Published online Apr 21, 2011. doi: 10.3748/wjg.v17.i15.1996
Revised: September 7, 2010
Accepted: September 14, 2010
Published online: April 21, 2011
AIM: To determine the prevalence of increased intraepithelial lymphocytes, using immunohistochemistry in patients with normal colonoscopy and near normal biopsy.
METHODS: We retrospectively reviewed all non-malignant colon mucosal biopsies between 2005 and 2007, reported as normal, chronic inflammation or melanosis coli in patients who were undergoing routine colonoscopy. Immunohistochemistry using CD3 was performed on all mucosal biopsies and an intraepithelial lymphocyte count (IEL) was determined. Cases with an IEL count of ≥ 20 IELs per 100 surface epithelial cells were correlated with demographic, clinical and follow-up data. A further subgroup was evaluated for lymphocytic colitis.
RESULTS: Twenty (8.3%) of 241 cases revealed an IEL count ≥ 20. Six (2.5%) patients were identified as having lymphocytic colitis (P < 0.001), of whom, five were missed on initial evaluation (P = 0.01). Four of these five patients were labeled with diarrhea-predominant irritable bowel syndrome (IBS). On follow-up, three of the remaining 20 cases were diagnosed with malignancy (renal cell carcinoma and myelodysplastic syndrome) and one had an unknown primary tumor with multiple liver metastases. Two cases of collagenous colitis with an IEL count < 10 were included in this study. Increased IELs were not confined to patients with diarrhea as a primary presenting symptom, but were also present in patients with abdominal pain (n = 7), constipation (n = 3) and loss of weight (n = 1).
CONCLUSION: Immunohistochemistry using CD3 is of value in identifying and quantifying IELs for the presence of microscopic colitis in patients with diarrhea-predominant IBS.
- Citation: Mohamed N, Marais M, Bezuidenhout J. Microscopic colitis as a missed cause of chronic diarrhea. World J Gastroenterol 2011; 17(15): 1996-2002
- URL: https://www.wjgnet.com/1007-9327/full/v17/i15/1996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i15.1996
Microscopic colitis is regarded as a common cause of chronic watery diarrhea, accounting for approximately 4%-13% of patients presenting with this symptom[1]. By definition the colon appears normal or nearly normal on colonoscopy, with set histopathological criteria required for the diagnosis on mucosal biopsy. Lymphocytic colitis and collagenous colitis constitute the two major subtypes of microscopic colitis that share many similarities, including almost identical clinical symptoms, together with a macroscopically normal colonic mucosa. Both entities demonstrate colonic intraepithelial lymphocytosis, increased inflammatory cells within the lamina propria, and preserved crypt architecture, but are distinguished by the presence of a thickened basement membrane in collagenous colitis.
In the past, microscopic colitis was thought to be a rare disorder and very little was known about its etiology or epidemiology. It has become apparent that microscopic colitis is now regarded as common cause of diarrhea in middle-aged and elderly patients. Many recent publications have shown that the incidence of microscopic colitis is on the increase. Epidemiological data have now been reported from seven major regions[2], with most of the reported data coming from North American and European studies. The incidence rates for collagenous colitis is 0.8-6.2/100 000 and lymphocytic colitis is 0.5-12.9/100 000[2]. According to various studies, the prevalence of collagenous colitis and lymphocytic colitis is 10-15.7/100 000 and 14.4/100 000, respectively[1,3-5]. There are very few data available from developing countries, with a few case series reported from India[6], Turkey[7] and Sri Lanka[8].
Currently there are no data regarding this disease in South Africa, where infectious diseases are more prevalent. Isolated cases have been reported from Nigeria[9,10]. In this region, microscopic colitis is underdiagnosed because of a lack of colonoscopic facilities and the assumption that most cases of chronic diarrhea are likely to be infective, therefore, most patients self medicate and do not present to a hospital[10,11].
