Published online Apr 21, 2011. doi: 10.3748/wjg.v17.i15.2013
Revised: January 4, 2011
Accepted: January 11, 2011
Published online: April 21, 2011
AIM: To evaluate the effect of multidisciplinary team (MDT) treatment modality on outcomes of patients with gastrointestinal malignancy in China.
METHODS: Data about patients with gastric and colorectal cancer treated in our center during the past 10 years were collected and divided into two parts. Part 1 consisted of the data collected from 516 consecutive complicated cases discussed at MDT meetings in Peking University School of Oncology (PKUSO) from December 2005 to July 2009. Part 2 consisted of the data collected from 263 consecutive cases of resectable locally advanced rectal cancer from January 2001 to January 2005. These 263 patients were divided into neoadjuvant therapy (NT) group and control group. Patients in NT group received MDT treatment, namely neoadjuvant therapy + surgery + postoperative adjuvant therapy. Patients in control group underwent direct surgery + postoperative adjuvant therapy. The outcomes in two groups were compared.
RESULTS: The treatment strategy was altered after discussed at MDT meeting in 76.81% of gastric cancer patients and in 58.33% of colorectal cancer patients before operation. The sphincter-preservation and local control of tumor were better in NT group than in control group. The 5-year overall survival rate was also higher in NT group than in control group (77.23% vs 69.75%, P = 0.049).
CONCLUSION: MDT treatment modality can significantly improve the outcomes of patients with gastrointestinal malignancy in China.
- Citation: Du CZ, Li J, Cai Y, Sun YS, Xue WC, Gu J. Effect of multidisciplinary team treatment on outcomes of patients with gastrointestinal malignancy. World J Gastroenterol 2011; 17(15): 2013-2018
- URL: https://www.wjgnet.com/1007-9327/full/v17/i15/2013.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i15.2013
Treatment of cancer has evolved toward a multidisciplinary team (MDT) approach[1-3]. The effect of MDT treatment modality on cancer is significantly better than that of conventional treatment modalities[4-6]. Although the MDT treatment modality has been successfully implemented in Western countries for decades, no report is available on its application in China. We conducted a study on the MDT treatment modality in a representative cancer center of China to evaluate its effect on outcomes of patients with gastrointestinal malignancy in China.
Data about patients with gastric and colorectal cancer were collected and divided into two parts. Part 1 consisted of the data collected from 516 consecutive complicated cases discussed at MDT meetings in Peking University School of Oncology (PKUSO) from December 2005 to July 2009. Complicated cases were defined as those with synchronic distant metastasis, marginally resectable or unresectable lesions, postoperative progression, and other conditions leading to difficulty in making treatment strategy. Records and treatment plans or recommendations for MDT treatment were used to investigate the effect of MDT treatment modality on clinical decision making and outcomes of patients with gastrointestinal malignancy (Table 1).
