Published online Apr 21, 2011. doi: 10.3748/wjg.v17.i15.1947
Revised: October 19, 2010
Accepted: October 26, 2010
Published online: April 21, 2011
AIM: To establish stable tetracycline-inducible pancreatic cancer cell lines.
METHODS: Suit-2, MiaPaca-2, and Panc-1 cells were transfected with a second generation reverse tetracycline-controlled transactivator protein (rtTA2S-M2), under the control of either a cytomegalovirus (CMV) or a chicken β-actin promoter, and the resulting clones were characterised.
RESULTS: Use of the chicken (β-actin) promoter proved superior for both the production and maintenance of doxycycline-inducible cell lines. The system proved versatile, enabling transient inducible expression of a variety of genes, including GST-P, CYP2E1, S100A6, and the actin capping protein, CapG. To determine the physiological utility of this system in pancreatic cancer cells, stable inducible CapG expressors were established. Overexpressed CapG was localised to the cytoplasm and the nuclear membrane, but was not observed in the nucleus. High CapG levels were associated with enhanced motility, but not with changes to the cell cycle, or cellular proliferation. In CapG-overexpressing cells, the levels and phosphorylation status of other actin-moduating proteins (Cofilin and Ezrin/Radixin) were not altered. However, preliminary analyses suggest that the levels of other cellular proteins, such as ornithine aminotransferase and enolase, are altered upon CapG induction.
CONCLUSION: We have generated pancreatic-cancer derived cell lines in which gene expression is fully controllable.
- Citation: Tonack S, Patel S, Jalali M, Nedjadi T, Jenkins RE, Goldring C, Neoptolemos J, Costello E. Tetracycline-inducible protein expression in pancreatic cancer cells: Effects of CapG overexpression. World J Gastroenterol 2011; 17(15): 1947-1960
- URL: https://www.wjgnet.com/1007-9327/full/v17/i15/1947.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i15.1947
The ability to precisely control the levels of specific proteins in cells, either positively through overexpression, or negatively through siRNA-mediated depletion, provides an invaluable opportunity to examine their function. The most widely used technique for stringently controlling gene expression levels is the tetracycline-regulated system, originally proposed by Gossen and Bujard[1]. Using this approach, cells are transfected with a plasmid encoding a tetracycline-controlled transactivator protein (tTA), and subsequently transfected with a plasmid carrying a gene of interest that has a tetracycline response element (TRE) in its 5’ regulatory region. The addition of a tetracycline derivative, doxycycline (dox) to the cell culture medium prevents the tTA from binding to the TRE, thus inactivating the system (tet-off).
The tet system has undergone several improvements since it was first introduced. One improvement is the creation of a reverse tTA (rtTA), which can bind to the TRE in the presence of dox (Tet-on), obviating the need for the continuous presence of dox in the cell culture medium[2-4]. Further improvements came with the introduction of the second generation rtTA, termed rtTA2s-M2[5], which enhanced the transactivation potential and reduced the affinity for the TRE in the absence of dox. This overcame the problem of leakiness; low level gene expression in the absence of dox, sometimes observed with the earlier generation tTA[5]. The system continues to be modified for new applications in cell lines[6,7] and in animals[8].
For certain cell types, such as pancreatic cancer cells, there are remarkably few reports of dox-inducible cells[9,10]. This reflects the difficulty in obtaining such cell lines, and efficient methods for generating them are required. In this study, we attempted to derive dox-inducible pancreatic cancer cell lines, using vectors expressing rtTA2S-M2 under the control of either a viral (CMV) promoter[5] or a chicken (β-actin) promoter[11]. Using the resulting tet-inducible system, we went on to generate pancreatic cancer cells stably overexpressing CapG, an actin capping protein, which was previously shown to be upregulated in pancreatic ductal adenocarcinoma[12]. CapG is activated by calcium and caps actin filaments, a function that is inhibited by membrane polyphosphoinositides[13]. Depletion of CapG from a variety of pancreatic cancer cell lines has been associated with decreased cell motility and wound healing capacity[12]. The dox-inducible overexpression of CapG enabled further characterisation of its role in pancreatic cancer cells, and exemplified the utility of this model system in studying the role of specific genes in pancreatic cancer cells.
Two Tet-on plasmids were used in this study. The rtTA2S-M2[5], which contains a viral CMV promoter driving the tet-transactivator rtTA2S-M2 (denoted here as CMV-rtTA2S-M2), was a kind gift from Professor W Hillen, University of Heidelberg. The pN1βactin-rtTA2S-M2-IRES-EGFP[11], in which the CMV promoter has been replaced with a strong chicken β-actin promoter, was a kind gift from Dr. A Welman, Paterson Institute for Cancer Research, Manchester. The full-length CapG coding sequence was amplified using a forward primer 5′-AGAACGCGTCAGCATGTACACAGCCAATTC-3′, which includes an MluI restriction site, highlighted in bold, and a reverse 5′-AGAGCGGCCGCCACCCTCATTTCCAGTCCT-3′ containing a NotI restriction site, in bold. The full size CapG amplicon was inserted directionally into the pTRE2hyg vector (Clontech, Saint-Germain-en-Laye, France) using the MluI and NotI restriction sites. The full-length S100A6 sequence was amplified using the following forward 5′-TCAGCCCTTGAGGGCTTCAT-3′ and reverse 5′-ATGGCATGCCCCCTGGATCA-3′ primers. The amplicon was ligated into the EcoRV restricted pTRE2hyg vector. Vector inserts were verified by sequencing (Eurofins MWG Operon, London, UK) and aligned using the Basic Local Alignment Search Tool (Blast). PTRE2hygGST-P, containing the full length coding sequence of human glutathione S-transferase P (GST-P), and pTRE2hygCYP2E1, containing the full-length coding sequence of cytochrome P-450 2E1, were described previously[14].
Pancreatic cancer cells, Suit-2, Panc-1, and MiaPaca-2 cells were maintained in RPMI-1640 medium supplemented with 10% foetal bovine serum, 10 mmol/L L-glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin (Sigma-Aldrich, Gillingham, UK). Growth media for cell lines stably expressing the rtTA protein was supplemented with 50 μg/mL G418 sulphate (Invitrogen, Paisley, UK). The media for the transgenic cell lines stably transfected with the inducible CapG was supplemented with 100 μg/mL hygromycin B (Sigma-Aldrich, Gillingham, UK). Cells were maintained at 37°Cand 5% CO2. For the induction of CapG protein expression in clones harbouring stable inducible CapG, cells were cultured for 24 h with antibiotic-free medium, and for an additional 24 h with or without 500 ng/mL dox (Sigma-Aldrich, Gillingham, UK) in RPMI-1640 media, supplemented with 10 % FBS and L-Glutamine.