At Tygerberg Hospital, a tertiary referral center, approximately 1700 colonoscopies are performed each year, which include colonoscopies for non-infective diarrhea-related causes. Colonic biopsies at our institution are often reported as chronic inflammation, indeterminate colitis, chronic colitis or normal in the investigation of diarrheal disease. It is possible that the diagnosis of LC may have been missed in a proportion of these cases, because under-reporting of cases is common and has been documented in other studies[12]. According to a Swedish study, in a third of cases the diagnosis was missed in the primary histological examination[13,14]. The important role of the pathologist was clearly illustrated in this study that showed the difficulties in diagnosing microscopic colitis, especially the lymphocytic subtype[14]. According to Nielson, terms such as “unspecific chronic inflammation” or “signs of chronic inflammatory bowel disease but not diagnostic” should be avoided[14].
Immunostaining does not seem to play a major role in the diagnosis of LC. According to Tysk et al[2] and Chang et al[15], in some uncertain cases, immunostaining may facilitate the assessment of intraepithelial counts. There have been no studies to validate the benefit of performing immunohistochemistry in uncertain cases. Currently, the histological diagnosis is based on hematoxylin and eosin (H and E) assessment of criteria: (1) increase in intraepithelial lymphocytes (IELs > 20/100 surface epithelial cells); (2) surface epithelial damage; and (3) infiltration of lymphocytes and plasma cells into the lamina propria, with no subepithelial collagen deposition, as identified in collagenous colitis[16,17]. The diagnosis of lymphocytic colitis remains a challenge because it is often difficult to identify and quantify lymphocytes due to orientation of the biopsy, or nuclei having similar cytological features to those of columnar cell nuclei. Immunohistochemistry staining with CD3 has been shown to play a role in the counting and identification of IELs in celiac disease; incidentally, there is also an association between microscopic colitis and celiac disease[18].
Only one recent study has examined the reproducibility of histological diagnosis in microscopic colitis. That study found excellent correlation in distinguishing microscopic colitis and non-microscopic colitis amongst pathologists using H and E- stained slides. That study claimed κ values of 0.90 and 0.83 for inter-observer agreement and 0.89 for intra-observer agreement[19]. However, the authors have stated that their high rate of concordance was due to their particular expertise within the field of gastroenterology. In their study, immunohistochemistry was not performed to assess whether it allows easier identification of IELs.
In the present study, we aimed to determine the prevalence of increased IELs with the use of immunohistochemistry and described the spectrum of disease in all non-malignant biopsies reported as normal or chronic inflammation over a 3-year period. By facilitating counting of IELs, we hoped to identify cases that may have the lymphocytic colitis subtype.
The study design was a retrospective analysis of all non-malignant colonoscopic biopsies diagnosed as normal or chronic inflammation in patients who underwent colonoscopy at the Tygerberg Hospital Gastroenterology Unit, for the period 2005-2007. Cases were retrieved from the Department of Pathology, Division of Anatomical Pathology, DisaLAB database for the 3-year period. Of the 1212 cases identified, only 247 met the criteria necessary for our analysis: (1) normal or chronic colitis on histology; (2) melanosis coli; or (3) microscopic colitis including collagenous and lymphocytic colitis. The melanosis coli category was incorporated as it is possible that reported cases of diarrhea might not be due to laxative abuse. Cases that were excluded were: (1) known cases of inflammatory bowel disease, malignancy, radiotherapy, infective diarrhea, rectal bleeding, and an abnormal colonoscopy.
Immunohistochemistry using antibodies against CD3 was performed on all cases as the primary evaluation method for IELs. The staining was performed on 4-μm thick, formalin-fixed, paraffin-embedded tissue sections, using the Bond max autoimmune stainer with the Bond Polymer Refine Detection system (DS9800). Antibodies against CD3 (Leica Biosystems, Newcastle, UK; NCL-L-CD2-565, dilution 1:300) were applied to each case. For epitope retrieval ER2 (Leica Biosystems) was used for 20 min.
