INTRODUCTION
The imbalance of colonic epithelial proliferation and apoptosis may lead to both ulcer- and carcinoma development of the mucosa. The final direction of this imbalance depends on complex pathogenetic pathways in which isolated lymphoid follicles (ILFs) seem to have a specific role.
Some steps of colonic epithelial regeneration are known, but the connection among them is not fully understood. The continuous reformation of the epithelial layer is important in avoiding the aggregation of pernicious mutations induced by intraluminal factors. In inflammation, the lack of regenerative factors and the disturbance of the regulation of regenerative mechanisms favour ulcer development. It has also been observed that in colonic inflammation there is a tight connection between the degree of epithelial damage and the number, diameter and cellular compounds of subepithelial lymphoid follicles[1,2]. The more severe the epithelial destruction that develops, the higher the number of ILFs that can be found in adjacent mucosa.
It has recently been reported that lymphoid follicles are also present in carcinomas of the lung[3], endometrium[4], liver[5], and colon[1]. They are supposed to have immune-mediated anti-tumoral effects, as their elevated number is in positive correlation with a better prognosis and a longer survival[3]. However, the density of lymphoid follicle-associated flat dysplastic aberrant crypt foci was significantly higher compared to the rest of the mucosa in azoxymethane-treated rats[6]. Several reports have investigated the association between lymphoid aggregates and colonic tumors in rodents[7,8]. The results indicate that colonic crypts overlying ILFs show a significantly higher proliferative activity, which may also influence genetically defected epithelial cells. Hence, the risk of carcinoma is increased in the colonic mucosa of ILFs compared to mucosa without ILFs. It has also been shown that the incidence of ILFs in early human colorectal cancers significantly differs by gender, location, macroscopic type and histology, but moreover, their localization significantly differs by their macroscopic type[9].
However, the exact role of ILFs in colonic epithelial repair and colorectal carcinogenesis is not yet known. Some data show[10] that the lack of lymphoid follicles results in abnormal crypt formation in the case of epithelial destruction. On the other hand, Apc gene mutation causes impairment of developmental and apparent differentiation blockade in proliferative tissues, including those of the lymphoid follicles[11]. Whether ILFs act as a regenerative pool containing putative stem cells in case of mucosal damage, or they are responsible only for the optimal cytokine milieu for the differentiation of immigrating stem cells or invasive carcinoma cells[12] need to be further examined.
THE ORGANIZATION OF THE GUT-ASSOCIATED LYMPHOID TISSUE
The gut-associated lymphoid tissue (GALT) is a component of the mucosa-associated lymphoid tissue, in which approximately 70 percent of the body’s immune cells are found[13,14]. GALT differentiates between pathogens and commensal bacteria.
The majority of GALT is composed of isolated and aggregated lymphoid follicles dispersed throughout the small and large intestines[15]. These lymphoid follicles, including Peyer’s patches (PPs) of the small intestine and ILFs of the large intestine, are composed of a specialised follicle associated epithelium (FAE), which overlies a subepithelial dome containing numerous macrophages, dendritic cells, T, B lymphocytes, and special antigen sampling microfold/M/cells[15-17]. The FAE has a crucial role in the initiation of the mucosal and systemic immune response[18]. ILFs have, in general, an average diameter of 0.1-0.7 mm and number of around 30 000 in humans[19].
ILFs are innervated sites of GALT. Functionally, antigen-triggered mast cell and eosinophil activation affects both the secretory and motor functions of the intestines[20], and these defensive reactions can be modulated by the enteric nervous system[21]. It has been recently recognised that there is a dense neuronal network at the level of the supra-follicular dome region, but not within the germinal centers in lymphoid follicles[22]. Neuronal alterations of PPs and ILFs, such as nerve-eosinophil associations or increasing neuronal cell adhesion molecule expression, may have consequences on the uptake of particular pathogens[16,23].
