HBeAg) positive, anti-HBe negative, anti-HBc positive], with fluctuating levels of alanine aminotransferase (ALT) (ranging from 82 to 252 U/L) and HBV DNA (ranging from 7.8 × 105 to 4.3 × 108 copies/mL). The other 25 patients were assigned as HBV carriers with positive HBsAg, various HBV DNA levels and normal ALTs. Standards of patient selection were based on 2009 AASLD (American Association for the Study of Liver Diseases) guidelines on chronic hepatitis B [18]. Another 35 healthy subjects were also included as controls. All individuals were negative for anti-hepatitis C virus and HIV-1, and for other markers of viral or autoimmune hepatitis. The study protocol was approved by the local Ethical Committee, and all subjects provided written informed consent. Full baseline characteristics and virological data are summarized in Table 1.
| Characteristics | Hepatitis B patients (n = 35) | HBV carriers(n = 25) | Healthy controls (n = 35) |
| Mean age (yr) | 34.4 ± 13.5 | 31.2 ± 13.3 | 34.0 ± 9.1 |
| Sex (male/female) | 29/6 | 13/12 | 21/14 |
| Ethnic background | Han 35 (100) | Han 25 (100) | Han 35 (100) |
| Serum ALT level (IU/L) | 281 ± 59.1 | 25.3 ± 11.5 | 21.4 ± 10.2 |
| HBV DNA level (log copies/mL) | 7.68 ± 0.98 | 8.43 ± 0.89 | < 3 |
| Positive for HBeAg | 35 (100) | 25 (100) | 0 (0) |
| Prior medication for HBV | 0 (0) | 0 (0) | 0 (0) |
Among the 35 chronic hepatitis B patients, 17 were then assigned to adefovir dipivoxil monotherapy at the oral dose of 10 mg/d (Deyang Huakang Pharmaceutical Co., Ltd., Sichuan, China), based on their clinical manifestations and patient agreement. All treated patients were followed for at least 48 wk after adefovir dipivoxil initiation; 14 of them were followed for 72 wk, and still remain on the follow-up list. Virological, biochemical and serological assessments, as well as T cell subset measurement of these patients, were carried out before treatment and at each follow-up point.
Serum HBV DNA load was assessed with real-time fluorescent quantitative polymerase chain reaction method (Real-Time-PCR) using a Lightcycler PCR system (FQD-33A, Bioer) with a lowest limit of detection of approximately 103 viral copies/mL. The experimental procedures were performed in strict accordance with the reagent kit (Shenzheng PG Biotech Co., Ltd.) package insert. The primer was provided in the kit, the reaction volume was 40 μL, and the reaction condition was 37°C for 5 min, 94°C for 1 min then 40 cycles as 95°C for 5 s and 60°C for 30 s. HBV markers (HBsAg, HBsAb, HBeAg, HBeAb, HBcAb) were measured by ELISA (enzyme-linked immunosorbent assay) method (Anthos 2010, Austria). The experimental methods followed the guidelines written in the reagent kit (Sino-American Biotech Co., Ltd.).
Peripheral blood T cell subset measurements were routinely performed during each follow-up review. Percentages of T cell subsets were determined on 100 μL ethylenediamine tetra-acetic acid (EDTA) blood sample, using a three-color direct immunofluorescence method (Beckman-Coulter, USA, EPICS2XL). In sample Tube 1, CD3+ T cells were autogated and analyzed for CD4 and CD8 cell expression using CD4/CD8/CD3 cell triple staining. In Tube 2, CD16/CD19/CD3 cell triple staining was used to identify B cells and natural killer (NK) cells. In Tubes 3, 4 and 5, CD38/CD8/CD3, CD28/CD8/CD3 and CD28/CD4/CD3 cell triple antibody cocktails were used, respectively, for analysis of CD38 and CD28 expression. Absolute counts of lymphocyte subpopulations were calculated according to complete blood cell counts on the same day.
SPSS version 11.0 was used. Baseline variables were assessed for all 95 subjects. For those who were included in the adefovir dipivoxil treatment and follow-up, virological and immunological parameters till the end of 72 wk were collected. Normal variables were summarized as means and standard deviations, and non-normal variables as medians and interquartile range (IQR) according to Kolmogorov-Smirnov Normality Test. Normal data were compared by Student t test or one-way ANOVA adjusted for multiple comparison, as appropriate. Multiple comparison of non-normal data was carried out by Kruskal Wallis test. All tests were two-sided, and a P-value ≤ 0.05 was considered significant. Associations between variables were assessed using Spearman’s rank correlations. Tested results of serum HBV DNA which were below the lower limit of detection (less than 1000 copies per milliliter) were all analyzed as being 1000 copies per milliliter for the ease of analysis.
