Published online Mar 28, 2011. doi: 10.3748/wjg.v17.i12.1606
Revised: September 27, 2010
Accepted: October 4, 2010
Published online: March 28, 2011
AIM: To investigate the spleen vascular involvement and the presence of liver fibrosis in a population of subjects with established systemic sclerosis (SSc).
METHODS: In a cross-sectional fashion, 17 patients with SSc were compared with 18 patients suffering from hepatitis C virus (HCV)-related liver cirrhosis, grade A and B Child-Pugh classification. Eighteen non elderly subjects, apparently healthy, were used as the control group. Splenic artery resistivity index (SARI) at doppler ultraSound, transient elastography of liver and nailfold capillaroscopy were the main outcomes.
RESULTS: Transient elastography values of SSc patients were similar to those of controls; 5.2 ± 1.1 vs 4.5 ± 1, (P = 0.07). Median Alanine amino transferase (ALT) concentrations of cirrhotic patients were greater than those of controls and SSc patients, i.e. 66.5 (36-89) U/L vs 29 (22-34) U/L and 31 (22-41) U/L, respectively, (P = 0.005). SARI determinations in cirrhotic patients, although significantly higher than those found in controls and SSc patients, showed some degree of overlap with SSc patients, i.e. 0.59 vs 0.52 and 0.57, respectively, (P = 0.04). Mean systolic blood pressure was significantly higher in SSc patients than in cirrhotics and controls, i.e. 142 mmHg vs 128.2 mmHg and 127 mmHg, respectively, (P = 0.005). Mean diastolic blood pressure behaved in a similar fashion, i.e. 84 mmHg vs 72.2 mmHg and 76.9 mmHg (P = 0.005). Nailfold Capillaroscopy grades and diastolic blood pressure values correlated well with SARI results.
CONCLUSION: An enhanced resistivity of the splenic artery was found in patients suffering from SSc; they did not have evidence of splenomegaly as well as no liver fibrosis or any other form of liver damage.
- Citation: Tarantino G, Spanò A, Loi G, Parisi A, Tarantino M, Brancaccio G, Gaeta GB, Riccio A. Is spleen circulation impaired in systemic sclerosis and what is the role of liver fibrosis? World J Gastroenterol 2011; 17(12): 1606-1613
- URL: https://www.wjgnet.com/1007-9327/full/v17/i12/1606.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i12.1606
Systemic sclerosis (SSc) is a disease characterized by a complex interplay of inflammation, fibrosis and vascular damage. In fact, the arterial microvessels of SSc display rarefaction and mural thickening, including medial smooth muscle and intimal cell hyperplasia[1]. Vasculopathy, showing surprising similarities in various areas, i.e. pulmonary, renal, cerebral and peripheral, induces pulmonary arterial hypertension (PAH), scleroderma renal crisis, severe cerebral vasculopathy and digital tip ulcers. Endothelin-1 is a potent mediator of vascular features in SSc[2], but immunological mechanisms also participate in SSc pathogenesis. They include a Th1/Th2 imbalance in CD4+ T cells[3], an increase of type 2 cytokine-producing T cells in target organs[4] and the presence of autoantibodies with fibroblast-activating capacities[5]. Finally, caveolin-1 has been thought to regulate the signaling of transforming growth factor-β[6], a key determinant in SSc. In fact, the core lesion of SSc is the uncontrolled fibrosis involving internal organs and derma.
There are some a priori reasons, mainly indirect causations, for predicting a reasonable incidence of hepatic fibrosis in patients with SSc. The hepatic mesenchymal tissue would be expected to share enhanced synthesis and/or deposition of collagen. The liver having a double blood supply, playing a central role in the induction of immune tolerance and also being a target for immune-mediated damage, it could actively participate in systemic diseases. Surprisingly, the extensive literature on SSc provides no definitive indications of the incidence and nature of associated hepatic fibrosis in the direct, the indirect (hepatoxicity) or the fortuitous (occult viral infection and associated autoimmune or metabolic co-morbidities) types. As the “companion” organ of the liver, the spleen plays an important role in splanchnic hemodynamics, beyond its established immunologic functions[7]. This occurs through changes in intrasplenic microvascular tone and through reflex activation of the splenic afferent sympathetic nerves[8]. Actually, it is recommended to perform a biopsy examination to assess the liver participation in SSc[9,10]. However, what about unravelling hepatic fibrosis when ethical and legal issues do not permit invasive tests? Systematic reviews have demonstrated that evaluation of liver stiffness by transient elastography (TE) is clinically useful for the diagnosis of various grades of fibrosis in the course of chronic liver diseases[11], even though it is better at excluding than at predicting cirrhosis[12]. Parameters of doppler ultrasound (DUS) are reckoned to be the new biomarkers of the liver-spleen circulatory axis[13,14], by way of substituting the estimation of hepatic venous pressure gradient (HVPG) that is an expensive technique[15] based on catheterism. Among these hemodynamic indices SARI has one of the closest correlations to HVPG < 12 (non-severe portal hypertension)[16]. On the other hand, enlargement of spleen may be associated with portal hypertension-related events in most patients with advanced chronic liver disease[17]. Thus, hypothesizing a diffuse vascular injury[18], and considering that mechanisms similar to those of SSc play a role in cirrhotic portal hypertension[19,20], it is conceivable that a hemodynamic alteration of the spleno-portal axis could be present in SSc patients. Therefore, in a population of subjects with established SSc, the presence of liver fibrosis was investigated by TE and assessment of the spleen vascular involvement was carried out by DUS; the results of DUS were compared with those found in subjects with an advanced chronic liver disease characterized by a hyperdynamic circulation and increased resistance in the hepatic and spleen vascular bed. Furthermore, we tried to track the possible association between DUS findings and other vascular parameters such as blood pressure and nailfold capillaroscopy (NFC).