VASCULARIZATION OF ILFS
ILFs have rich blood and lymphatic vascularization[14,19]. Vasculogenesis may play a dual role in mucosal organization, in that it is not only necessary for nutritional and metabolic processes, but the homing of the repopulating bone marrow derived stem cells to the site of tissue damage may happen via blood vessels. In the case of cancer development, the vascular system is essentially involved in tumor growth, invasion and metastasis formation.
Revascularization is a key point of colonic mucosal repair. During inflammatory stages, due to cytokine action and intercellular adhesion, molecules signalling some of the vessels differentiate into high endothelial venules (HEVs)[24,25]. In the case of lymphocytes and neutrophils, it is supposed that they firstly reach the inflammatory sites via a transcellular pathway through the HEVs[26], but an intercellular pathway is also known[27]. Upon epithelial injury the circulating bone marrow derived cells (BMDCs) migrate to the stromal layer of the damaged colonic wall, presumably via HEVs at an increased number regulated by overexpressed inflammatory chemokines[28].
Based on the result of Witmer et al[29], it has been suggested that in lymphoid tissues, including GALT, the signaling system of the vascular endothelial growth factor (VEGF) and its receptor play a permanent role in the vasculogenesis of ILFs. Whereas the inhibition of VEGF has shown promising results in sporadic colon cancer, it has been recently published that VEGF receptor signaling acts as a direct growth factor for tumor cells in colitis-associated cancer, providing a molecular link between inflammation and the development of colon cancer[30].
BONE MARROW DERIVED STEM CELLS OF ILFS
Based on the former results[31-33], emerging evidence suggests that bone marrow derived stem cells contribute to tissue regeneration partly by promoting neovascularization or arteriogenesis. After human hematopoietic cell transplantation epithelial tissue chimerism appears[34-36].
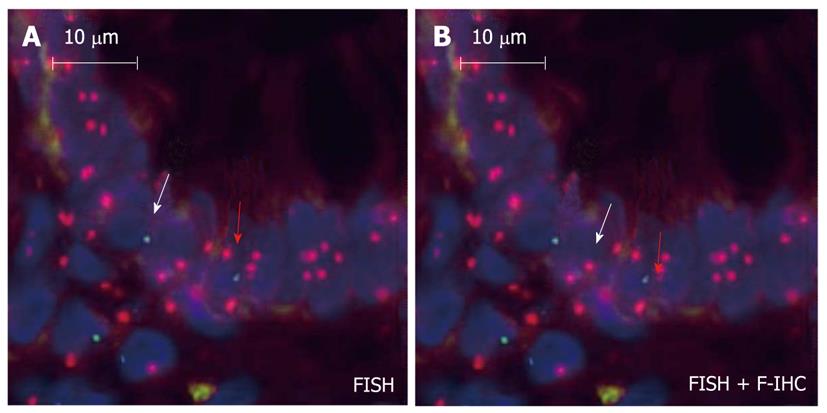
The bone marrow origin of epithelial cells may be supposed by observations in which epithelial cell markers and leukocyte markers showed that double positive cells were found in inflamed mucosa adjacent to lymphoid aggregates[2,37-39]. The presence of cytokeratin, epithelial growth factor receptor, hepatocyte-derived growth factor receptor or CDX2 co-expression in CD45+ cells of ILFs may support the mesenchymal origin of epithelial stem cells. Based on these results, it seems that ILFs are involved in the homing and differentiation of BMDCs in the case of colonic mucosal damage (Figure 1).
Figure 1 Intraepithelial male donor bone marrow origin CD45-/Y-FISH+ cell (white arrow) and CD45+/Y-FISH+ intraepithelial lymphocyte (red arrow) in the colonic biopsy specimen of a female acceptor.
A: Chromosomal detection (green: Y-chromosome, red: X-chromosome; fluorescence in situ hybridization); B: CD45 and cytokeratin (green: cytokeratin, red: CD45; fluorescence immunohistochemistry; 130 × magnification).