Cells were transfected using Lipofectamine 2000 (Invitrogen, Paisley, UK) at a 1:3 DNA: lipofectamine ratio, following the manufacturer’s protocol. Briefly, cells were plated at a density of 3 × 106 per 10 cm2 dish and grown for 24 h, placed in serum- and antibiotic-free medium and transfected with 5 μg of CMV-rtTA2S-M2 or 20 μg pN1βactin-rtTA2S-M2-IRES-EGFP or with pTRE2hygCapG (5 μg or 20 μg). Cells were selected for integration of rtTA2S-M2 coding sequences by treating with G418 (300 μg/mL; Invitrogen, Paisley, UK) for two weeks. Single colonies were separated into 96-well plates, and positive clones identified using a luciferase assay (described below), expanded, and cryopreserved. Stable CapG-inducible cell populations were selected by incubation with hygromycin B (200 μg/mL). After two weeks, single colonies were separated into 96-well plates, cultured until confluence, and subsequently transferred to 24- and 6-well plates, respectively. Tet-on CapG-overexpressing clones were identified by Western Blotting for CapG, using dox as an inducer (see below). The transient transfection of the tetracycline responsive vectors pTRE2hygGST-P, pTRE2hygCYP2E1, and pTRE2hygS100A6 was undertaken as described above, and 24 h later expression was induced using 500 ng/mL dox in PBS. PBS alone was used as a vehicle control. Twenty-four hours following doxycycline induction, cells were lysed, and evidence of induction of protein expression was assessed using Western blotting, as described below.
To test CMV-rtTA2S-M2 or pN1βactin-rtTA2S-M2-IRES-EGFP-transfected clones for dox inducibility, cells were seeded into 96-well plates and transfected with 50 ng pTRE2hygluc vector (Clontech, Saint-Germain-en-Laye, France) using a 1:3 DNA: Lipofectamine ratio. Twenty-four hours later, clones were treated with 500 ng/mL dox in PBS or with PBS only as a control and incubated for 24 h. Cells were harvested in 50 μL 1 × Glo Lysis buffer (Promega, Southampton, UK) and assayed for luciferase activity according to the manufacturer’s instructions, using a plate reader (PerkinElmer, Bucks, UK). Each clone was assayed in triplicate and experiments were repeated at least three times. Data were analysed statistically using Student’s two-tailed paired t-test.
Cells were lysed in SDS lysis buffer (0.1 mol/L Tris/HCl pH 6.8, 2% SDS) containing protease inhibitor (Roche Diagnostics Ltd., Burgess Hill, UK). For separation into cytoplasmic and nuclear fractionations, the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Rockford, IL, USA) were used. For analysis of phospho-proteins, cells were lysed in RIPA buffer, supplemented with protease and phosphatase inhibitor (Roche Diagnostics Ltd., Burgess Hill, UK). Protein concentrations were determined using a Bradford assay (Bio-Rad, Hemel Hempstead, UK). For Western blotting, membranes were blocked with 5% milk (Bio-Rad, Hemel Hempstead, UK) in PBS containing 0.1% Tween 20 (PBST) for 1 h at RT. As primary antibodies, polyclonal chicken anti-CapG (1:7000, GenWay Biotech, San Diego, USA), monoclonal mouse anti-β-actin (1:200 000, Sigma-Aldrich, Gillingham, UK), rabbit anti-GAPDH (1:400, Santa Cruz Biotechnology, Heidelberg, Germany), monoclonal mouse anti α-tubulin (1:3000; Sigma Aldrich, Gillingham, UK), monoclonal mouse anti-GST-P (1:2500; Sigma Aldrich, Gillingham, UK), rabbit anti-CYP2E1 (1:2500; Sigma Aldrich, Gillingham, UK) and rabbit anti-S100A6 polyclonal antibody (1:3000; DAKO UK Ltd., Ely, UK) were used. The primary antibodies were incubated overnight at 4°C with gentle shaking. As secondary antibodies, goat anti rabbit HRP (1:3000, Dako UK Ltd., Ely, UK), goat anti mouse HRP (1:3000, Dako UK Ltd., Ely, UK), or goat anti chicken HRP (1:14 000, GenWay Biotech, San Diego, USA) were used. For Cofilin and Ezrin the Actin-Reorganization Kit (Cell Signalling Technology Inc., Danvers, Maryland, USA) was used according to the manufacturer’s protocol. Bound horse radish peroxidase was detected using enhanced chemiluminescence western lightning reagent (Perkin-Elmer Life Sciences, Waltham, USA). For quantification purposes, X-ray films were scanned using a GS-800- densitometry scanner (Bio-Rad, Hemel Hempstead, UK) and evaluated using the Quantity One software (Bio-Rad, Hemel Hempstead, UK), or directly developed using a Kodak Gel Logic 1500 imaging station and analysed using Kodak Molecular Imaging software (Carestream Health UK, Hemel Hempstead, UK).
The dox-inducible CapG-expressing clones, Sβtet29Cap35, Sβtet29Cap43, and negative control Sβtet29 were incubated for 18 h with or without 500 ng/mL dox and solubilised in lysis buffer (7 mol/L urea, 2 mol/L thiourea, 4% 3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulphonate, 40 mmol/L Tris base and 1% dithiothreitol) by sonication. Extracted proteins (200 μg) were focused on pH 3-10 non-linear strips, 18 cm in length, as described[15], and then separated on 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis gels. Gels were stained with colloidal Coomassie Blue. Gel images were scanned using a GS-800 scanner, acquired using PDQuest software (Bio-Rad, Hemel Hempstead, Herts, UK) and analysed using Progenesis Workstation software V2002.1 (NonLinear Dynamics, Newcastle, UK).
The proliferation of Sβtet29Cap35, Sβtet29Cap43 and control Sβtet29 clones was determined by MTS assay (EZ4U nonradioactive cell proliferation and cytotoxicity assay, Fa. Biomedica, Vienna, Austria) following the manufacturer’s protocol. This assay is based on the reduction of the nontoxic yellow tetrazolium salt (450 nm) to an intensively coloured red formazan derivate (620 nm). Reduction requires functional mitochondria, an indicator of cell viability and cell proliferation. Cells (n = 3000) were plated in 100 μL antibiotic free RPMI medium onto 96-well plates. Dox was added at a final concentration of 0 or 500 ng/mL. After 48 h, 100 μL substrate was added to each well, and cells incubated before absorbance readings were measured at 0, 1, 2, 3, and 4 h at 450 and 620 nm in an Multiskan EX plate reader (Thermo Scientific, Basingstoke, UK). Within each experiment, each condition was plated in twelve replicates and experiments were repeated on three independent occasions. Data were analysed statistically using Student’s two-tailed paired t-test.