Seventy-two subjects formed the initial study population. Twenty-six consecutive patients who fulfilled the American College of Rheumatology (formerly, the American Rheumatism Association) criteria for SSc[21], having been classified as having limited cutaneous (lc) SSc or diffuse cutaneous (dc) SSc according to the criteria described by LeRoy et al[22], were selected. Three SSc patients were disallowed as they were on penicillamine therapy. Six other patients were excluded, two of whom due to contextual HCV infection, one to alcohol abuse and three to non-alcoholic fatty liver disease (NAFLD) presence in diabetics. The remainder (17 patients), who were on low doses of prednisone ≤ 10 mg and vasodilators, acted as the SSc group. Mean duration of disease was 3.5 years.
Twenty-six patients suffering from HCV-related liver cirrhosis, genotype 1b, grade A and B Child-Pugh classification, were selected to be compared with the SSc patients. They were diagnosed on the basis of liver biopsy (8 cases), the remainder on the grounds of appropriate tests. Briefly, the presence of cirrhosis was established by appropriate clinical (spider nevi, hepato-splenomegaly), laboratory (low serum total cholesterol, prothrombin activity and pseudocholinesterase levels, reduced white blood cell and platelet count, globulin/albumin ratio > 1) as well as imaging data of the liver (coarse echo-texture, nodularity presence, increased caudate/right lobe ratio, hypertrophy of the left lobe, characterized by a rounded inferior marginal edge, and portal vein enlargement with decreased flow velocity, absence of a normal doppler waveform, hepatofugal flow). Eight patients were excluded as they were cryoglobulinemic and likely suffering from vasculitis. Eighteen patients were finally considered as forming the cirrhotic group. A cohort of non elderly subjects (18 cases), apparently healthy, was used as a control group. Laboratory data and instrumental measurements were strictly carried out within one month of each other. The protocol was consistent with the principles of the Declaration of Helsinki, and participants gave their informed consent. The study had no external funding source.
Liver stiffness was assessed by means of a Fibroscan (Echosens, France). At least 10 measurements per patient were obtained, using the standard probe. These were averaged and reported as kPa. A success rate of at least 60% was considered necessary. In our population, special care was taken in order to make sure there was no A-shaped wave on the elastogram, which indicates an incorrectly accepted (non-automatically rejected) measurement, leading to an overestimation of the stiffness produced by influence of the surrounding rib bone and soft tissue.
Spleen longitudinal diameter (SLD) was performed by postero-lateral scanning at US, using a Tecnos (EsaOte, Italy). The Maximum Length (ML, the optically greatest overall longitudinal dimension obtained from one of the two poles) and the Cranio-Caudal Length (CCL, the optically maximal transversal dimension intercepting one of the two poles) were measured; the resulting values were then averaged, since the two measurements do not always coincide. SLD (ML+CCL/2) was also measured in controls to set reference intervals as mean values of TE.
SARI values at DUS were automatically calculated with the following formula: RI = peak systolic velocity-end systolic velocity/peak systolic velocity. The probe was positioned below the left costal arch or in the left costal spaces. Color Doppler allowed identification of the main branches of the splenic artery. Antihypertensive drugs were withdrawn at least three days before the test.
All nailFold capillaroscopy (NFC) procedures were performed in a stereomicroscope (Olympus-SZ40) under 10-20x magnification according to the protocol proposed by Andrade et al[23]. All the ten digits of the hands were examined, except when prevented by extremely poor visibility. The following parameters were analyzed: (1) number of capillary loops/mm; (2) vascular deletion score; (3) number of enlarged loops (about four times the normal afferent, transition, and efferent limb widths); and (4) number of giant capillary loops (10 or more times the normal width of capillary limbs). Enlarged and giant loops were counted together. The vascular deletion score was assessed according to a well-known method[24], in which a deletion area is defined as the absence of two or more consecutive loops. Each finger was rated from 0 to 3: grade 0, no deletion area; 1, one or two discrete deletion areas; 2, more than two discrete deletion areas; and 3, extensive and confluent deletion areas. For each patient the NFC parameters were calculated as the average obtained in all analyzed digits.