As shown in Table 2, the T lymphocyte counts in chronic hepatitis B patients and HBV carriers were 1145 ± 380 and 1190 ± 278 cells/μL, respectively, both significantly lower than those of the healthy controls (P < 0.01). The absolute counts of CD4+ T cells and CD8+ T cells in chronic hepatitis B patients and HBV carriers were also lower than those of the controls. Yet the proportions thereof showed no significant differences between these three groups. The average counts of NK cells in chronic hepatitis B patients showed a moderate decrease compared to those of the carriers and controls. There were no significant differences in B cell counts between the different groups.
| T subsets | CHB patients (n = 35) | HBV carriers (n = 25) | Healthy controls (n = 35) |
| White blood cells × 109/L | 4.96 ± 1.18b | 5.60 ± 0.96b | 6.53 ± 1.42 |
| Lymphocytes × 109/L (%) | 1.60 ± 0.51b (32.7 ± 8.7) | 1.72 ± 0.44b (31.2 ± 8.8) | 2.13 ± 0.49 (33.1 ± 6.6) |
| Natural killer cells /μL (%) | 197 ± 102a (12.2 ± 5.1) | 272 ± 189 (15.1 ± 8.4) | 310 ± 181 (14.6 ± 7.8) |
| B cells/μL (%) | 228 ± 148 (14.3 ± 6.3) | 220 ± 113 (12.6 ± 4.6) | 242 ± 95 (11.4 ± 3.8) |
| CD3+ T cells/μL (%) | 1145 ± 380b (71.5 ± 7.1) | 1190 ± 278b (70.4 ± 9.6) | 1516 ± 382 (71.4 ± 7.6) |
| CD3+CD4+ T cells/μL (%) | 573 ± 194b (36.3 ± 6.9) | 673 ± 148b (40.1 ± 6.9) | 816 ± 259 (38.2 ± 7.0) |
| CD3+CD8+ T cells/μL (%) | 489 ± 213a (30.1 ± 8.0) | 445 ± 157b (25.9 ± 6.1) | 609 ± 177 (28.9 ± 5.7) |
| CD4+/CD8+ | 1.35 ± 0.62 | 1.64 ± 0.50 | 1.39 ± 0.44 |
| CD8+CD38+ T cells/μL (%) | 301 ± 152 (62.0 ± 14.7bd) | 285 ± 121 (51.8 ± 18.5) | 274 ± 81 (46.3 ± 11.9) |
| CD4+CD28+ T cells/μL (%1) | 532 ± 191b (96.0) | 622 ± 149a (95.7) | 744 ± 221 (94.7) |
| CD8+CD28+ T cells/μL (%) | 235 ± 125a (51.1 ± 15.5) | 245 ± 100a (55.7 ± 16.0) | 306 ± 94 (51.3 ± 11.7) |
Levels of CD8+CD38+ T cells were also examined for each group of patients. We noticed a marked increase of CD8+CD38+ T cell proportions in chronic hepatitis B patients (62.0% ± 14.7%), compared to those of the HBV carriers (51.8% ± 18.5%) and healthy controls (46.3% ± 11.9%) (P < 0.01) (Figure 1). No such differences were observed in absolute CD8+CD38+ T counts between these patients. The CD8+CD38+ T cell levels of the HBV carriers and healthy controls showed no differences.
The counts of functional subsets CD4+CD28+ and CD8+CD28+ T cells were both markedly decreased in chronic hepatitis B patients and HBV carriers. However, again, no significant differences were observed as to the proportions thereof compared with the normal controls.