| Variables | Rectal cancer | Colon cancer | Gastric cancer |
| Pre-operation | 68 (33.10) | 40 (30.77) | 69 (38.12) |
| Postoperative progression1 | 121 (59.02) | 62 (47.69) | 101 (55.80) |
| Other condition | 16 (7.80) | 28 (21.54) | 11 (6.08) |
| Total | 205 (100) | 130 (100) | 181 (100) |
| Baseline characteristics | NT group | Control group | P value | |
| (n = 101) | (n = 162) | |||
| Sex | ||||
| Male | 57 | 88 | 0.737 | |
| Female | 44 | 74 | ||
| Age (yr)1 | 55 (51-59) | 55 (50-60) | 0.664 | |
| Distance of tumor from anal verge | ||||
| < 5 cm | 35 (34.7) | 37 (22.8) | 0.051 | |
| 5-12 cm | 66 (65.3) | 125 (77.2) | ||
| Surgery | ||||
| APR | 25 | 32 | 0.422 | |
| LAR | 76 | 130 | ||
| Preoperative serum CEA level | ||||
| Normal | 52 (51.5) | 82 (50.6) | 0.745 | |
| Abnormal | 35 (34.7) | 52 (32.1) | ||
| Unknown | 14 (13.9) | 28 (17.3) | ||
| Pretreatment staging tools | ||||
| MRI | 61 (60.4) | 66 (40.7) | < 0.001 | |
| ERUS | 28 (27.7) | 34 (21.0) | ||
| CT | 12 (11.9) | 62 (38.3) | ||
| Pretreatment TNM stage | ||||
| IIA(T3 N0) | 24 (23.8) | 54 (34.0) | 0.278 | |
| IIB (T4 N0) | 4 (4.0) | 4 (2.5) | ||
| IIIA (T1-2 N1) | 3 (3.0) | 8 (4.9) | ||
| IIIB (T3-4 N1) | 32 (31.7) | 37 (22.8) | ||
| IIIC (AnyT N2) | 38 (37.6) | 58 (35.8) | ||
| Pathologic TNM stage | ||||
| I (T1-2 N0) | 35 (34.7) | 12 (7.4) | < 0.01 | |
| IIA (T3 N0) | 26 (25.7) | 54 (33.3) | ||
| IIB (T4 N0) | 1 (1.0) | 0 (0) | ||
| IIIA (T1-2 N1) | 6 (5.9) | 7 (4.3) | ||
| IIIB (T3-4 N1) | 17 (16.8) | 40 (24.7) | ||
| IIIC (AnyT N2) | 16 (15.8) | 49 (30.2) | ||
| Histologic differentiation | ||||
| High | 2 (2.0) | 20 (12.3) | 0.013 | |
| Moderate | 70 (69.3) | 110 (67.9) | ||
| Poor | 24 (23.8) | 24 (14.8) | ||
| Mucinous and signet | 5 (5.0) | 8 (4.9) | ||
| Lymphovascular invasion | ||||
| Present | 21 (20.8) | 50 (30.9) | 0.074 | |
| Absent | 80 (79.2) | 112 (69.1) |
Part 2 consisted of the data collected from 263 consecutive cases of resectable locally advanced rectal cancer from January 2001 to January 2005. Patients included in this study were those with resectable rectal cancer located 12 cm or less from the anal verge, histologically identified primary carcinoma of the rectum, no clinical evidence of preoperative distant metastasis, transabdominal radical resection based on the principle of total mesorectal excision (TME)[7], and R0 resection. Finally, 263 eligible patients included in this study (Table 2) were divided into neoadjuvant therapy (NT) group and control group according to whether they underwent neoadjuvant radiotherapy.
Patients in NT group received neoadjuvant therapy + surgery + postoperative adjuvant therapy. The total preoperative radiation dose was 30 Gy (30 Gy/10 fractions, bioequivalent dose 36 Gy) recommended by the Chinese Anti-Cancer Association (CACA)[8], and 5-FU or capecitabine was used in postoperative chemotherapy.
Contrast to MDT treatment, the conventional treatment strategy for locally advanced rectal cancer in China is surgery followed by postoperative chemoradiotherapy which was commonly used in China 5 years ago. Patients in NT group were evaluated before operation by special MDT members while those in control group were not evaluated.
Patients were followed-up every three months for the first 2 years after surgery followed by every six months for 5 years. Serum carcinoembryonic antigen (CEA) level was measured and abdominal ultrasound, pelvic MRI, chest radiograph were performed every six months, and colonoscopy was performed annually during the follow-up. The follow-up time ranged from six to ninety-six months, with a median time of seventy-two months. The outcomes of patients with gastrointestinal malignancy were evaluated at the end of 5-year follow-up with a follow-up rate of 87.8% (231/263).
Demographic and clinicopathologic data were analyzed by χ2 test. Kaplan-Meier life table and log-rank test were used to compare the disease-free survival (DFS) and overall survival (OS) rates. Cox proportional hazards regression was used in multivariate analysis. Statistical analysis was performed using the SPSS version 16.0 software. P < 0.05 was considered statistically significant.