The cause of metastasis remains elusive despite a vast amount of information on cancer cells. According to recent research, cancer cell fusion with macrophages or immigrating BMDCs provides an explanation[40,41]. BMDCs fused with tumor cells were present not just in animal tumor xenografts where they were associated with metastases, but in human carcinomas, including colon cancer. BMDC-tumor cell fusion explains the epidermal-mesenchymal transition in cancer since BMDCs express mesodermal traits and epithelial-mesenchymal transition regulators (i.e.: Twist, SPARC). If BMDC-tumor cell fusion underlies invasion and metastasis in human cancer, new therapeutic strategies would be mandated.
DENDRITIC CELLS IN ILFS
Follicular dendritic cells (FDCs) in lymphoid follicles retain native antigens in the form of immune complexes on their membrane for months, and present these antigens to B cells during the secondary response[42,43]. The origin and cell lineage of FDCs are controversial. Whereas their immune functions and expression of hemopoietic cell-associated antigens suggest that they belong to the hemopoietic lineage[44], their spindle-shaped morphology “in vitro”, lack of CD45, and presence of antigens expressed by fibroblasts[45] indicate that FDCs may be mesenchymal cells. Based on studies with mouse radiation chimeras, Humphrey et al[46] concluded that FDCs were not derived from the bone marrow, but came from a local mesenchymal precursor. However, Kapasi et al[44], using mice homozygous for the SCID mutation, which lack T, B lymphocytes, and FDCs, demonstrated that after reconstitution with bone marrow from donor mice, the FDCs of the reconstituted mice expressed the donor phenotype. These authors concluded that FDC precursors came from bone marrow.
According to the results of Muñoz-Fernández et al[47], FDCs seem to be a specialized form of myofibroblasts and derive from bone marrow stromal cell progenitors. The authors were able to isolate and culture 18 follicular dendritic cell lines from human tonsils. These cells were CD45-negative and expressed antigens associated with FDCs (CD21, CD23, CD35, CD40, CD73, BAFF, ICAM-1, and VCAM-1) and antigens specific for FDC (DRC-1, CNA.42, and HJ2). These cell lines were also able to bind B cells and secrete CXCL13, and they had functional activities characteristic of FDCs. Nevertheless, the additional expression of STRO-1, together with CD10, CD13, CD29, CD34, CD63, CD73, CD90, ICAM-1, VCAM-1, HLA-DR, alkaline phosphatase, and α-smooth muscle actin (α-SMA) indicated that FDCs are closely related to bone marrow stromal cell progenitors. The expression of α-SMA also relates FDCs with myofibroblasts. Like myofibroblasts, FDC lines expressed stress fibers containing α-SMA and were able to contract collagen gels under the effect of TGFβ1 and platelet-derived growth factor.
In various inflammation models, tissue-derived dendritic cells have been shown to migrate from the inflammatory site via lymphatics to secondary lymphoid organs where they interact with lymphocytes[48]. Based on their dual phenotype, follicular dendritic cells may represent a transformation switch point among immigrating bone marrow derived stem cells in ILFs and the surrounding subepithelial myofibroblasts.
The origin of dendritic cells (DCs) in tumors remains obscure. Recent studies indicate that conventional DCs in lymphoid tissues arise from a distinct population of committed conventional DC precursors (pre-cDCs) that originate in bone marrow and migrate via blood. Diao et al[49] showed that pre-cDCs are precursors for conventional DCs in tumors, and they migrate from blood into the tumor where they generate conventional DCs. The chemokine CCL3, which is markedly upregulated in tumors (including colon cancer) and in tumor-infiltrating stromal and immune cells, promotes pre-cDC recruitment. Both pre-cDCs and their conventional DC progeny actively proliferate within the tumor, and have the ability to mature and stimulate Ag-specific lymphocytes. This finding suggests that in several cases the migration of pre-cDCs to tumors may represent a normal response to inflammation. Further studies are needed to delineate the role of pre-cDCs in other inflammatory processes and to compare them with monocytes, which are currently considered the main source of inflammatory DCs in peripheral tissues[50,51].
MYOFIBROBLASTS SORROUNDING ILF ADJACENT EPITHELIUM
Subepithelial myofibroblasts (SEMFs) exist as a syncytium that extends throughout the colonic lamina propria, merging with the pericytes surrounding the blood vessels[52,53]. SEMFs are involved in two epithelial repair processes[54,55]. One process is called restitution[56]. This is an important response to minor to moderate injury. The other process is observed when the wound is deep, and the subepithelial tissues and the basement membrane need to be reconstituted[55].