All immunohistochemical slides were randomly assigned a study number and an IEL count was performed. For a lymphocyte to be counted, the nucleus had to be visible with cytoplasmic and membrane staining. Intercryptal areas were counted and areas overlying lymphoid follicles were avoided. Only cases with ≥ 20 per 100 IELs were further investigated. In addition, all H and E-stained sections were re-evaluated for basement membrane thickening. In suspected cases, a Masson Trichrome stain was performed and the basement membrane measured with an Olympus ocular micrometer. Poorly orientated biopsies were excluded from evaluation.
Data regarding presenting history, microscopic diagnosis, patient age, sex and follow-up of patients with ≥ 20 IELs were recorded. A subcategory of patients presenting with chronic diarrhea was identified and further evaluated for microscopic colitis. The results were correlated with clinical findings.
Histological diagnosis of lymphocytic colitis was confirmed with ≥ 20 IELs per 100 surface epithelial cells, with normal being < 5[16,19,20]. In addition, a mixed inflammatory infiltrate in the lamina propria that consisted of lymphocytes and plasma cells with surface epithelial damage was noted[3,16]. Diagnosis of collagenous colitis was established with a subepithelial collagen layer reaching or exceeding 10 μm[16,21,22].
The study protocol was approved by the University of Stellenbosch Ethics committee.
Microsoft Excel was used to capture the data and STATISTICA version 9 was used to analyze the data. Summary statistics were used to describe the demographic variables and certain laboratory parameters. Medians and means were used as the measures of central location and SDs and quartiles as indicators of spread. For demographic variables such as laboratory parameters, 95% CIs were calculated. Incidence rates in the population studied were determined as proportions and these were compared to determine whether they were significantly different from zero. P < 0.05 represented statistical significance in hypothesis testing.
Immunohistochemical evaluation of 241 cases revealed a mean lymphocyte count of 7.7 (95% CI = 6.4-8.9). Twenty cases (8.3%) were identified as having an IEL count of ≥ 20 per 100 surface epithelial cells (P < 0.001) (Table 1).
| No. of cases | 1P value | |
| Cases marked as normal, chronic inflammation or melanosis coli | 241 | |
| IELs > 20 | 20 | < 0.001 |
| Known case of LC | 1 | 0.158 |
| Missed LC | 5 | < 0.001 |
| Collagenous colitis | 2 | 0.078 |
| Microscopic colitis (Total No. of cases) | 8 | < 0.001 |
These 20 patients were further categorized and the clinicopathological features summarized in Table 2. Six (2.5%) of the 241 patients were identified as having lymphocytic colitis (P < 0.001). Five (2%) of these patients were only diagnosed in this review and were therefore missed on initial evaluation (P = 0.01). Four of the five patients were labeled with irritable bowel syndrome (IBS). On follow-up, 3/5 patients had persistent diarrhea, despite ongoing investigations. On review, their diagnosis was changed to microscopic colitis. The remaining 2/5 patients were lost to follow-up. Clinical symptoms that were not in keeping with irritable bowel syndrome in this group included increase stool frequency of up to three times per day and abdominal pain that woke the patient at night. These patients were not evaluated for response to treatment.