The working model of MDT in our center includes two major components: weekly MDT meetings to discuss complicated clinical cases and interdisciplinary consultations for preoperative and postoperative evaluation and therapy. Most patients receive MDT therapy according to interdisciplinary consultations while only complicated cases are discussed at MDT meetings. Although the MDT team modalities are different, the treatment strategies for patients are made by the same team in our center. The key members of MDT team include a surgeon, a medical oncologist, a radiation oncologist, a radiologist, a pathologist, and specialized nurses. Attendance of the key members at MDT meetings is not compulsory but enhanced by a special coordinator who is responsible for organizing and recording the MDT meetings. The discussion processes and conclusions for each patient are recorded in special tables.
Complicated cases of gastric cancer (n = 181), colon cancer (n = 130), and rectal cancer (n = 205) were discussed at MDT meetings during the last 5 years (Table 1). Among the discussed cases, outpatients accounted for 84.69% (n = 437) and inpatients accounted for 15.31% (n = 79), respectively. For each disease classification, patients with postoperative recurrence or metastasis accounted for 48%-59%, suggesting that such patients are needed to be discussed at MDT meetings.
The MDT team modality directly influenced the clinical decision making. Of the 69 preoperative patients with gastric cancer discussed at MDT meetings, 53 (76.81%) underwent neoadjuvant chemotherapy instead of direct surgery. Of the 63 preoperative patients with extensive lesions or synchronous distant metastasis of colorectal cancer who underwent MDT treatment, including chemotherapy, chemoradiotherapy, or target therapy, 7 with initially inoperable liver metastasis underwent radical resection after MDT treatment.
To verify the comparability of outcomes in NT and control groups, the major demographic and tumor variables were analyzed (Table 2). No difference was found in gender and age of the patients, tumor location, preoperative serum carcinoembryonic antigen (CEA) level, pretreatment clinical stage and lymphovascular invasion (LVI) of tumor between the two groups. The histological differentiation of tumor appeared poorer in control group than in NT group, implying that the prognosis of patients with gastrointestinal malignancy is potentially better in control group than in NT group. However, multivariate analysis demonstrated that it was not a major factor for the clinical outcome of such patients, indicating that the outcomes of patients in the two groups are comparable.
The staging tools used for pretreatment evaluation of the two groups differed significantly. Magnetic resonance imaging (MRI) was more frequently used in NT group than in control group (60.4% vs 40.7%, P < 0.05), while computed tomography (CT) was more commonly used in control group than in NT group.
Although no significant difference was found in pretreatment stage between the two groups, the proportion of pathologic stage I was higher in NT group than in control group (34.7% vs 7.4%), while that of stage III was higher in control group than in NT group (Table 2).
Among the patients with low rectal cancer less than 5 cm from the anal verge (n = 72), the sphincter preservation rate was 37.14% (13/35) and 13.51% (5/37), respectively, for the NT group and control group (P < 0.05, Table 3).
| Clinical outcome | NT group | Control group | Odds ratio | P value |
| (n = 101) | (n = 162) | (95% CI) | ||
| Sphincter preservation1 | 13 (37.14) | 5 (13.51) | 3.78 (1.18-12.13) | 0.041 |
| Local recurrence | 4 (3.96) | 18 (11.11) | 0.33 (0.11-1.00) | 0.042 |
| Distant metastasis | 22 (21.78) | 36 (22.22) | 0.87 (0.48-1.57) | 0.933 |
| 5-yr disease-free survival rate | 77 (76.24) | 109 (67.28) | - | 0.039 |
| 5-yr overall survival rate | 78 (77.23) | 113 (69.75) | - | 0.049 |
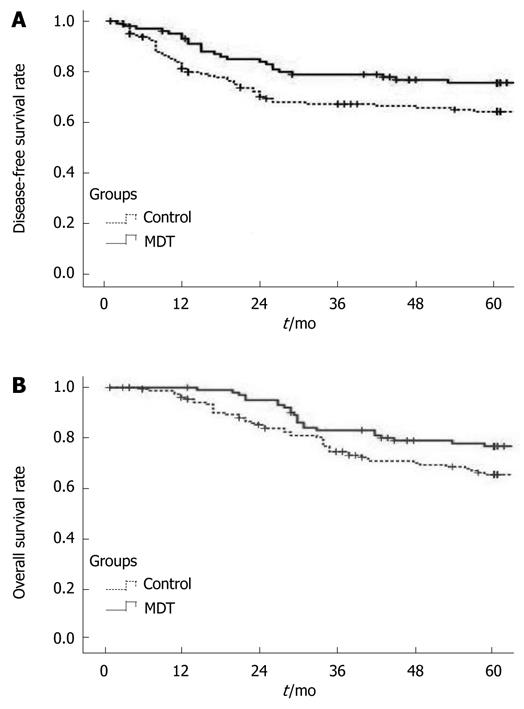
The local recurrence rate was 3.96% (4/101) and 11.11% (18/162), the 5-year DFS rate was 76.24% and 67.28% (P < 0.05, Figure 1), and the 5-year OS rate was 77.23% and 69.75% (Figure 1, Table 3), for the NT group and control group, respectively.