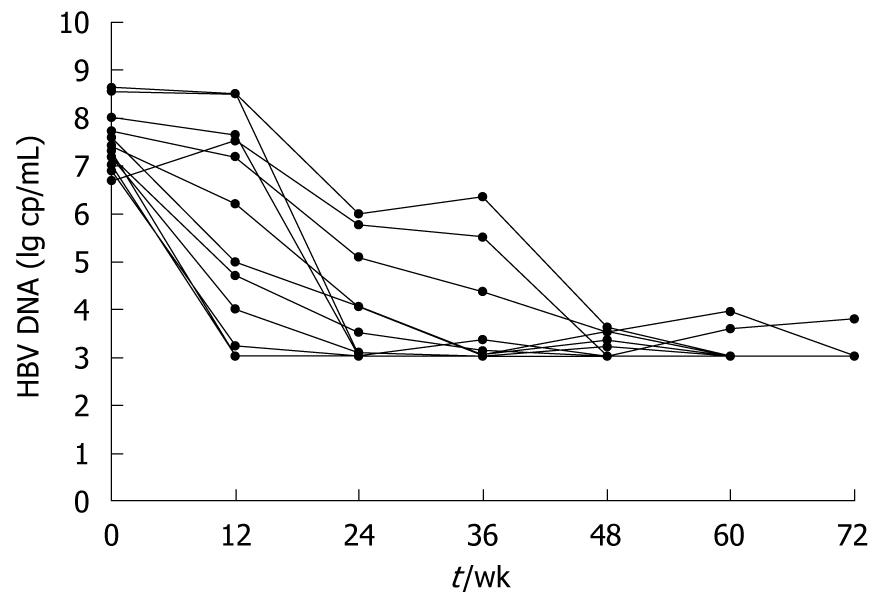
Median change in serum HBV DNA load is presented as log10 copies/mL. Among all the 17 patients receiving adefovir dipivoxil, 13 have achieved significant viral suppression, with their median HBV DNA decreased to undetectable levels (< 103 copies/mL) since 24 wk of treatment (Figure 2). However, the other 4 failed to respond to the therapy, either with no virological improvement or with a later rebound. As will be discussed later, changes in T lymphocyte subsets were analyzed based on patients’ virological responses.
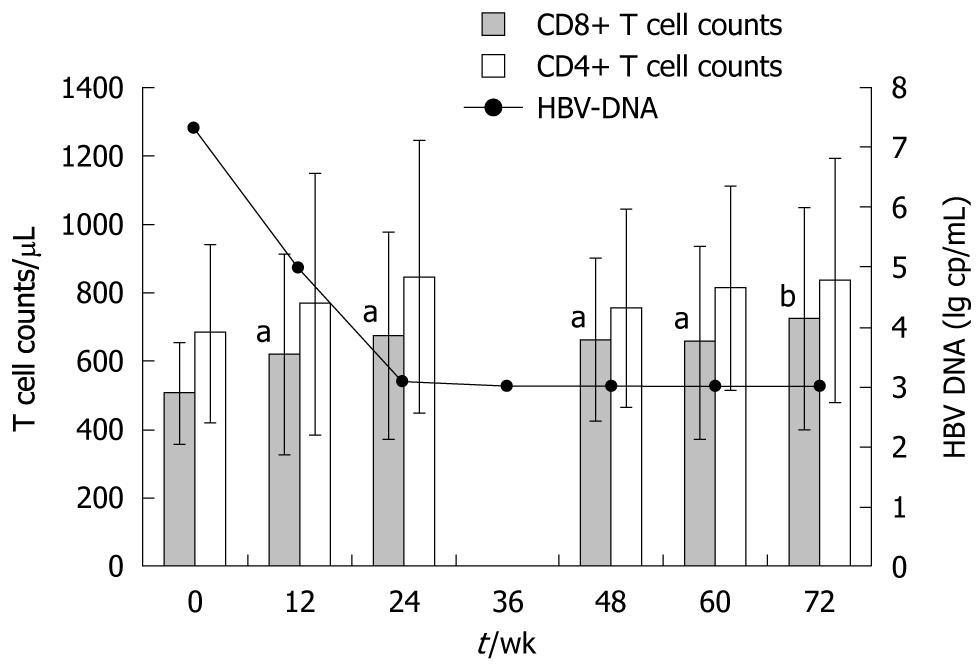
CD3+CD8+ and CD3+CD4+ T lymphocyte subsets: As is shown in Figure 3, in those who responded well to adefovir dipivoxil, we observed a marked increase of 34.5% in absolute CD8+ T cell mean counts, from 505 ± 144 to 723 ± 324 cells/μL (P < 0.01) at the end of 72 wk of treatment. This increase began in the first 12 wk of treatment (P < 0.05), concomitant with the reduction of HBV DNA loads. Meanwhile, percentage of CD8+ T cells was also elevated at the end of 72 wk, from 30.7% to 33.2% (P < 0.05). In contrast, patients with poor response to adefovir dipivoxil showed no increase of CD8+ T cells.