| Age | Sex | IEL count | Presenting symptom | Original biopsy diagnosis | Follow-up period for 2 yr |
| 32 | F | 22 | Chronic diarrhea | Normal | Lymphocytic colitis12 |
| 32 | F | 27 | Chronic diarrhea | Normal | Lymphocytic colitis2 |
| 55 | F | 27 | Constipation | Normal | Resolve |
| 65 | M | 26 | Abdominal pain | Normal | Myelodysplastic syndrome |
| 22 | F | 31 | Constipation | Normal | Lost to follow up |
| 35 | M | 38 | Chronic diarrhea | Normal | Lymphocytic colitis1 |
| 42 | F | 30 | Constipation | Normal | Lost to follow up |
| 59 | F | 28 | Abdominal pain | Normal | Resolve |
| 59 | F | 40 | Chronic diarrhea | Normal | Multiple liver lesions |
| 56 | M | 31 | Chronic diarrhea | Normal | Ulcerative colitis |
| 79 | F | 20 | Abdominal pain | Melanosis coli | Diverticulitis |
| 19 | F | 28 | Abdominal pain | Normal | Behcet’s disease |
| 24 | F | 24 | Chronic diarrhea | Normal | Lymphocytic colitis2 |
| 51 | M | 48 | Chronic diarrhea | Normal | Metastatic renal cell carcinoma |
| 56 | F | 30 | Chronic diarrhea | Normal | Lymphocytic colitis2 |
| 34 | F | 30 | Abdominal pain | Normal | Resolved |
| 33 | F | 30 | Chronic diarrhea | Lymphocytic colitis | Lymphocytic colitis3 |
| 71 | M | 20 | Loss of weight | Normal | Diverticulitis |
| 59 | F | 25 | Abdominal pain | Normal | Resolve |
| 35 | F | 35 | Abdominal pain | Melanosis coli | Pancreatitis |
| Age | Sex | IEL count | Original diagnosis | Comorbid disease | Follow-up period for 2 yr | |
| Diagnosis | Diarrhea | |||||
| 54 | F | 10 | Chronic inflammation | Diabetic hypertension | Lactose intolerant | Persistent |
| 70 | F | 12 | Melanosis coli | Diabetic | Autonomic neuropathy | Persistent |
| 22 | M | 13 | Chronic inflammation | Lost to FU | Lost to FU | |
| 64 | M | 15 | Melanosis coli | Diabetic, asthmatic | Autonomic neuropathy | Persistent |
| 59 | F | 16 | Melanosis coli | Schistosomiasis contact | Irritable bowel syndrome | Persistent |
| 64 | F | 18 | Normal | Diabetic, asthmatic, previous sigmoidectomy for benign stricture | Hypothyroid | Persistent |
We included two patients who were later diagnosed with malignant disease (myelodysplastic syndrome and renal cell carcinoma) after a 2-year follow-up. A third patient developed multiple liver lesions with an unknown primary tumor. In addition, three patients initially reported as having normal colonoscopy were diagnosed with diverticular disease (n = 2) and ulcerative colitis (n = 1). Subsequent review of the surgical notes indicated an error in documentation.
Among the remaining eight patients, the primary presenting symptom resolved in four. Two patients with abdominal pain were later diagnosed with pancreatitis and Behcet’s disease. It is not clear if the latter patient’s abdominal pain was related to her condition. Two patients were lost to follow-up.
The two (0.8%) cases of collagenous colitis that were included in the total study population of 241 (P = 0.07) had an IEL count of 7 and 8, respectively. No additional cases of collagenous colitis were identified by selective staining with the Masson Trichrome technique.
The most common presenting complaint was chronic diarrhea in 9/20 cases, abdominal pain in 7/20, and constipation in 3/20, followed by loss of weight in 1/20. Seventeen cases were originally reported as normal on histology; one of lymphocytic colitis and two of melanosis coli (Table 2).
In addition, patients with an IEL > 10 and < 19 who presented with chronic diarrhea were documented to identify possible cases of paucicellular lymphocytic colitis (Table 3). Although no patients could be confidently diagnosed in this subgroup, comorbid disease such as diabetes accounted for several cases of diarrhea within this subgroup.