Multivariate analysis demonstrated that the pretreatment serum CEA level, pathologic TNM stage, and LVI were the major factors for the long-term survival rate of patients with gastrointestinal malignancy (Table 4). Other variables, including neoadjuvant radiotherapy, were not the independent factors for the OS rate.
| Variables | Hazard ratio | 95% CI | P value |
| Pretreatment CEA level | 1.429 | 1.044-1.956 | 0.026 |
| Pathologic TNM stage | 1.440 | 1.137-1.825 | 0.002 |
| Lymphovascular invasion | 0.468 | 0.286-0.765 | 0.002 |
| Sex | 1.164 | 0.726-1.867 | 0.529 |
| Age | 0.700 | 0.424-1.156 | 0.163 |
| Distance of tumor from anal verge | 0.994 | 0.854-1.157 | 0.934 |
| Pretreatment TNM stage | 0.949 | 0.727-1.239 | 0.703 |
| Surgery form (LAR or APR) | 0.853 | 0.575-1.264 | 0.427 |
| Histologic differentiation | 0.969 | 0.822-1.142 | 0.706 |
| NT | 0.878 | 0.519-1.483 | 0.626 |
Treatment of cancer increasingly requires the cooperation of specialists from various disciplines[9], although surgery still plays a critical role in cancer treatment. Currently, most doctors around the world have recognized the effect of MDT approach[10,11] and endorse it as a principal treatment modality for cancer[1,2]. Although the composition of MDT in China is similar to that in Western countries, there are many distinct differences in working models of China. First, no special rules or guidelines are available on MDT in China, thus it is not compulsory for all cancer patients to receive MDT treatment. Second, not all but some big cancer centers adopt MDT treatment modality without consistent indications for discussion at MDT meetings in different hospitals. In general, MDT is still under development in China[12,13].
Our cancer center is one of the earliest hospitals adopting MDT approach in China. It is difficult to quantify improvement in outcomes of cancer patients, especially those with complicated clinical conditions, after MDT treatment. In this study, data on cases of locally advanced rectal cancer, which is considered the most successful and mature model of MDT approach[14-16], were collected to evaluate the effect of MDT treatment on the clinical outcomes of patients with gastrointestinal malignancy. Cases discussed at MDT meeting were reviewed to assess the influence of MDT treatment modality on the treatment strategy for patients with gastrointestinal malignancy. The data included in the two parts were completely independent without any overlap.
Several studies demonstrated that MDT approach can optimize the decision making, enhance the quality of cancer care, and improve the clinical outcomes of cancer patients[1,11,17,18]. Our data indicate that MDT meetings change a considerable proportion of treatment strategies, including neoadjuvant therapy for preoperative patients and MDT treatment modality for patients with tumor recurrence and metastasis. In this study, 7 patients with inoperable liver metastasis of colorectal cancer underwent R0 resection after MDT treatment. However, the limited time of MDT meetings and the large number of patients who need to be discussed at MDT meetings made it impossible to discuss and evaluate all patients, thus the vast majority of patients were evaluated before operation and neoadjuvant therapy was evaluated according to the interdisciplinary consultations.