Fluctuations of CD3+CD4+ T cell counts and proportions were also examined throughout the treatment. No significant changes were observed in either population. Absolute counts of CD4+ T cells at each time point are also shown in Figure 3.
As regards NK cells and B lymphocytes, neither the absolute numbers nor the proportions thereof showed any significant changes after the treatment.
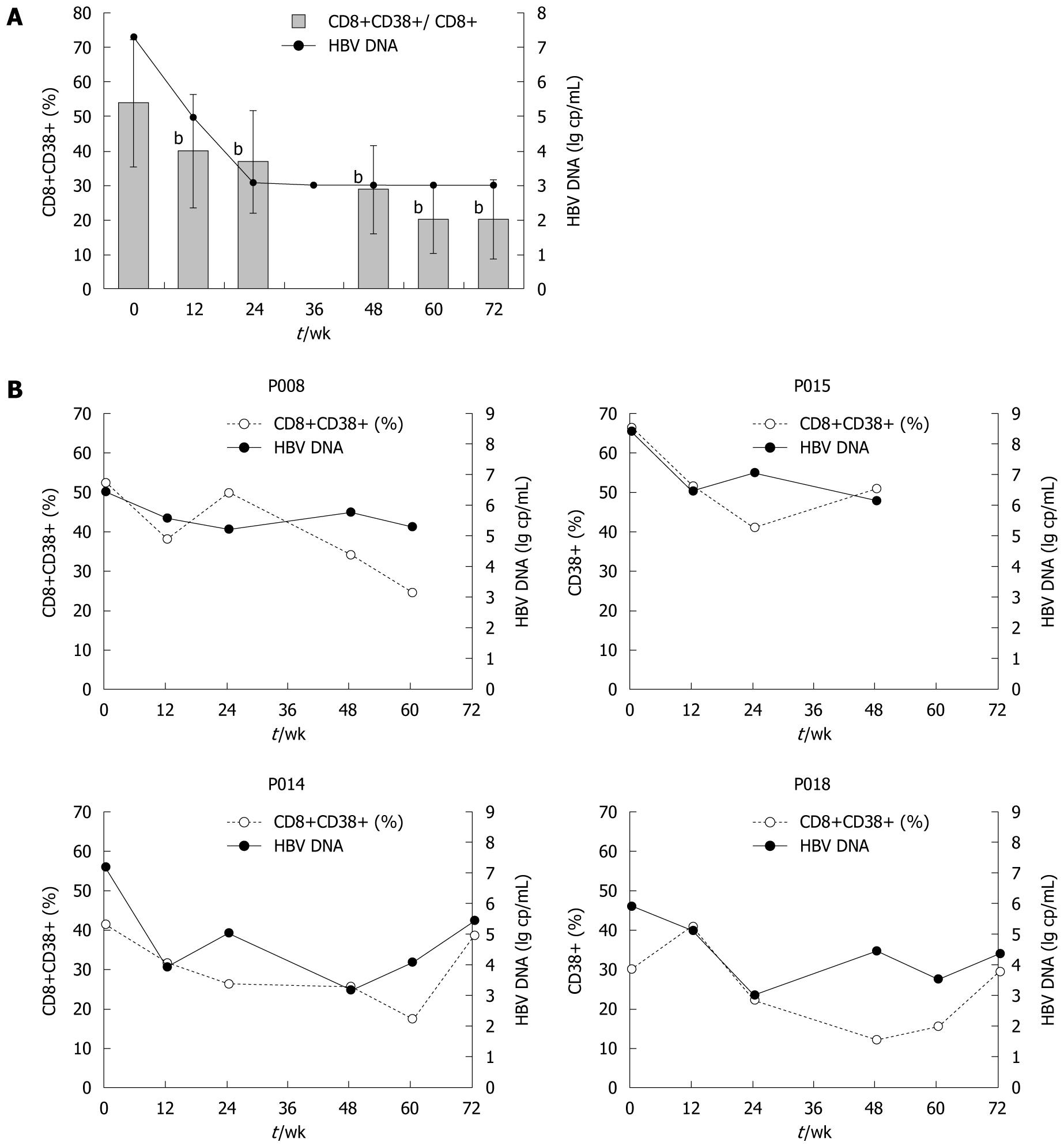
CD8+CD38+ T cell subsets: In those who achieved good control of HBV viremia with adefovir dipivoxil, both the proportions and absolute counts of CD8+CD38+ T cells declined dramatically in peripheral blood (Figure 4A). From the first 12 wk after starting therapy, levels of CD8+CD38+ T cells began to decrease sharply. By week 24, the proportions of CD8+CD38+ T cells fell significantly from 53.9% ± 18.4% to 36.8% ± 14.8%, while the absolute counts fell from 271 to 215 cells/μL (P < 0.01), and the same trends were sustained afterwards. By week 72, proportion of CD8+CD38+ T cell count had decreased as much as 62.8% from baseline to 20.1%. Absolute counts thereof showed a similar pattern of change. In contrast, the other 4 patients who did not achieve successful responses, though initially having some reduction of CD8+CD38+ T cell levels, showed CD8+CD38+ T cell fluctuations as their HBV load became unstable. CD8+CD38+ T cell changes of those who failed the therapy are depicted in Figure 4B.