According to recent studies[54,57,58], myofibroblasts are thought to derive from two major sources, bone marrow or locally activated fibroblasts, in response to transforming growth factor-β1. In the case of serious tissue injury (i.e. active ulcerative colitis) the regeneration capacity of local stem cells is not enough to complete tissue repair. In this case, bone marrow derived mesenchymal stem cells migrate into the gastrointestinal wall where they may contribute to the repair progress[59,60] as differentiated mesenchymal cells (e.g. myofibroblasts)[61].
Despite the increasing number of publications illustrating the role of tumor-associated stromal cells in cancer progression, there still exists a significant ambiguity with respect to the identification of cancer-associated fibroblasts, myofibroblasts and peritumoral fibroblasts in the cancer tissue. SEMFs appear early in the cancer’s development. The mutual interaction (through direct cell-cell contacts and paracrine signals) between cancer cells and SEMFs is essential for invasive growth and is translated into a poor clinical prognosis[62].
TOLL-LIKE RECEPTOR EXPRESSION IN ILFS
Beside immune functions, PPs and ILFs are supposed to be involved in mucosal repair via Toll-like receptors (TLRs). In ILFs, TLRs are expressed on the cells of the monocyte/macrophage system, on some kinds of T cells, as well as on intestinal epithelial, endothelial and stromal cells[63]. Using the dextran sodium sulfate (DSS) model of colitis, mice lacking TLR2, TLR4 or MyD88 all developed more severe colitis than wild type mice when exposed to orally administered DSS[64]. These findings suggest that signaling from commensal bacteria throughout TLRs resulted in protection from DSS colitis through enhanced epithelial cell proliferation, and worked as a compensatory factor against epithelial damage[64].
TLRs can also bind endogenous ligands including necrotic cells, heat shock proteins, and extracellular matrix components[65-67]. Necrotic cells may activate NF-κB through TLR2, leading to the expression of tissue repair-associated genes[65]. It is supposed that necrosis induced inflammation in tissue damage may provide danger signals functioning as inducers of tissue repair responses through TLRs. The TLR ligands released from necrotic cells have not been identified, although heat shock proteins produced by damaged cells are known to be TLR ligands[66]. Components of the extracellular matrix, such as hyaluronan, can be an endogenous ligand for TLR4[67]. Increased hyaluronan production has been demonstrated in both DSS colitis in mice and in human Crohn’s disease[68]. It is possible that TLR activation may occur in the absence of microbial products[68]. In the case of inflammatory mucosal damage, ILFs may induce repair mechanisms via endogenous TLR activation.
TLR4 was also shown to be expressed on human colon carcinoma cells and functionally active. It may play important roles in promoting immune escape of human colon carcinoma cells by inducing immunosuppressive factors and apoptosis resistance, and it may also promote the proliferation and migration of cancer cells[69,70].
The analysis of isolated tumor cells from primary colon cancers showed co-expression of TLR7 and TLR8 with CD133 and gave evidence for a subpopulation of colon cancer-initiating cells[71]. Persistent TLR-specific activation of NF-κB in colorectal cancer, particularly in tumor-initiating cells, may sustain further tumor growth and progression through perpetuated signaling known from inflammatory and tissue repair mechanisms with consecutive self-renewal in pluripotent tumor cells. Activation through self-ligands or viral RNA fragments from tumor-associated lymphoid aggregates may putatively maintain this inflammatory process, suggesting a key role in cancer progression.
THE EFFECT OF THE PRESENCE OF ILFS ON MUCOSAL REPAIR
The epithelia of intestinal crypts associated with ILFs and PPs have an increased proliferation rate[10,14]. Saxena et al[10] showed that PPs in rats have a facilitative effect on the healing of intestinal wounds by promoting both epithelial cell migration on the defect and epithelial cell proliferation in the crypts adjacent to the wound and by decreasing the rate of wound contraction.