It is widely believed that accurate and integrative evaluation before operation, as well as active strategies for adjuvant therapy used by MDT members, are the primary factors for improving the clinical outcomes of cancer patients[2,3,16,19,20]. The meticulous and reliable assessment of patients with locally advanced rectal cancer before operation by MDT members is closely associated with the treatment strategy. It was reported that MRI is more accurate in clinical staging of tumor and in predicting of circumferential resection margin (CRM) when it is used in evaluation of rectal cancer[21-23]. In this study, the strategy for preoperative evaluation of the two groups differed significantly. MRI was used more frequently in NT group than in control group (60.4% vs 40.7%, P < 0.01), suggesting that MRI can improve the accuracy of clinical tumor staging in NT group.
It has been shown that neoadjuvant radiotherapy for rectal cancer can improve the local control of cancer before operation[24-27], which constituted the major difference between MDT and traditional treatment modalities in this study. Neoadjuvant radiotherapy can decrease the size or stage of low rectal cancer, thus preserving the anus[28-30]. In this study, the sphincter preservation, the local control of cancer, and the 5-year OS rate were better in NT group than in control group, suggesting that patients may benefit from MDT treatment.
Finally, although our study showed the advantages of MDT treatment modality for gastrointestinal cancer, its widespread use in China is still problematic. First, administrative support is insufficient in some places, leading to organizational problems and even its discontinuation. Second, MDT meetings are time-consuming and incomplete attendance is a barrier to success. However, these problems will not hinder the popularity and application of MDT treatment modality in China.
In conclusion, MDT treatment modality can significantly improve the clinical strategies for the treatment of gastrointestinal malignancy, and Chinese patients can benefit from it.
Treatment of cancer has evolved toward a multidisciplinary team (MDT) approach. Although the MDT treatment modality has been successfully implemented in Western countries for decades, it is not widely applied in China. The authors conducted a study on the MDT treatment modality in a representative cancer center in China to evaluate its effect on clinical decision making and outcomes in patients with gastrointestinal malignancy.
MDT treatment modality is the major concern in cancer treatment, and this study addressed it for cancer in China.
MDT treatment modality, systematically introduced in this study, can significantly improve the clinical strategies for the treatment of gastrointestinal malignancy, and Chinese patients can benefit from it.
MDT treatment modality is of high values for patients with gastrointestinal malignancy and can be commonly used in hospitals of China in treatment of cancer patients.
This paper is very good and shows that neoadjuvant therapy can improve the outcomes of patients with gastrointestinal malignancy, thus providing a novel therapy for gastrointestinal cancer.
Peer reviewer: Dr. Yeng S Ang, MD, Department of Gastroenterology, Royal Albert Edward Infirmary, Wigan Lane, Wigan, Greater Manchester WN1 2NN, United Kingdom
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Carter S, Garside P, Black A. Multidisciplinary team working, clinical networks, and chambers; opportunities to work differently in the NHS. Qual Saf Health Care. 2003;12 Suppl 1:i25-i28. |
| 2. | Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK? Lancet Oncol. 2006;7:935-943. |
| 3. | Moehler M, Lyros O, Gockel I, Galle PR, Lang H. Multidisciplinary management of gastric and gastroesophageal cancers. World J Gastroenterol. 2008;14:3773-3780. |
| 4. | Forrest LM, McMillan DC, McArdle CS, Dunlop DJ. An evaluation of the impact of a multidisciplinary team, in a single centre, on treatment and survival in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2005;93:977-978. |