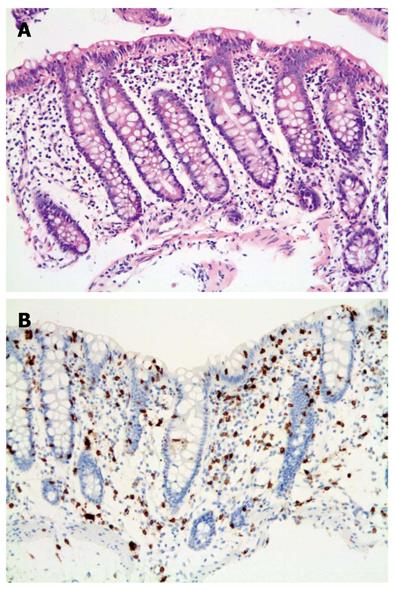
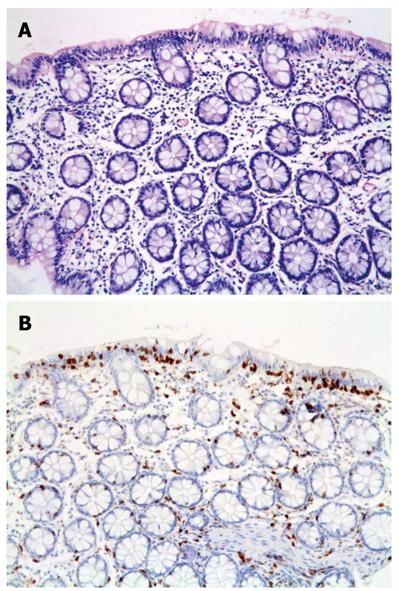
The histological findings among the different groups were very similar with IELs apparent in all 20 cases by H and E staining. However, the lymphocytes were more easily identified with the aid of CD3 (Figure 1). Poor staining quality and tangential biopsies accounted for misidentification of lymphocytes by H and E staining (Figure 2). Chronic inflammation was mild to moderate within the lamina propria.
In the lymphocytic colitis group, chronic inflammation was regarded as moderate in the lamina propria and 3/6 cases showed surface epithelial damage. Crypt branching was absent. Again, lymphocytes were more easily counted and identified with the immunohistochemical stain compared to H and E.
The two cases of collagenous colitis had a thick collagen band that measured 10 and 11 μm with visible entrapment of capillaries and mild to moderate chronic inflammation in the lamina propria.
Pigment was confirmed in two cases of melanosis coli but was subtle. The site of most biopsies could not be verified because it was not documented. None of the patients with microscopic colitis was evaluated for response to medical treatment.
An increase in the awareness of the entity of microscopic colitis has resulted in it being recognized as a known cause of chronic watery diarrhea[2,13,23]. Although the incidence of this disease seems to be rising, there has been very little documentation of this entity from our hospital. The importance of recognizing this condition is crucial, firstly, because chronic diarrhea is a debilitating illness, and secondly, treatment of this condition is no longer empirically based, as several recent randomized, double-blind, placebo-controlled trials have shown budesonide to be effective in the treatment of this disorder[24-27].
Our study is novel in the sense that we reviewed all our non-malignant colon biopsies reported as normal or chronic inflammation, to identify patients with chronic diarrhea that might have had microscopic colitis. Using this bottom up approach, we focused on lymphocytic colitis. Neither the incidence nor the prevalence of this disease could be estimated using this approach, because our sample population did not consist of patients presenting exclusively with chronic diarrhea. Instead, we identified secondary causes of intraepithelial lymphocytosis that included diverticular disease, ulcerative colitis, and malignancy. These secondary causes need to be excluded before making a diagnosis of microscopic colitis[14]. Other secondary causes of intraepithelial lymphocytosis, not identified in this study but described by Nielson, include Crohn’s disease, colonic infections and amyloidosis[14]. According to Fenoglio-Preiser[28], there have also been reports of lymphocytic-colitis-like histology in patients with constipation, which is similar to our findings. We have also identified patients with abdominal pain as another group presenting with lymphocytosis. Although the clinical symptoms of constipation and abdominal pain resolved in a few cases, others were later identified as having significant pathology. Our results suggest that any normal colonoscopy with a finding of intraepithelial lymphocytosis should be carefully monitored for future disease.