As is apparent from Figure 4A, the drop in CD8+CD38+ T cell levels during the first 24 wk of treatment coincided with a marked change in serum HBV DNA load, with a median reduction from 7.30 log copies/mL to undetectable level. Regardless of virological responses to adefovir dipivoxil, in both patient groups the shape of the CD8+CD38+ T cell fluctuation curve followed that of the HBV DNA level. Also, there was a close relationship between changes in viral load and changes in CD8+CD38+ T cell levels. Further analysis from all available data paired for viral loads and T cell subsets indicated that CD8+CD38+ T cell subpopulations in chronic hepatitis B patients are significantly and positively related to serum HBV DNA log values (Figure 5, r = 0.438, P = 0.001).
Moreover, changes in CD8+CD38+ T cells were examined in 11 individuals who had achieved and maintained a viral load below 103 copies/mL, including at least two assessments of CD8+CD38+ T cells during the period of virological suppression. Of these 11 patients, 9 (82.2%) experienced a further fall in CD38+ T cell counts and proportions, whereas changes in HBV DNA were undetectable, all below 103 copies/mL. The mean change in CD8+CD38+/CD8+ percentage from the first available count to the last was -10.6% (P < 0.01), indicating that reductions in CD8+CD38+ T cell proportions had continued even after serum HBV DNA become undetectable.
CD4+CD28+ and CD8+CD28+ T cell subsets: We also examined changes of CD28+ subpopulations both in CD8+ and CD4+ T lymphocytes before and after adefovir dipivoxil treatment. No statistically significant changes took place during the treatment (Data not shown).
In addition to HBV DNA level and liver function, chronic hepatitis B is further characterized by marked changes in lymphocyte subpopulations and their activation status, which has only been poorly described in the literature. In the present study, we have identified discoordinate T cell profiles in chronic hepatitis B patients, with decreased counts of CD8+ T cells and robust CD8+ T activation, determined by an increase in the proportions of CD8+CD38+ T cells.
CD8+ and CD4+ T cells are two major components of the cellular immune system. As mentioned above, multiple studies have revealed that CD8+ T cells play an important role in clearance of the virus and progression of the disease. However, reports of CD8+ T cell subset changes in chronic hepatitis B patients have been rather conflicting. In the present study, we examined lymphocyte absolute counts as well as relative proportions in all 55 HBV infected patients and 35 healthy controls. We observed reductions of both CD8+ and CD4+ T cell levels in chronic hepatitis B patients and HBV carriers, which might reflect the T cell disturbance and suppression. Furthermore, in conjunction with the adefovir dipivoxil monotherapy, a marked elevation of CD8+ T cell levels took place, which demonstrated a partial restoration of T cell subsets and T cell immunity after the treatment. Prior studies have suggested that antiviral therapy can also overcome CD8+ T cell hypo-responsiveness in chronic HBV infection[14,15]. We believed that there could be some improvement of T cell functions with adefovir dipivoxil treatment. However, right at this point, more functional studies are needed in order to make a conclusion. On the other hand, neither counts nor proportions of CD4+ T cells demonstrated any significant changes throughout the therapy in our study, despite the initial reduction of CD4+ T cells in chronically infected patients. However, some have suggested that functions of HBV specific CD4+ T cells would be improved in response to antiviral treatment[19]. The peripheral CD4+/CD8+ ratios were much the same level in these three groups, perhaps because of the relatively localized infection and immunity that takes place in subjects with HBV infection. In addition, some prior studies have indicated that CD4+/CD8+ ratio of liver-derived lymphocytes, instead of peripheral lymphocytes, appeared to be more related to the level of HBV replication[20]. In this sense, our analysis of the peripheral T cell composition had its limitations in directly evaluating the local immune functions. However, it is still a much easier way for evaluating the immune status in a more general clinical practice.