In rats, a difference in epithelial apoptosis between the FAE of PPs and intestinal villi was described[72]. Onishi et al[72] showed that the progression of the apoptotic process in the epithelial cells of FAE occurs later than in the intestinal villi, so the possibility of epithelial differentiation might remain in FAE, unlike in intestinal villi. PPs are supposed to have a regulatory effect on the epithelial proliferation as well[73].
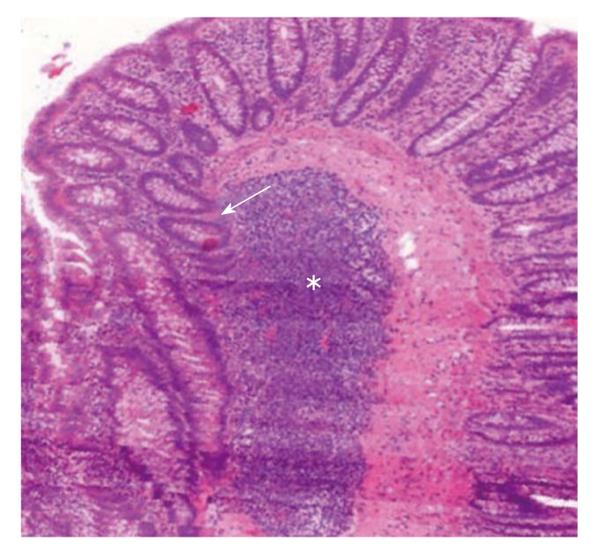
The Wnt signaling pathway is critical for regulating a number of basic cell functions, such as cell proliferation, cell fate, polarity, differentiation, and migration, leading to morphogenesis and organogenesis[74,75]. There is strong genetic evidence that Wnt signaling play critical roles in the regulation of epithelial stem cells in the intestinal tract[76]. The Wnt target gene Lgr5 has been recently identified as a novel stem cell marker of the intestinal epithelium and the hair follicle[77]. In the intestine, Lgr5 is exclusively expressed in cycling crypt base columnar cells[77]. Many Wnt family proteins are expressed in hematopoietic tissues, and can also be secreted by lymphoid cells[78,79]. The Wnt-Lgr5 pathway may be a potential switch between the ILFs and colonic epithelial renewal. Lymphoid cells of ILFs may produce Wnts which are essential components of a milieu in which bone marrow derived stem cells immigrated to ILFs to engage in epithelial differentiation (Figure 2).
Figure 2 3D reconstruction of a human colonic surgical sample (MIRAX Viewer, 3D, 3DHISTECH Ltd.
, Budapest). A large subepithelial isolated lymphoid follicle (white star) can be seen. Colonic crypts (white arrow) with no connection to the luminal surface “outgrow” from the isolated lymphoid follicle.
THE EFFECT OF THE PRESENCE OF ILFS ON COLORECTAL CARCINOGENESIS
Results from experimental colon cancer studies indicated that ILFs might promote the development of adenocarcinomas[7,8]. However, studies in experimental animals have also shown that the intestinal lymphoid system plays an important role in immunologic defense mechanisms; that is, antigenic stimuli result in germinal center formation, antibody production, and finally enlargement of the follicles[80]. In humans, the presence of tumor-infiltrating lymphocytes is associated with an improved prognosis in colorectal cancers, as does the presence of high level DNA microsatellite instability[81]. These results suggest that ILFs in early colorectal neoplasms play an important role in defense rather than in promotion.
In a recent study, Fu et al[9] found that the incidence of ILFs in early human colorectal neoplasms significantly differs by gender, location, macroscopic type, and histology, but moreover, their localization significantly differs by macroscopic type.