For cell cycle analysis, cells were plated in triplicate 12 h prior to treatment with or without 500 ng/mL doxycycline for an additional 24 h or 72 h. Cells were harvested, washed twice with ice-cold PBS, and fixed with ice-cold 70% ethanol for 1 h. Cells were centrifuged at 1000 r/min for 4 min, and cell pellets washed twice with PBST. Cell pellets were resuspended in PBS containing 100 μL RNAse A (10 mg/mL) and incubated for 5 min at 4°C. Cells were then stained with 900 μL of a PBS solution containing propidium iodide (55 μg/mL) and 10 000 cells analysed by flow cytometry.
Sβtet29Cap35, Sβtet29Cap43, and, as the control, Sβtet29 cells were plated into 12-well plates at 5 × 106 cells per well and incubated for 24 h with or without 500 ng/mL doxycycline. Wounds were made using a P200 plastic pipette tip and photographed (t = 0 h) before being returned to 37°C. Wounds were photographed again eight hours later. The distance migrated by the cell monolayer was measured and the results expressed as a migration index (i.e. the distance migrated by the experimental condition, e.g. dox stimulated CapG inducible cells, divided by the distance migrated by the control treated cells, e.g. dox stimulated control cells). Experiments were performed in triplicate and repeated on three independent occasions. Statistical significance between the different conditions was determined using Student’s paired t-test, with significance set at P≤ 0.05.
Motility assays were performed using 24-well cell culture inserts (modified Boyden chambers, BD Biosciences, Oxford, UK) with 8 μm pore size. CapG-inducible Sβtet29Cap35, Sβtet29Cap43, and, as the control, Sβtet29 cells were plated in T25 flasks in RPMI media supplemented with 10% FBS and incubated with or without 500 ng/mL dox for 24 h. Cells were counted and 5 × 104 cells were plated into 24-well Boyden chamber inserts in 500 μL serum-free RPMI medium. The medium in the lower chamber was supplemented with 1% FBS. For cells undergoing dox induction, treatment was continued by supplementing both upper and lower chambers with 500 ng/mL dox. The Boyden chambers were incubated at 37°C, 5% CO2 for 18 h. Cells that had migrated to the lower side of the transwell insert were fixed and stained using Diff-Quik (Siemens Healthcare Diagnostics, Deerfield, USA) and counted on a Leica CME microscope (Leica, Microsystems UK, Milton Keynes, UK) at × 40 total magnification. All experimental conditions were performed in triplicate with three independent repeats. Experiments were statistically analyzed using paired Student’s t-test (StatView, version 5.0.1, Adept Scientific Plc., Letchworth, UK).
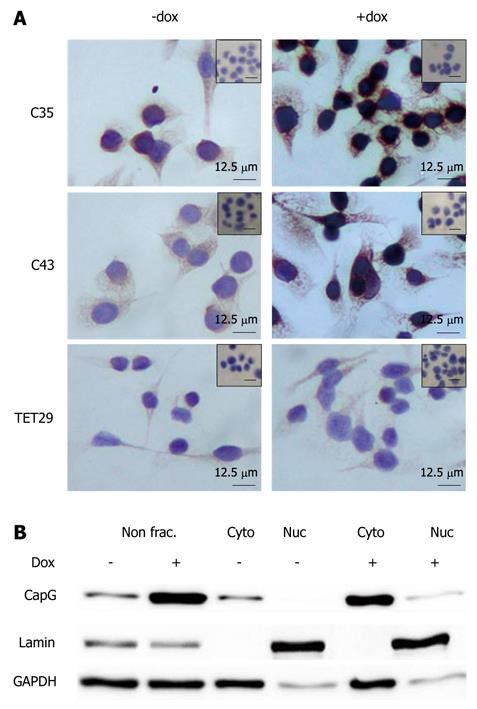
For immunohistochemistry (IHC), cells were grown on glass chamber slides (LabTek II, Nunc GmbH, Langenselbold, Germany) overnight at 37°C and cultured for an additional 24 h with or without 500 ng/mL dox. Cells were fixed using acetone which had been equilibrated at -20°C for 10 min and washed with PBST. Cells were permeabilized by incubation with 0.1% Triton X-100 in PBS for 10 min at RT. Peroxidase was blocked by a 3% H2O2/methanol treatment for 30 min in the dark. Slides were washed with PBST and unspecific binding blocked by incubation in 1:10 diluted goat serum in PBST for 30 min at RT. The primary antibody, chicken anti CapG antibody (1:1000; GenWay Biotech, San Diego, USA), was incubated in 1% BSA/PBST overnight at 4°C in a humidified chamber. Slides were washed with PBST and incubated with the secondary anti-chicken HRP conjugated (1:1000; GenWay Biotech, San Diego, USA) for 1 h at room temperature in 3% BSA/PBS. The staining was developed with DAB and counterstained with hematoxylin.
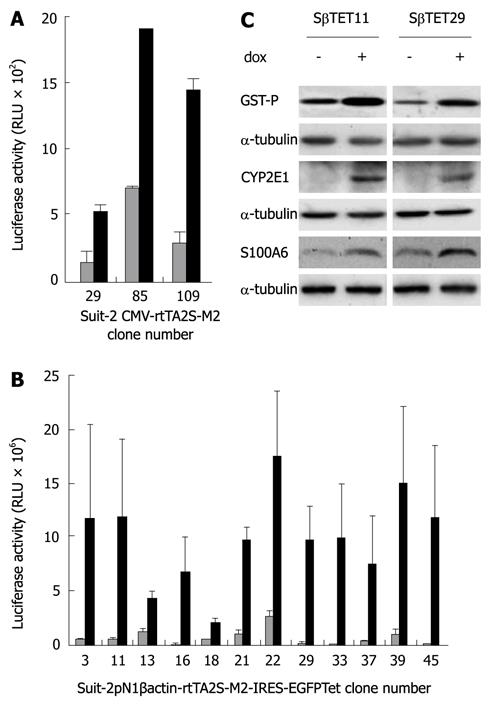
Pancreatic cancer cells, Suit-2, Panc-1 and MiaPaca-2 were transfected with plasmids CMV-rtTA2S-M2 and pN1βactin-rtTA2S-M2-IRES-EGFP, in which rtTA2S-M2 expression is under the control of the CMV and β-actin promoters respectively. Cells were selected for resistance to G418. Emergent clones were tested for dox inducibility by transient transfection with a luciferase expression plasmid under the control of a TRE (pTRE2hygluc). Elevated luciferase activity in the presence of dox when compared to the untreated (-dox) control was taken as evidence of expression of the rtTA protein. Of the Suit-2 cells transfected with CMV-rtTA2S-M2, 167 G418-resistant clones were screened, although only three of these clones (29, 85 and 109) showed evidence of statistically significant dox-inducible luciferase activity (Figure 1A, P < 0.05, Student’s paired t test). Of the Suit-2 cells transfected with pN1βactin-rtTA2S-M2-IRES-EGFP, 49 G418-resistant Suit-2 clones were screened. Nineteen of these clones showed a greater than five-fold dox-inducibility of luciferase activity compared to the untreated (-dox) control. The luciferase measurement for twelve representative clones is shown in Figure 1B. The induction of luciferase was significantly increased in all clones shown (P < 0.05, Student’s paired t test) except for clone 18 (P < 0.09).