The further evaluation of each functional and activated T cell subset in chronic HBV infection highlighted the group of CD8+CD38+ activated T cell subsets. We have found much higher proportions of circulating CD8+CD38+ T cells in chronic hepatitis B patients. CD38 is a surface glycoprotein existing on many immune cells. As a marker of cell activation, it is associated with many infectious diseases such as HIV, EBV or other infections. In certain infections, CD8+CD38+ T cells undergo a rapid up-and-down pattern after the infection once the immune control of acute phase is achieved[21]. However, persistency of immune activation and CD38+ expression throughout the acute and chronic phase is also possible, which may reflect the failure of the host’s immune system to fully suppress viral replication. According to our observation of the peripheral CD8+CD38+ T cells in chronic hepatitis B patients, chronic HBV infection showed a mode of persistent T cell activation, just like that of HIV infection. However, elevations of CD8+CD38+ T cells in chronic hepatitis B patients were not so high as those of HIV-, EBV- or cytomegalovirus-infected patients[5,6,13,22], perhaps due to the relatively localized infection of HBV in liver tissue in contrast to other systemic infections. On the other hand, HBV carriers, though also chronically infected, had similar levels of CD8+CD38+ T cells as healthy controls. This difference of CD8+ T activation may help to explain the relative immune quiescence of HBV carriers.
In further assessment of the 17 patients receiving adefovir dipivoxil monotherapy, we found that not only did the treatment effectively inhibit HBV replication, but it also resulted in a concomitant, profound and rapid decline in the CD8+CD38+ T cell levels. This reduction in CD8+CD38+ T cells began to take place after the first 12 weeks of treatment, and in the majority of treated patients, CD8+CD38+ T cell levels finally became fully normalized. This recovery of CD8+CD38+ T cell proportions confirmed the abnormal T cell activation as a result of the virological failure in active HBV infection. A further correlation analysis showed a positive and significant relationship between the CD8+CD38+ T cell proportions and serum HBV DNA, which indicated a T phenotype drift with viral stimulation. However, at this stage, it remains unclear whether the immune activation we observed was only a secondary change of viral insults, or part of HBV pathogenesis. Based on the variance of T cell activation between hepatitis B patients and HBV carriers with similar levels of viremia, CD8+CD38+ activated T cells most probably represent multiple interactions between viral and host factors. Studies of immune activation in HIV have suggested that CD8 T cell activation levels can predict the rate of disease progression independent of viral load, though the causes of this immune activation are likely multifactorial[23,24]. Furthermore, some have tried to target HIV-associated immune activation by using immunomodulatory agents in addition to antiretroviral therapy[25-27]. The role of immune activation in chronic HBV infection has been poorly described. Here, our study of CD8+CD38+ activated T cells raises the possibility that T immune activation helps to shape the fate of chronic HBV infection and, therefore, use of immunomodulatory agents may help with the control of hepatitis B virus. However, a larger sample size and more specific and functional studies are needed to establish this conclusion.
Moreover, a continued decline in CD8+CD38+ T cell levels was observed even after HBV DNA became undetectable and CD8+CD38+ T cells became normalized in successfully treated patients. This finding is interesting, because we have also observed similar phenomena in other treated viral infections. It could be an indication of presentations of the residual virus below the lower limit of viremia detection. Another possible explanation is that the CD8+CD38+ T cell levels under normal conditions may actually present an overall balance between the human immune system and occasional environmental insults, and that non-specific antiviral medications such as adefovir dipivoxil may also have an effect on these other pathogens, thus reducing the chances of immune activation in these patients. A longer follow-up of these patients during and after treatment may help to better explain the dynamics of the CD8+CD38+ T cell subsets.