In squamous cell carcinomas of the esophagus, cyclin A expression in the germinal center cells of ILFs beneath the superficial tumorous lesions was shown to be an immunological signal toward the proliferation and progression of the tumors[82]. Gutfeld et al[83] found that the cells of colonic ILFs, inflammatory cells, ganglion cells, and endothelial cells express serum amyloid A, an acute phase reactant, whose level in the blood is elevated in response to trauma, infection, inflammation, and neoplasia, on both mRNA and protein levels. The serum amyloid A mRNA expression in epithelial cells was found to gradually increase as it progressed through different stages of dysplasia to overt carcinoma. While expression of the serum amyloid A1 and -4 genes in colon carcinomas was confirmed by RT-PCR analysis, this expression was barely detectable in normal colon tissues. Their findings indicate local and differential expression of serum amyloid A in human colon cancer and tumor-associated ILFs, and suggest its role in colorectal carcinogenesis.
MESENCHYMAL-EPITHELIAL AND EPITHELIAL-MESENCHYMAL TRANSITION IN ILFS
Epithelial-mesenchymal transition (EMT) is a physiological mechanism present during development, and is also encountered in several pathological situations such as renal interstitial fibrosis, endometrial adhesion, and cancer metastasis[84]. A reverse phenomenon, mesenchymal-epithelial transition (MET) also takes place during normal development in processes such as somitogenesis, kidney development and coelomic cavity formation[85]. In adult organisms, it has been proposed that restrictive mechanisms repress EMT and MET[86]. During tumor development, these mechanisms appear to fail, allowing EMT described in metastasis generation[87].
In inflammation, MET can also be altered because mesenchymal stem cells are mobilized to these sites of injury and consequently subjected to the inflammatory response[88]. BMDCs could differentiate into mature-appearing epithelial cells in response to tissue damage[89]. It was recently published that versican, a large chondroitin sulfate proteoglycan, mediates MET[90]. The results of Hirose et al[91] indicate that versican can bind specific chemokines through its chondroitin sulfate chains and that the binding tends to down-regulate the chemokine function. This raises the possibility that versican may act as a regenerative factor in colonic mucosa, and may be an important switch point between ILFs and MET. The presence of CDX2 and cytokeratin positive subepithelial cells in the marginal zone of ILFs also suggests that MET may take place in these immune formations[2].
Stroma-tissue, including lymphoid aggregates and ILFs surrounding the cancer cells, plays an important role in the tumor behavior. Mesker et al[92] analyzed the expression of markers involved in pathways related to stroma production and EMT (β-catenin, TGF-β-R2, SMAD4) in high-risk colorectal cancer patients, and found that patients with stroma-high and SMAD4 loss are of high risk. The anti-EMT effect of SMAD4 was also proven in colon carcinoma cells[93].
CONCLUSION
Based on the summarized results of literature, it seems that ILFs act like a switch between colonic mucosal regeneration and colorectal carcinogenesis.
Subepithelial revascularization after mucosal damage takes place partly under the direction of ILFs with the prominent help of vascular endothelial growth factor and its receptors. Immigrating stem cells from bone marrow may leave circulation via high endothelial venules in ILFs and their surroundings. Their differentiation throughout mesenchymal-to-epithelial transition may also happen in ILFs, and follicular dendritic cells, as well as the subepithelial myofibroblasts, seem to be crucial parts of colonic crypt formation and epithelial renewal.
Vasculogenesis in ILFs supports not just tumor growth and the metastatic process, but the VEGF receptor signaling acts like a direct growth factor for tumor cells. The fusion of BMDCs immigrating to ILFs with tumor cells may explain EMT in colorectal cancers. The presence of ILFs, dendritic cells and subepithelial myofibroblasts may also result in a specific milieu for tumor formation, growth and invasion.
Better understanding of the role of ILFs in mucosal repair may lead to the development of new therapeutic agents for inflammatory colon diseases that not only decrease the activity of inflammation, but also accelerate epithelial barrier recovery, hence dramatically decreasing clinical symptoms. Moreover, by revealing the exact connections between ILFs and colorectal carcinogenesis, the basis of individualized anti-cancer immunotherapies may be established.
Peer reviewer: Akira Andoh, MD, Department of Internal Medicine, Shiga University of Medical Science, Seta Tukinowa, Otsu 520-2192, Japan
S- Editor Tian L L- Editor Rutherford A E- Editor Ma WH