In the present study, the diagnosis of lymphocytic colitis was missed in five patients at the initial histological evaluation. It is particularly interesting to note that four of these patients were labeled as having IBS, in view of the biopsy being reported as normal. In a population-based cohort from Olmsted County, approximately one half of patients with microscopic colitis met the symptom-based criteria for IBS[29]. It is therefore not surprising that there is symptomatic overlap between these two entities. The recommendations from the Olmsted County study are that patients with diarrhea-predominant IBS should undergo colonoscopy to exclude microscopic colitis[29]. Similarly, Madisch et al[30]have shown that 30% of patients with microscopic colitis had clinical symptoms that overlap with IBS. We can therefore conclude that patients with microscopic colitis can be misdiagnosed with IBS.
Even though there is very little inter- and intra-observer variability in the histological diagnosis[19], the diagnosis of microscopic colitis can be challenging at times, especially due to the morphological heterogeneity described in microscopic colitis. Since the initial description of lymphocytic colitis in 1989, there have been several atypical forms of microscopic colitis described[15], including a paucicellular variant[31]. In this variant, patients still have the same clinical symptoms, but the IEL count is less, with only 10-12 IELs/100 enterocytes cited[32]. We feel that, in these cases, immunostaining might be of more diagnostic value in determining a low IEL that is not so apparent by H and E staining. A recent study has challenged the notion of regarding paucicellular lymphocytic colitis as a variant of classical lymphocytic colitis, based on the demonstration of a distinct immunological difference[33]. This group also has indirectly claimed that immunostaining displays a clear contrast between immunoreactive lymphocytes and negative epithelial cells. However, the comparison between H and E staining and immunohistochemistry was not directly evaluated in their study[33]. Our study was not designed to identify cases of paucicellular lymphocytic colitis, but it is an area that requires further study.
As stated earlier, the prevalence of microscopic colitis is difficult to estimate from our study due to our selection criteria and referral bias. However, this study does indicate that microscopic colitis, especially the lymphocytic colitis subtype, is underdiagnosed at our institution (P < 0.05). For a true estimate of the prevalence of this disorder, further studies are needed, combining data from all referral centers in the region. Other factors not taken into account in this study are the site of the biopsy and drug history. It is well known that lymphocytic colitis and collagenous colitis can be patchy in distribution, and the topographic gradient of IELs decreases from the right colon to the rectum[34]. Therefore, representative biopsies should be taken from each part of the colon and submitted in a separate container. Concomitant drug use can cause or worsen drug-induced microscopic colitis. It is important to recognize these drugs because drug withdrawal may improve symptoms. Among the more common drugs implicated are non-steroidal anti-inflammatory drugs, lansoprazole, clozapine, ranitidine, ticlopidine, carbose and flutamide[32]. Future studies at our institution need to take these factors into account.
We identified that intraepithelial lymphocytosis may be an early manifestation of a disease other than microscopic colitis within our defined population. IEL count alone is not specific for microscopic colitis and the biopsy findings need to be correlated with clinical information for a more specific diagnosis. In cases in which there is a history of chronic watery diarrhea, the use of CD3 immunohistochemistry may be of additional value in making the diagnosis of lymphocytic colitis. We suggest that patients with diarrhea-predominant IBS should have a routine colonoscopy and be evaluated for microscopic colitis.
Microscopic colitis was previously considered a rare disorder, but it now accounts for approximately 10% of cases of chronic watery diarrhea. Cases are often under-recognized despite there being well-established histopathological criteria. It is suspected that colon mucosal biopsies are often under-reported as chronic inflammation, normal or colitis, not otherwise specified.
Intra-epithelial lymphocytes (IELs) are crucial to the histological diagnosis of the lymphocytic colitis subtype. Immunohistochemistry has been shown to be of value in the quantification of IELs in celiac disease, but not in the identification and quantification of IELs in lymphocytic colitis.
A recent randomized, double-blind, placebo-controlled study has confirmed that budesonide is effective in the treatment of lymphocytic colitis. It has therefore become increasingly important to recognize this condition. This is believed to be the first time that normal colon biopsies were retrospectively reviewed and evaluated for IELs. The authors demonstrated that a subset of patients with chronic diarrhea was identified as having lymphocytic colitis using this approach.