The functional subsets of CD8+CD28+ and CD4+CD28+ T cells were also examined in our study. CD28+ acts as a co-stimulating molecule on the surface of T cells. However, according to a few currently available evaluations[12,28], unlike HIV infection which leads to the down-regulation of CD28 on the surface of T cells[29,30], HBV infection seems to have no significant impact on CD28 expression. In our study, the proportions of CD8+CD28+ and CD4+CD28+ T cells in the three groups were similar to each other. The absolute counts, though lower in chronic hepatitis patients and HBV carriers, were actually reflections of the decreased CD8+ and CD4+ T cell levels. Further treatment, which resulted in good control of the virus, did not change CD28 expression on the CD4+ or CD8+ T cells either, which further confirmed our observations before the treatment.
Chronic Hepatitis B virus (HBV) infection is a global health concern. Studies have identified that T cellular immune responses are essential for disease control. However, profiles of T cell subsets in chronic HBV infection, especially those of activated T cell subsets, have not been fully revealed. The authors studied the peripheral T cell subsets in chronic HBV infection, and their dynamics in response to adefovir dipivoxil monotherapy.
T cellular immune responses are essential for pathogenesis of chronic HBV infection; impaired balance of peripheral T subpopulations has been reported at various stages of chronic HBV infections, and can be partially restored after antiviral therapy.
The study systemically assessed the peripheral T lymphocyte subsets in different types of chronic HBV infection. For the first time, the authors highlighted the group of CD8+CD38+ activated T cells, and their dynamics before and after treatment. The authors found T cell activation was linked with active HBV infection, and declined with successful antiviral treatment, which suggested the crucial role of immune activation in HBV pathogenesis.
The authors clarified differences of peripheral T cell subsets between different clinical types of chronic HBV infection, and changes thereof in response to treatment. HBV-associated immune activation may be crucial for the pathogenesis of HBV infection. This adds to current knowledge of T immune functions in chronic HBV infection, and helps with a better understanding of HBV pathogenesis. The abnormal T immune activation status in chronic hepatitis B suggests the possible use of immunomodulatory agents as further treatment.
This is an interesting and well-written manuscript that clearly shows the link between CD8+38+ T-cells and HBV DNA levels. The authors described a decrease in total CD4 and CD8 T cell counts in chronic hepatitis B along with a higher proportion of CD8/CD38 cells after treatment. The amount of activated cells decreased in patients responding to an antiviral therapy while the amount of CD8 cells rose. The authors suggest HBV-associated immune activation may be a crucial part of the pathogenesis and a promising target of treatment.
Peer reviewer: Shinji Shimoda, MD, PhD, Medicine and Biosystemic Science, Kyushu University Graduate School of Medical Sciences, 3-1-1 Maidashi, Higashi-Ku, Fukuoka 812-8582, Japan
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
| 1. | World Health Organization, Department of Communicable diseases surveillance and response. Hepatitis B. WHO Fact Sheets. Accessed: July 28 2010; Available from: http://www.who.int.. |
| 2. | Maini MK, Boni C, Ogg GS, King AS, Reignat S, Lee CK, Larrubia JR, Webster GJ, McMichael AJ, Ferrari C. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117:1386-1396. |
| 3. | Baumert TF, Thimme R, von Weizsäcker F. Pathogenesis of hepatitis B virus infection. World J Gastroenterol. 2007;13:82-90. |
| 4. | Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439-1449. |
| 5. | Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269-1280. |
| 6. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. |
| 7. | Bertoni R, Sidney J, Fowler P, Chesnut RW, Chisari FV, Sette A. Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J Clin Invest. 1997;100:503-513. |
| 8. | You J, Zhuang L, Zhang YF, Chen HY, Sriplung H, Geater A, Chongsuvivatwong V, Piratvisuth T, McNeil E, Yu L. Peripheral T-lymphocyte subpopulations in different clinical stages of chronic HBV infection correlate with HBV load. World J Gastroenterol. 2009;15:3382-3393. |