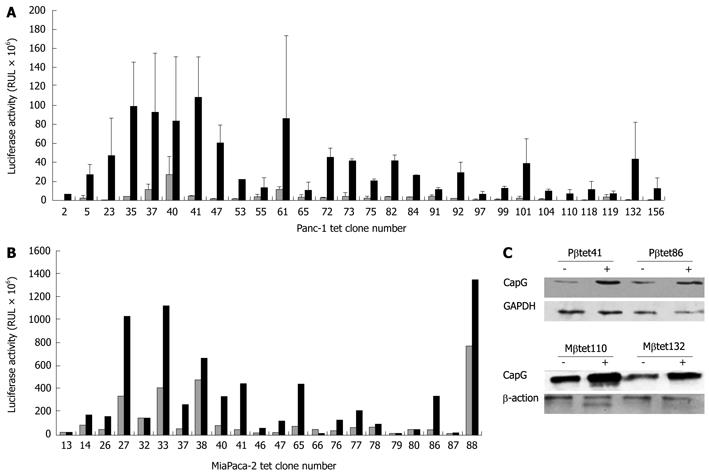
Stably-transfected Panc-1 (Figure 2A) and MiaPaca-2 (Figure 2B) cell clones harbouring the pN1βactin-rtTA2S-M2-IRES-EGFP Tet-on plasmid were also identified. Thirteen G418-resistant CMV-rtTA2S-M2 Panc-1 clones emerged; however, none showed evidence of dox-inducible luciferase activity. No G418-resistant CMV-rtTA2S-M2 MiaPaca 2 clones were obtained despite four transfection and selection attempts.
We next wished to determine the versatility of stable dox-inducible pancreatic cells for the expression of a variety of heterologous genes. To do this, two pN1βactin-rtTA2S-M2-IRES-EGFP-derived Suit-2 clones (Sβtet11 and Sβtet29), which showed good inducibility, were transiently transfected with plasmids containing the genes for glutathione S transferase P (GST-P), cytochrome P450 2E1 (CYP2E1), and S100A6, each under the control of a TRE. These genes were chosen because of our previous experience of their inducible expression in hepatoma cells (GST-P, CYP2E1)[14] or because of our interest in them as genes that are overexpressed in pancreatic cancer (S100A6 and CapG)[12,16,17]. Their inducibility was measured using dox. Western blotting (Figure 1C) revealed that dox treatment of the transiently transfected Sβtet11 and Sβtet29 clones resulted in induction of GST-P, CYP2E1, and S100A6. Two inducible pN1βactin-rtTA2S-M2-IRES-EGFP-derived Panc-1 clones and two pN1βactin-rtTA2S-M2-IRES-EGFP-derived MiaPaca-2 clones were also evaluated by transient transfection of a plasmid carrying a pTRE-dependent CapG gene, and induction of expression with dox. Both Panc-1 and MiaPaca-2 clones showed increases in the CapG protein following dox treatment (Figure 2C).
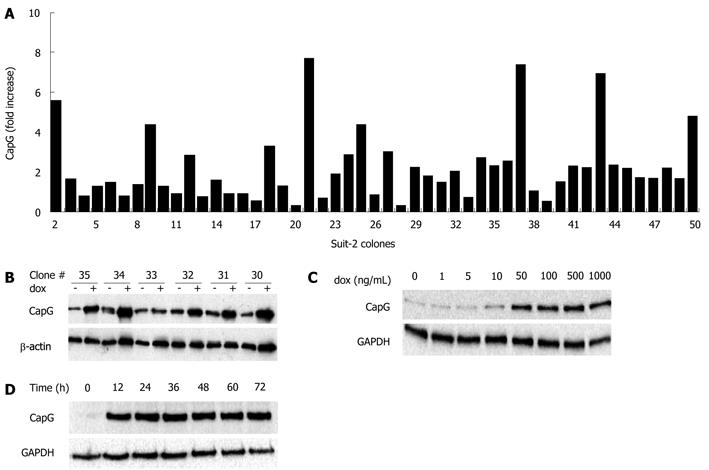
Having observed good transient inducible gene expression, we wished to determine whether stable inducible CapG overexpressing cells could be derived. Transgenic cells stably expressing the inducible form of CapG (pTRE2hygCapG) were constructed using Suit-2 pN1βactin-rtTA2S-M2-IRES-EGFP derived Tet-on cell clones (Sβtet11 and Sβtet29). Hygromycin B-resistant clones (n = 49) were screened for plasmid integration by treating with 500 ng/mL dox for 24 h and measuring CapG protein overexpression by Western blotting. The fold increase in CapG relative to expression of actin is shown in Figure 3A. Six CapG clones (clones 30-35) are shown on a representative Western blot presenting the dox-dependent CapG inducible expression (Figure 3B). From the 49 clones analysed, 20 (40%) showed no change in CapG expression, whereas 22 (44%) showed a 1.5 to 3 fold increase in CapG expression following dox treatment. For eight clones (16%), a greater than three-fold increase in CapG protein level was observed. For a proportion of clones (38%), actin levels appeared to be reproducibly induced following dox treatment/CapG induction. Since CapG is an actin-capping protein, it was decided that subsequent experiments would use alternative internal control proteins, such as tubulin or GAPDH.
The time and dose-dependent inducibility of CapG protein expression was investigated. Concentration dependency was examined by inducing CapG overexpression with different concentrations of dox (0-1000 ng/mL) for 36 h (Figure 3C). CapG expression was not induced at low concentrations of dox (1-10 ng/mL), but was induced at concentrations of dox above 50 ng/mL. There was no concentration-dependent increase in the amount of CapG protein induced. For the time course study (Figure 3D) and further experiments, a concentration of 500 ng/mL dox was chosen. An increase in CapG protein level was observed after 12 h of dox treatment and was stable for at least a further 60 h (from 12 to 72 h) (Figure 3D).