| 5. | Junor EJ, Hole DJ, Gillis CR. Management of ovarian cancer: referral to a multidisciplinary team matters. Br J Cancer. 1994;70:363-370. |
| 6. | MacDermid E, Hooton G, MacDonald M, McKay G, Grose D, Mohammed N, Porteous C. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis. 2009;11:291-295. |
| 7. | Heald RJ, Karanjia ND. Results of radical surgery for rectal cancer. World J Surg. 1992;16:848-857. |
| 8. | Chinese Anti-Cancer Association. The surgical guideline of low rectal cancer. Chin J Gastrointest Surg. 2005;8:88-90. |
| 9. | Rougier P, Neoptolemos JP. The need for a multidisciplinary approach in the treatment of advanced colorectal cancer: a critical review from a medical oncologist and surgeon. Eur J Surg Oncol. 1997;23:385-396. |
| 10. | Minsky BD. Multidisciplinary case teams: an approach to the future management of advanced colorectal cancer. Br J Cancer. 1998;77 Suppl 2:1-4. |
| 11. | Blazeby JM, Wilson L, Metcalfe C, Nicklin J, English R, Donovan JL. Analysis of clinical decision-making in multi-disciplinary cancer teams. Ann Oncol. 2006;17:457-460. |
| 12. | Chan PK, Fischer S, Stewart TE, Hallett DC, Hynes-Gay P, Lapinsky SE, MacDonald R, Mehta S. Practising evidence-based medicine: the design and implementation of a multidisciplinary team-driven extubation protocol. Crit Care. 2001;5:349-354. |
| 13. | Shen J, Liu M, Zhang J, Su W, Ding G. Relapse in MB leprosy patients treated with 24 months of MDT in south west China: a short report. Lepr Rev. 2006;77:219-224. |
| 14. | Aschele C, Lonardi S. Multidisciplinary treatment of rectal cancer: medical oncology. Ann Oncol. 2007;18 Suppl 9:ix114-ix121. |
| 15. | Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM, Haustermans K, Maingon P, Overgaard J, Pahlman L. Multidisciplinary Rectal Cancer Management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol. 2009;92:148-163. |
| 16. | Cervantes A, Rodríguez-Braun E, Navarro S, Hernández A, Campos S, García-Granero E. Integrative decisions in rectal cancer. Ann Oncol. 2007;18 Suppl 9:ix127-ix131. |
| 17. | Davies AR, Deans DA, Penman I, Plevris JN, Fletcher J, Wall L, Phillips H, Gilmour H, Patel D, de Beaux A. The multidisciplinary team meeting improves staging accuracy and treatment selection for gastro-esophageal cancer. Dis Esophagus. 2006;19:496-503. |
| 18. | Stephens MR, Lewis WG, Brewster AE, Lord I, Blackshaw GR, Hodzovic I, Thomas GV, Roberts SA, Crosby TD, Gent C. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal cancer. Dis Esophagus. 2006;19:164-171. |
| 19. | Burton S, Brown G, Daniels IR, Norman AR, Mason B, Cunningham D. MRI directed multidisciplinary team preoperative treatment strategy: the way to eliminate positive circumferential margins? Br J Cancer. 2006;94:351-357. |
| 20. | Cervantes A, Roselló S, Rodríguez-Braun E, Navarro S, Campos S, Hernández A, García-Granero E. Progress in the multidisciplinary treatment of gastrointestinal cancer and the impact on clinical practice: perioperative management of rectal cancer. Ann Oncol. 2008;19 Suppl 7:vii266-vii272. |
| 21. | Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003;90:355-364. |
| 22. | Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. |
| 23. | Salerno G, Daniels IR, Moran BJ, Wotherspoon A, Brown G. Clarifying margins in the multidisciplinary management of rectal cancer: the MERCURY experience. Clin Radiol. 2006;61:916-923. |
| 24. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. |
| 25. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. |
| 26. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. |
| 27. | Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625. |
| 28. | Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, Goodman KA, Minsky BD, Wong WD. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg. 2009;249:236-242. |
| 29. | Gerard JP, Chapet O, Nemoz C, Hartweig J, Romestaing P, Coquard R, Barbet N, Maingon P, Mahe M, Baulieux J. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: the lyon R96-02 randomized trial. J Clin Oncol. 2004;22:2404-2409. |