| 9. | Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R, Fiaccadori F. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest. 1998;102:968-975. |
| 10. | You J, Sriplung H, Geater A, Chongsuvivatwong V, Zhuang L, Li YL, Lei H, Liu J, Chen HY, Tang BZ. Impact of viral replication inhibition by entecavir on peripheral T lymphocyte subpopulations in chronic hepatitis B patients. BMC Infect Dis. 2008;8:123. |
| 11. | Boni C, Penna A, Ogg GS, Bertoletti A, Pilli M, Cavallo C, Cavalli A, Urbani S, Boehme R, Panebianco R. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology. 2001;33:963-971. |
| 12. | Savarino A, Bottarel F, Malavasi F, Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T-cell activation or an active player in virus/host interactions? AIDS. 2000;14:1079-1089. |
| 13. | Bofill M, Borthwick NJ. CD38 in health and disease. Chem Immunol. 2000;75:218-234. |
| 14. | Tilling R, Kinloch S, Goh LE, Cooper D, Perrin L, Lampe F, Zaunders J, Hoen B, Tsoukas C, Andersson J. Parallel decline of CD8+/CD38++ T cells and viraemia in response to quadruple highly active antiretroviral therapy in primary HIV infection. AIDS. 2002;16:589-596. |
| 15. | Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808-816. |
| 16. | Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800-807. |
| 17. | Zeng M, Mao Y, Yao G, Wang H, Hou J, Wang Y, Ji BN, Chang CN, Barker KF. A double-blind randomized trial of adefovir dipivoxil in Chinese subjects with HBeAg-positive chronic hepatitis B. Hepatology. 2006;44:108-116. |
| 18. | Lok ASF, McMahon BJ. AASLD Practice guidelines chronic hepatitis B: update 2009. Hepatology. 2009;50:1-36. |
| 19. | Lau GK, Cooksley H, Ribeiro RM, Powers KA, Shudo E, Bowden S, Hui CK, Anderson J, Sorbel J, Mondou E. Impact of early viral kinetics on T-cell reactivity during antiviral therapy in chronic hepatitis B. Antivir Ther. 2007;12:705-718. |
| 20. | Pham BN, Mosnier JF, Walker F, Njapoum C, Bougy F, Degott C, Erlinger S, Cohen JH, Degos F. Flow cytometry CD4+/CD8+ ratio of liver-derived lymphocytes correlates with viral replication in chronic hepatitis B. Clin Exp Immunol. 1994;97:403-410. |
| 21. | Lynne JE, Schmid I, Matud JL, Hirji K, Buessow S, Shlian DM, Giorgi JV. Major expansions of select CD8+ subsets in acute Epstein-Barr virus infection: comparison with chronic human immunodeficiency virus disease. J Infect Dis. 1998;177:1083-1087. |
| 22. | Belles-Isles M, Houde I, Lachance JG, Noël R, Kingma I, Roy R. Monitoring of cytomegalovirus infections by the CD8+CD38+ T-cell subset in kidney transplant recipients. Transplantation. 1998;65:279-282. |
| 23. | Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:332-340. |
| 24. | Hazenberg MD, Otto SA, van Benthem BH, Roos MT, Coutinho RA, Lange JM, Hamann D, Prins M, Miedema F. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881-1888. |
| 25. | Simonelli C, Nasti G, Vaccher E, Tirelli U, Zanussi S, De Paoli P, Comar M, Giacca M. Hydroxyurea treatment in HIV-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:462-464. |
| 26. | Margolis DM, Kewn S, Coull JJ, Ylisastigui L, Turner D, Wise H, Hossain MM, Lanier ER, Shaw LM, Back D. The addition of mycophenolate mofetil to antiretroviral therapy including abacavir is associated with depletion of intracellular deoxyguanosine triphosphate and a decrease in plasma HIV-1 RNA. J Acquir Immune Defic Syndr. 2002;31:45-49. |
| 27. | Read SW, DeGrezia M, Ciccone EJ, DerSimonian R, Higgins J, Adelsberger JW, Starling JM, Rehm C, Sereti I. The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS One. 2010;5:e11937. |
| 28. | Minguela A, Miras M, Bermejo J, Sánchez-Bueno F, López-Alvarez MR, Moya-Quiles MR, Muro M, Ontañón J, Garía-Alonso AM, Parrilla P. HBV and HCV infections and acute rejection differentially modulate CD95 and CD28 expression on peripheral blood lymphocytes after liver transplantation. Hum Immunol. 2006;67:884-893. |
| 29. | Choremi-Papadopoulou H, Panagiotou N, Samouilidou E, Kontopidou F, Viglis V, Antoniadou A, Kosmidis J, Kordossis T. CD28 costimulation and CD28 expression in T lymphocyte subsets in HIV-1 infection with and without progression to AIDS. Clin Exp Immunol. 2000;119:499-506. |
| 30. | Ostrowski SR, Gerstoft J, Pedersen BK, Ullum H. A low level of CD4+CD28+ T cells is an independent predictor of high mortality in human immunodeficiency virus type 1-infected patients. J Infect Dis. 2003;187:1726-1734. |