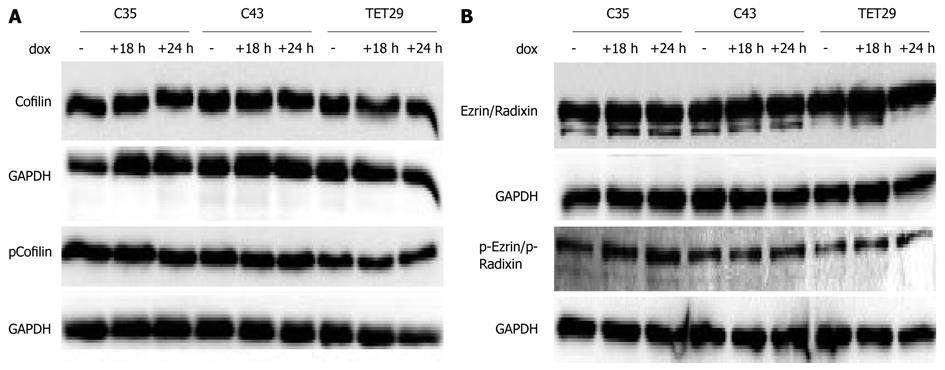
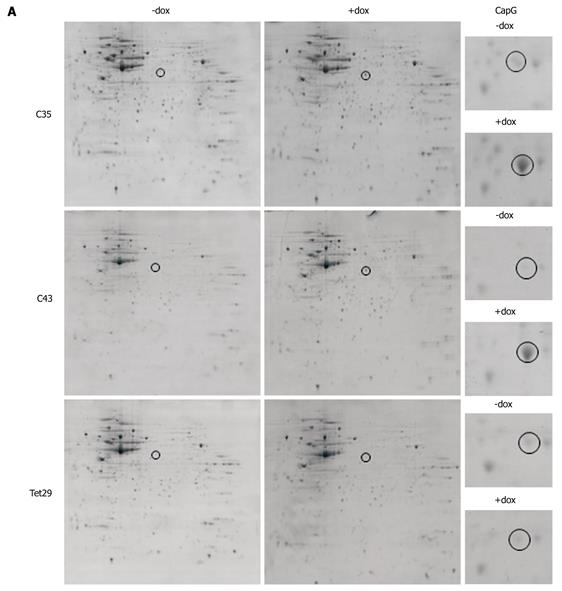
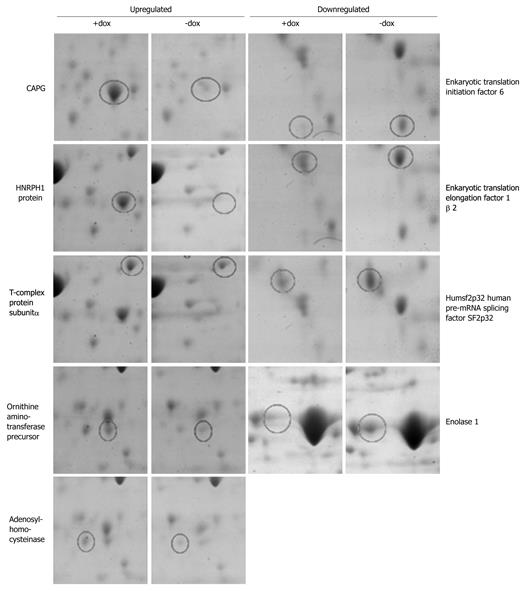
We next sought to determine whether artificially increasing the levels of CapG would alter the levels or activation status of other actin-modulating proteins, such as Cofilin and Ezrin/Radixin. We found that neither protein was changed in level or in phosphorylation status when CapG levels were increased (Figure 4A and B). This led us to ask a broader question of whether elevated CapG affected the expression of any cellular proteins. CapG overexpression was analysed by 2D-gel electrophoresis in two stable CapG inducible clones (Sβtet29Cap35 and Sβtet29Cap43), and, as the control, the rtTA protein expressing clone from which those two clones were derived (TetSβtet29). An increase in the intensity of a 38 kD protein (Figure 5A) was observed on 2-D gels following dox treatment of Sβtet29Cap35 and Sβtet29Cap43, but in not the control TetSβtet29, indicating that treatment with doxycycline alone is not sufficient to induce the expression of CapG, i.e. the cells must harbour a doxycycline-inducible CapG construct. Matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) confirmed the identity of this protein as CapG (Figure 5B). This finding is consistent with our previously obtained Western blot data (Figure 3). A number of other proteins also differentially expressed in cells induced to express high levels of CapG were also identified by MALDI-MS (Figure 6). HNRPH1, T-complex protein 1 subunit alpha, Adenosylhomocysteinase, and Ornithine aminotransferase were upregulated in CapG overexpressing cells. Conversely, Enolase, P27BBP protein, eukaryotic translation elongation factor 1 beta 2, and human pre-mRNA splicing factor SF2p32 were downregulated in CapG overexpressing cells. As none of these proteins are associated with the cytoskeleton, they were not validated further.
The localisation of CapG in the inducible clones Sβtet29Cap35, Sβtet29Cap43 and the control clone Sβtet29 was examined by immunohistochemistry. In untreated (-dox) cells, CapG was expressed homogeneously in the cytoplasm (Figure 7A), and nuclei were devoid of staining. After treatment of cells with doxycyline for 24 h, the intensity of CapG staining (brown colour) increased in the cytoplasm and around the nuclear membrane of Sβtet29Cap35 and Sβtet29Cap43 cells, suggesting CapG overexpression in the cytoplasmic compartment of these cells. As expected, the control clone Sβtet29 did not show any change in staining intensity upon treatment with dox, and no staining was observed in the nucleus. The relative levels of CapG in the cytoplasm and nucleus were quantitatively evaluated. The nuclear and cytoplasmic fractions of Sβtet29Cap35 cells were enriched and Western blotting for CapG performed. The levels of CapG in non-fractionated extracts in the non-induced and induced state are shown Figure 7B, lanes 1 and 2 respectively. Lanes 3, 4 and 5, 6 show nuclear and cytoplasmic CapG levels in the uninduced and induced states respectively. The enrichment of Lamin A is used as a marker of the nuclear fraction and GAPDH as a marker of the cytoplasmic fraction. A low level contamination of the nuclear fraction by the cytoplasmic fraction was observed. CapG protein was found exclusively in the cytoplasmic fraction in the non-induced state (lane 3). Following 24 h treatment with dox, the level of CapG was greatly increased in the cytoplasmic fraction (lane 5), with a very small proportion observed in the nuclear fraction (lane 6). The low level of CapG observed in the nuclear fraction may be due to contamination from the cytoplasmic fraction.