The value of this study demonstrates that immunohistochemistry is a useful adjunct to hematoxylin and eosin staining in the evaluation of IELs required for the diagnosis of lymphocytic colitis.
Microscopic colitis is an umbrella term that comprises lymphocytic and collagenous subtypes. Although the latter is distinguished histologically by a thickened membrane, the clinical symptoms and colonoscopy findings are identical.
This study illustrates well that patients with diarrhea-predominant irritable bowel syndrome should have a colon biopsy with close scrutiny of mucosal lymphocytes to exclude microscopic colitis.
Peer reviewer: Jackie Wood, PhD, Department of Physiology and Cell Biology, College of Medicine and Public Health, The Ohio State University, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, Ohio 43210-1218, United States
S- Editor Sun H L- Editor Kerr C E- Editor Ma WH
| 1. | Pardi DS, Smyrk TC, Tremaine WJ, Sandborn WJ. Microscopic colitis: a review. Am J Gastroenterol. 2002;97:794-802. |
| 2. | Tysk C, Bohr J, Nyhlin N, Wickbom A, Eriksson S. Diagnosis and management of microscopic colitis. World J Gastroenterol. 2008;14:7280-7288. |
| 3. | Bohr J, Tysk C, Eriksson S, Järnerot G. Collagenous colitis in Orebro, Sweden, an epidemiological study 1984-1993. Gut. 1995;37:394-397. |
| 4. | Fernández-Bañares F, Salas A, Forné M, Esteve M, Espinós J, Viver JM. Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Am J Gastroenterol. 1999;94:418-423. |
| 5. | Loftus EV. Microscopic colitis: epidemiology and treatment. Am J Gastroenterol. 2003;98:S31-S36. |
| 6. | Misra V, Misra SP, Dwivedi M, Singh PA, Agarwal V. Microscopic colitis in patients presenting with chronic diarrhea. Indian J Pathol Microbiol. 2010;53:15-19. |
| 7. | Erdem L, Yildirim S, Akbayir N, Yilmaz B, Yenice N, Gultekin OS, Peker O. Prevalence of microscopic colitis in patients with diarrhea of unknown etiology in Turkey. World J Gastroenterol. 2008;14:4319-4323. |
| 8. | Satarasinghe RL, Fernando HR, Jayamaha DH, Samarasinghe I, De Silva AP. Collagenous colitis in adult Sri Lankans: experience from the Indian subcontinent. Gut. 2006;55:436. |
| 9. | Otegbayo JA, Oluwasola AO, Akang EE. Collagenous colitis in an adult patient with chronic diarrhoea: case report. East Afr Med J. 2001;78:272-274. |
| 10. | Ekrikpo UE, Otegbayo JA, Oluwasola AO. Lymphocytic colitis presenting as difficult diarrhoea in an African woman: a case report and review of the literature. J Med Case Reports. 2010;4:31. |
| 11. | Otegbayo JA, Otegbeye FM, Rotimi O. Microscopic colitis syndrome--a review article. J Natl Med Assoc. 2005;97:678-682. |
| 12. | Tagkalidis P, Bhathal P, Gibson P. Microscopic colitis. J Gastroenterol Hepatol. 2002;17:236-248. |
| 13. | Olesen M, Eriksson S, Bohr J, Järnerot G, Tysk C. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993-1998. Gut. 2004;53:346-350. |
| 14. | Nielsen OH, Vainer B, Schaffalitzky de Muckadell OB. Microscopic colitis: a missed diagnosis? Lancet. 2004;364:2055-2057. |
| 15. | Chang F, Deere H, Vu C. Atypical forms of microscopic colitis: morphological features and review of the literature. Adv Anat Pathol. 2005;12:203-211. |
| 16. | Lazenby AJ, Yardley JH, Giardiello FM, Jessurun J, Bayless TM. Lymphocytic (“microscopic”) colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol. 1989;20:18-28. |