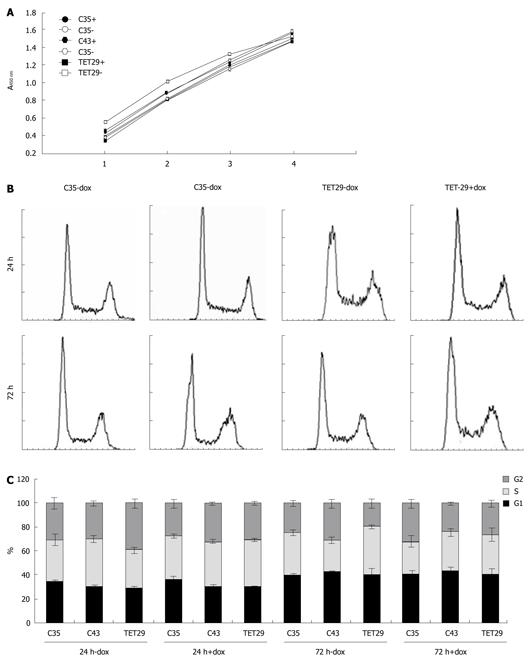
The viability of Sβtet29Cap35, Sβtet29Cap43, and Sβtet29 cells was measured 48 h after plating with or without 500 ng/mL dox. The MTS solution was added (t = 48 h) and the absorbance measured at 0, 1, 2, 3, and 4 h later. The kinetics for the turnover of the MTS solution was similar in all cell lines, as can be seen by the slope of the lines (0.3577 ± 0.031, Figure 8A). This indicates that 500 ng/mL dox was not toxic in any of the cell clones, including those in which dox caused induction of CapG (Sβtet29Cap35, Sβtet29Cap43).
Cell cycle analysis at 24 and 72 h after dox treatment did not result in any significant change in cell cycle profile. A representative figure showing the cell cycle profile of Sβtet29Cap35 (C35) and the parental cell line Sβtet29 (TET29) cultured for 24 or 72 h with or without 500 ng/mL dox is presented (Figure 8B). The percentage of cells in G1, G2 and S-Phase for all three experiments is summarized in Figure 8C and Table 1. There was a considerable change in cell cycle phases between the two time points (24 and 72 h), as cells are cultured for 48 h (24 h ± dox) or 96 h (72 h ± dox). There was no difference in the cell cycle phases between the groups cultured with or without dox. This shows that neither dox nor CapG overexpression affected the cell cycle of Suit-2 cells.
| - doxycycline | + 500 ng/mL doxycycline | |||||
| G1-phase | G2-phase | S-phase | G1-phase | G2-phase | S-phase | |
| 24 h | ||||||
| C35 | 34.8 ± 1.2 | 30.6 ± 5.2 | 34.7 ± 5.0 | 36.2 ± 3.2 | 27.3 ± 3.6 | 36.6 ± 1.1 |
| C43 | 30.8 ± 0.9 | 29.8 ± 2.5 | 39.4 ± 3.1 | 30.7 ± 1.6 | 31.7 ± 0.9 | 37.6 ± 1.8 |
| TET29 | 29.0 ± 1.7 | 38.9 ± 3.5 | 32.1 ± 2.4 | 30.2 ± 0.7 | 30.2 ± 1.5 | 39.6 ± 0.9 |
| 72 h | ||||||
| C35 | 39.8 ± 1.1 | 24.7 ± 2.4 | 35.5 ± 2.8 | 41.1 ± 3.1 | 31.8 ± 3.6 | 27.1 ± 5.0 |
| C43 | 42.6 ± 0.8 | 30.3 ± 3.5 | 27.1 ± 2.8 | 43.0 ± 4.1 | 24.0 ± 1.8 | 33.0 ± 3.0 |
| TET29 | 40.4 ± 5.4 | 19.4 ± 3.7 | 40.2 ± 1.9 | 41.6 ± 4.3 | 25.9 ± 3.1 | 32.5 ± 5.6 |
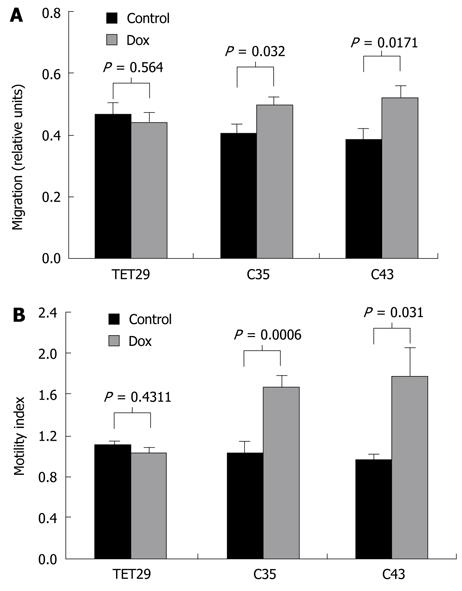
To investigate the effect of CapG overexpression on cell motility, wound healing and translocation assays were performed. When CapG was induced following dox treatment, we observed a significant increase in the distance travelled by the Sβtet29Cap35 and Sβtet29Cap43 cell clones. The distance travelled by Sβtet29Cap35 increased by 23% ± 5% and for Sβtet29Cap43 by 35% ± 7%. When treated with dox, the control clone Sβtet29 did not show any change in wound healing capacity (Figure 9A).
In Boyden chamber translocation assays, we observed a significant increase in cell migration by the Sβtet29Cap35 and Sβtet29Cap43 cell clones when CapG was induced following dox treatment (Figure 9B). The capability of Sβtet29Cap35 to cross through the membrane pores increased by 62% ± 10% and for Sβtet29Cap43 cells by 84% ± 16%. The control clone Sβtet29 did not show any change in cell migration capacity when treated with dox (Figure 9B).
In this study, we have described the generation and use of a tetracycline-inducible expression system in pancreatic cancer cell lines. Our use of both a CMV and a β-actin promoter-driven second generation reverse tetracycline transactivator protein (the rtTA2S-M2) led us to conclude that the latter was superior in terms of both production and maintenance of dox-inducible cell lines. Although we did not formally investigate mechanisms that might explain this, it is possible that the viral CMV promoter is silenced through methylation, whilst the chicken β-actin promoter remains active[6].
Recently, Zhang et al[10], successfully used a commercially available Tet-on system with a CMV-driven tetracycline transactivator protein, in Panc-1 cells. The authors reported dox-induced overexpression of the zinc finger transcription factor INSM1, although the fold increase in INSM1 protein level, as shown by Western blotting, was modest compared to the fold increases observed in the current study. This may reflect differences in the proteins under investigation in the respective studies, although it could be due to enhanced inducibility when using the β actin-rtTA2S-M2-IRES-EGFP vector.
The pancreatic cancer cell lines Suit-2, MiaPaca-2 and Panc-1 were all capable of stably harbouring the pN1βactin-rtTA2S-M2-IRES-EGFP vector. Moreover, inducible gene overexpression was observed for GST-P, CYP2E1, and S100A6 in Suit-2 and for CapG in all three investigated cell lines. This indicates that the system functions for a variety of different proteins. Equally, the method was efficient with 80% of pN1βactin-rtTA2S-M2-IRES-EGFP transfected Suit-2 clones and 60% of MiaPaca-2 clones showing greater than five-fold luciferase induction. Only Panc-1 cells showed relatively low efficiency, with only 14.4% of the selected cell clones showing a greater than five-fold luciferase increase. A clear advantage of the Tet-on system is the time and dose-dependent activation of expression of the gene of interest. Investigation of stable CapG overexpressing Suit-2 cell clones showed that CapG protein expression was dox-inducible in a time dependent manner. The level of CapG increased from 12 h after induction, and was maintained up to 72 h after induction. A similar time-dependent induction of gene expression in the liver carcinoma cells, HepG2 has been described previously[14]. However, we did not observe a linear correlation between dox concentration and protein expression, as was reported in the HepG2 cells[14]. Instead, we detected little increase in CapG protein concentration between 0-10 ng/mL dox, and overexpression of CapG with concentrations of 50 ng/mL and greater (up to 1000 ng/mL). Possible reasons for these different results could be related to the particular protein overexpressed or the different promoter used to drive the rtTA2S-M2 in both studies. The HepG2 Tet-on clones harboured CMV driven rtTA2S-M2, unlike the pancreas clones in this study.
Concerns have been raised about the toxicity of dox, the agent used to activate gene expression in the Tet-on vector system[18]. The cell growth of PC-12 cells was reduced after 96h incubation with concentrations ranging from as low as 0.2 to 100 μg/mL. Growth perturbations were observed with WI-38 VA-13 cells, but only with concentrations of dox greater than 2 μg/mL[18]. It appears that the concentration of dox which mediates a toxic effect is cell-line-dependent. At the concentrations of dox used in this study, no loss of proliferation was observed 48 h after dox treatment. These data were supported by cell cycle analyses profiles, which showed no change in cell cycle following dox treatment for 24 h and 72 h.
The function of CapG in the cytoplasm is well described. CapG caps and therefore blocks rapidly growing actin filaments, promoting the elongation of shorter actin filaments. It thus contributes to cell motility[19] and membrane ruffling[20]. Increased levels of CapG protein have been described in several tumors, including pancreatic ductal adenocarcinoma[12,21] and glioblastomas[22]. Transient CapG knockdown experiments in pancreatic cancer cell lines Suit-2, MiaPaca-2, and Panc-1 cell lines led to a significant decrease in wound healing capacity and cell motility[12]. Furthermore, Van den Abbeele et al[23]
showed that downregulation of CapG in the breast cancer cells MDA-MB 231 and the prostate cancer cells PC-3 also decreased invasion and motility. Here, we show for the first time that overexpression of CapG in a pancreatic cancer cell line caused a significant increase in motility and a modest, but significant, increase in wound healing capacity. This is consistent with a previous report of an increase in invasion after CapG overexpression in Madin-Darby Canine Kidney Epithelial Cells MDCK cells[24]. Interestingly, it has been reported that active nuclear import of CapG is necessary for CapG to promote invasion[24]. Prevention of nuclear accumulation of CapG in MDCK cells abolished collagen invasion, whereas restoring the nuclear import also restored collagen invasion of these cells. We found no evidence for elevated nuclear levels of CapG following overexpression in Suit-2 derived cells. Immunohistochemical staining localized CapG to the cytoplasm in Suit-2 cells. Dox treatment of stable inducible CapG expressors led to an increase in cytoplasmic staining, with increased CapG around the nuclear membrane, but CapG did not accumulate in the nucleus. Subcellular fractionation further validated this finding, showing increased CapG protein levels in the cytoplasmic fraction after dox treatment. We observed increased motility, suggesting that elevated cytoplasmic CapG may be sufficient for increased cancer cell motility.
CapG has been shown to interact with microtubule-dependent organelles during the cell cycle[25], possibly mediating cross-talk between the actin cytoskeleton and microtubule-based organelles involved in mitosis. CapG is imported into the nucleus by NTF2, Ran GTPase and nucleoporin Nuc62[26], a phenomenon that was observed in HEK293T, HeLa, and MDCK-AZ cells. We found no changes in the cell cycle following induction of CapG overexpression. This suggests that an excess of CapG is not sufficient to alter cell cycle dynamics, but it does not exclude a role for CapG in cell cycle processes.
In summary, we have shown that the pN1βactin-rtTA2S-M2-IRES-EGFP Tet-on vector system can be stably transfected in a variety of pancreatic cancer cell lines, permitting the investigation of dox inducible gene overexpression in a time and dose-dependent manner. Our use of this system to overexpress CapG in Suit-2 cells resulted in accumulation of CapG protein in the cytoplasmic cellular compartment with associated increases in wound healing and cell motility, but no alterations to the cell cycle or to cellular proliferation.
Pancreatic cancer cells have traditionally been recalcitrant to the tetracycline gene regulatory system, a method that allows precise control of the level of expression of specific proteins, enabling functional analysis of those proteins. In this study, the authors compare the performance, in pancreatic cancer cells, of a stable Tet-on system employing a cytomegalovirus (CMV) promoter with a system employing a chicken β-actin promoter. In doing so, the authors generated cells exhibiting stable inducible expression of the actin capping protein CapG, which plays a role in cell migration. This protein was recently shown to be overexpressed in pancreatic cancer tissue, and its downregulation is associated with loss of motility.
Use of the chicken (β-actin) promoter proved superior to the CMV promoter for both the production and maintenance of tetracycline-inducible pancreatic cancer cell lines. The authors observed that the system was versatile, enabling transient inducible expression of a variety of genes. Moreover, with the newly developed Tet-on system, the authors showed that CapG overexpression in pancreatic cancer cells led to increased cell motility, but did not alter the cell cycle, or cellular proliferation.
The Tet-on system using a chicken (β-actin) promoter allowed the generation of stable inducible pancreatic cancer cell lines, providing an important tool for investigating doxycycline induced overexpression of proteins associated with pancreatic cancer. Furthermore, the study illustrated that cytoplasmic CapG overexpression in pancreatic cancer cells increases motility of these cells.
The stable Tet-on pancreatic cancer cell lines developed are an excellent system that will provide quantitative and temporal information on the role of given proteins in these cells.
Panc-1, Suit-2 and MiaPaca-2 are pancreatic cancer cell lines. CapG belongs to the family of actin regulatory proteins. CapG caps the ends of barbed actin filaments and therefore contributes of the actin associated motility of cells. Nuclear CapG is involved in the cell cycle regulation.