| 17. | Warren BF, Edwards CM, Travis SP. ‘Microscopic colitis’: classification and terminology. Histopathology. 2002;40:374-376. |
| 18. | Mino M, Lauwers GY. Role of lymphocytic immunophenotyping in the diagnosis of gluten-sensitive enteropathy with preserved villous architecture. Am J Surg Pathol. 2003;27:1237-1242. |
| 19. | Limsui D, Pardi DS, Smyrk TC, Abraham SC, Lewis JT, Sanderson SO, Kammer PP, Dierkhising RA, Zinsmeister AR. Observer variability in the histologic diagnosis of microscopic colitis. Inflamm Bowel Dis. 2009;15:35-38. |
| 20. | Pardi DS. Microscopic colitis: an update. Inflamm Bowel Dis. 2004;10:860-870. |
| 21. | Jaskiewicz K, Rzepko R, Adrych K, Smoczyński M. Microscopic colitis in routine colonoscopies. Dig Dis Sci. 2006;51:241-244. |
| 22. | Lazenby AJ. Collagenous and lymphocytic colitis. Semin Diagn Pathol. 2005;22:295-300. |
| 23. | Tangri V, Chande N. Microscopic colitis: an update. J Clin Gastroenterol. 2009;43:293-296. |
| 24. | Miehlke S, Madisch A, Bethke B, Morgner A, Kuhlisch E, Henker C, Vogel G, Andersen M, Meier E, Baretton G. Oral budesonide for maintenance treatment of collagenous colitis: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2008;135:1510-1516. |
| 25. | Meining A, Schwendy S, Becker V, Schmid RM, Prinz C. In vivo histopathology of lymphocytic colitis. Gastrointest Endosc. 2007;66:398-399, discussion 400. |
| 26. | Miehlke S, Madisch A, Karimi D, Wonschik S, Kuhlisch E, Beckmann R, Morgner A, Mueller R, Greinwald R, Seitz G. Budesonide is effective in treating lymphocytic colitis: a randomized double-blind placebo-controlled study. Gastroenterology. 2009;136:2092-2100. |
| 27. | Bonderup OK, Hansen JB, Teglbjaerg PS, Christensen LA, Fallingborg JF. Long-term budesonide treatment of collagenous colitis: a randomised, double-blind, placebo-controlled trial. Gut. 2009;58:68-72. |
| 28. | Fenoglio-Preiser CM, Noffsinger AE, Stemmerman GN, Lantz PE, Isaacson PG. Gastrointestinal pathology: An atlas and text. 3rd ed. Philadelphia: Wolters Kluwer, Lippincott Williams 2008; 847. |
| 29. | Limsui D, Pardi DS, Camilleri M, Loftus EV Jr, Kammer PP, Tremaine WJ, Sandborn WJ. Symptomatic overlap between irritable bowel syndrome and microscopic colitis. Inflamm Bowel Dis. 2007;13:175-181. |
| 30. | Madisch A, Bethke B, Stolte M, Miehlke S. Is there an association of microscopic colitis and irritable bowel syndrome--a subgroup analysis of placebo-controlled trials. World J Gastroenterol. 2005;11:6409. |
| 31. | Goldstein NS, Bhanot P. Paucicellular and asymptomatic lymphocytic colitis: expanding the clinicopathologic spectrum of lymphocytic colitis. Am J Clin Pathol. 2004;122:405-411. |
| 32. | Carmack SW, Lash RH, Gulizia JM, Genta RM. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Adv Anat Pathol. 2009;16:290-306. |
| 33. | Fernández-Bañares F, Casalots J, Salas A, Esteve M, Rosinach M, Forné M, Loras C, Santaolalla R, Espinós J, Viver JM. Paucicellular lymphocytic colitis: is it a minor form of lymphocytic colitis? A clinical pathological and immunological study. Am J Gastroenterol. 2009;104:1189-1198. |
| 34. | Kirby JA, Bone M, Robertson H, Hudson M, Jones DE. The number of intraepithelial T cells decreases from ascending colon to rectum. J Clin Pathol. 2003;56:158. |