There are precious few published papers describing the use of stable tetracycline-inducible pancreatic cancer cells for the overexpression of proteins. This reflects the difficulty that scientists in this field have faced with the available systems. The authors describe the successful generation of stable tetracycline-inducible pancreatic cancer cells, using a chicken β-actin promoter to express the reverse tetracycline-controlled transactivator protein. This is an important advance, illustrating an improvement to the system which other pancreatic cancer researchers could also benefit from.
Peer reviewers: Dr. Bruno Christ, Professor, 1st Department of Medicine, University of Halle-Wittenberg, Heinrich-Damerow-Straße 1, D-06120 Halle/Saale, Germany; Mark D Gorrell, PhD, Professor, Centenary Institute of Cancer Medicine and Cell Biology, Locked bag No. 6, Newtown, NSW 2042, Australia
S- Editor Sun H L- Editor Stewart GJ E- Editor Ma WH
| 1. | Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547-5551. |
| 2. | Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766-1769. |
| 3. | Hinrichs W, Kisker C, Düvel M, Müller A, Tovar K, Hillen W, Saenger W. Structure of the Tet repressor-tetracycline complex and regulation of antibiotic resistance. Science. 1994;264:418-420. |
| 4. | Orth P, Cordes F, Schnappinger D, Hillen W, Saenger W, Hinrichs W. Conformational changes of the Tet repressor induced by tetracycline trapping. J Mol Biol. 1998;279:439-447. |
| 5. | Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA. 2000;97:7963-7968. |
| 6. | Welman A, Barraclough J, Dive C. Generation of cells expressing improved doxycycline-regulated reverse transcriptional transactivator rtTA2S-M2. Nat Protoc. 2006;1:803-811. |
| 7. | Yu J, Müller H, Hehn S, Koschmieder S, Schönig K, Berdel WE, Serve H, Müller-Tidow C. Construction and application of an inducible system for homogenous expression levels in bulk cell lines. PLoS One. 2009;4:e6445. |
| 8. | Roth S, Franken P, van Veelen W, Blonden L, Raghoebir L, Beverloo B, van Drunen E, Kuipers EJ, Rottier R, Fodde R. Generation of a tightly regulated doxycycline-inducible model for studying mouse intestinal biology. Genesis. 2009;47:7-13. |
| 9. | Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108-8125. |
| 10. | Zhang T, Liu WD, Saunee NA, Breslin MB, Lan MS. Zinc finger transcription factor INSM1 interrupts cyclin D1 and CDK4 binding and induces cell cycle arrest. J Biol Chem. 2009;284:5574-5581. |
| 11. | Welman A, Cawthorne C, Barraclough J, Smith N, Griffiths GJ, Cowen RL, Williams JC, Stratford IJ, Dive C. Construction and characterization of multiple human colon cancer cell lines for inducibly regulated gene expression. J Cell Biochem. 2005;94:1148-1162. |
| 12. | Thompson CC, Ashcroft FJ, Patel S, Saraga G, Vimalachandran D, Prime W, Campbell F, Dodson A, Jenkins RE, Lemoine NR. Pancreatic cancer cells overexpress gelsolin family-capping proteins, which contribute to their cell motility. Gut. 2007;56:95-106. |
| 13. | Johnston PA, Yu FX, Reynolds GA, Yin HL, Moomaw CR, Slaughter CA, Südhof TC. Purification and expression of gCap39. An intracellular and secreted Ca2(+)-dependent actin-binding protein enriched in mononuclear phagocytes. J Biol Chem. 1990;265:17946-17952. |
| 14. | Goldring CE, Kitteringham NR, Jenkins R, Lovatt CA, Randle LE, Abdullah A, Owen A, Liu X, Butler PJ, Williams DP. Development of a transactivator in hepatoma cells that allows expression of phase I, phase II, and chemical defense genes. Am J Physiol Cell Physiol. 2006;290:C104-C115. |
| 15. | Shekouh AR, Thompson CC, Prime W, Campbell F, Hamlett J, Herrington CS, Lemoine NR, Crnogorac-Jurcevic T, Buechler MW, Friess H. Application of laser capture microdissection combined with two-dimensional electrophoresis for the discovery of differentially regulated proteins in pancreatic ductal adenocarcinoma. Proteomics. 2003;3:1988-2001. |
| 16. | Nedjadi T, Kitteringham N, Campbell F, Jenkins RE, Park BK, Navarro P, Ashcroft F, Tepikin A, Neoptolemos JP, Costello E. S100A6 binds to annexin 2 in pancreatic cancer cells and promotes pancreatic cancer cell motility. Br J Cancer. 2009;101:1145-1154. |
| 17. | Vimalachandran D, Greenhalf W, Thompson C, Lüttges J, Prime W, Campbell F, Dodson A, Watson R, Crnogorac-Jurcevic T, Lemoine N. High nuclear S100A6 (Calcyclin) is significantly associated with poor survival in pancreatic cancer patients. Cancer Res. 2005;65:3218-3225. |
| 18. | Ermak G, Cancasci VJ, Davies KJ. Cytotoxic effect of doxycycline and its implications for tet-on gene expression systems. Anal Biochem. 2003;318:152-154. |
| 19. | Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61:2614-2623. |
| 20. | Witke W, Li W, Kwiatkowski DJ, Southwick FS. Comparisons of CapG and gelsolin-null macrophages: demonstration of a unique role for CapG in receptor-mediated ruffling, phagocytosis, and vesicle rocketing. J Cell Biol. 2001;154:775-784. |
| 21. | Iacobuzio-Donahue CA, Maitra A, Olsen M, Lowe AW, van Heek NT, Rosty C, Walter K, Sato N, Parker A, Ashfaq R. Exploration of global gene expression patterns in pancreatic adenocarcinoma using cDNA microarrays. Am J Pathol. 2003;162:1151-1162. |
| 22. | Lal A, Lash AE, Altschul SF, Velculescu V, Zhang L, McLendon RE, Marra MA, Prange C, Morin PJ, Polyak K. A public database for gene expression in human cancers. Cancer Res. 1999;59:5403-5407. |
| 23. | Van den Abbeele A, De Corte V, Van Impe K, Bruyneel E, Boucherie C, Bracke M, Vandekerckhove J, Gettemans J. Downregulation of gelsolin family proteins counteracts cancer cell invasion in vitro. Cancer Lett. 2007;255:57-70. |
| 24. | De Corte V, Van Impe K, Bruyneel E, Boucherie C, Mareel M, Vandekerckhove J, Gettemans J. Increased importin-beta-dependent nuclear import of the actin modulating protein CapG promotes cell invasion. J Cell Sci. 2004;117:5283-5292. |
| 25. | Hubert T, Van Impe K, Vandekerckhove J, Gettemans J. The actin-capping protein CapG localizes to microtubule-dependent organelles during the cell cycle. Biochem Biophys Res Commun. 2009;380:166-170. |