Because transthoracic echocardiography (TTE) is a reproducible method, it was used as a non-invasive diagnostic tool to determine pulmonary arterial pressure and helped us exclude any secondary causes of PAH in SSc patients with unexplained dyspnea. According to a peak velocity of tricuspid regurgitation (VTR) ≥ 3.0 m/s at TTE, which is consistent with international guidelines[25], PAH was diagnosed. Once the diagnosis of PAH was established, together with several parameters (ventricular function, exercise parameters, peak oxygen uptake or peak systolic blood pressure), NYHA functional classes were selected to predict prognosis in these patients.
The systolic/diastolic blood pressure (SBP, DBP) was the average of three consecutive readings taken by the physician during the day, during usual practice hours, after subjects had rested for five minutes in the sitting position.
ALT activity was determined by in-house standard procedures. Sera samples were tested for anti-nuclear antibodies (ANA) using indirect immunofluorescence and HEp-2 cells as antigen substrate (Antibodies Inc., Davis, CA). Anti-centromere antibodies (ACA) were determined by their distinctive IIF pattern on HEp-2 cells. Autoantibodies to topoisomerase I (ATA) were determined by passive immunodiffusion against calf thymus extract (Inova Diagnostics, San Diego, CA).
Liver stiffness, age, SARI, SLD, systolic blood pressure and diastolic blood pressure data derived from a normally distributed population (Shapiro-Wilk test (S-W), P = 0.94; S-W, P = 0.98; S-W, P = 0.23; S-W, P = 0.064; S-W, P = 0.26; S-W, P = 0.11, respectively) were expressed as mean plus SD. A variable not normally distributed, such as ALT (S-W, P = 0.001) was expressed as median (range). NFC grades were considered ordinals and managed in the same way. The difference of means was evaluated by Two-Sample t test, two-tailed probability. Frequencies were evaluated by χ2, or in case of more than two groups by χ2 for trends. Tracking the degree of association between single parameters, i.e. NFC scores and SARI determinations, Spearman’s rho for non uniform intervals was used. The Pearson’s coefficient (r) was employed to analyze the correlation between blood pressure values and SARI determinations. One-way analysis of variance (ANOVA) with the Student-Newman-Keuls test for all pairwise comparisons was performed to examine differences among groups; when dealing with a variable not normally distributed the Kruskal-Wallis test with post-hoc analysis was adopted. To predict the presence of PAH, logistic regression (enter method), with relative odds ratio (OR) and 95% confidence intervals (CI), was employed, utilizing as independent variables ATA positivity. Following the clinical and laboratory standards institute 2008 guidelines (C28-A3) for smaller samples, the 90% CIs were estimated to set our normal range of TE and SLD at US. The concordance correlation coefficient (ρc), which measures precision and accuracy, was adopted to evaluate the degree of pair observations at US. Statistical analysis was performed operating on medcalc version 10.4.8® (Frank Schoonjans) software package.
In order to allow readers to gauge the internal validity of this study, we emphasize that the minimal required sample size, with a type I error of 0.05 and power of 68%, when breaking the population into two groups (i.e. controls and SSc patients) by SARI values (means = 0.56 and 0.52 with a pooled SD of 0.06), was calculated in 18 subjects. Additionally, to weigh how well the study findings apply to the patients (external validity) we stress that the fifty-three subjects, divided into three cohorts well balanced for gender and age, were studied in a cross-sectional fashion.
Demographic, clinical and instrumental characteristics of the full population are represented in Table 1. TE values of SSc patients were similar to those of controls, 5.2 ± 1.1 vs 4.5 ± 1, P = 0.07, two-sample t test, two-tailed probability.
| Variables | SSc | Liver cirrhosis | Controls | P-value |
| The number of patients | 17 | 18 | 18 | |
| Age (yr) | 47.9 ± 12.6 | 49.7 ± 9.5 | 44.4 ± 9.8 | 0.060 |
| Females | 15 | 16 | 14 | 0.3801 |
| Type diffuse/cutaneous | 12/5 | ND | ND | |
| GI involvement | 9 | 0 | ND | 0.003 |
| PAH presence | ND | 3 | ND | |
| C-P score A/B | ND | 12/6 | ND | |
| SLD at US (mm) | 102.3 ± 9.7 | 130.4 ± 4.6 | 100.9 ± 6.6 | 0.000 |
| SARI at DUS | 0.56 ± 0.06 | 0.59 ± 0.02 | 0.52 ± 0.01 | 0.040 |
| TE kPa | 5.2 ± 1.1 | ND | 4.5 ± 1 | 0.070 |
| NFC grade 0/1/2/3 | 3/7/2/5 | ND | ND | |
| Hypertension | 6 | 0 | 0 | 0.0011 |
| ALT (U/L), median (range) | 31 (22-41) | 66.5 (36-89) | 29 (22-34) | 0.005 |
| ATA/ACA/ANA positivity | 3/4/2010 | ND | ND |
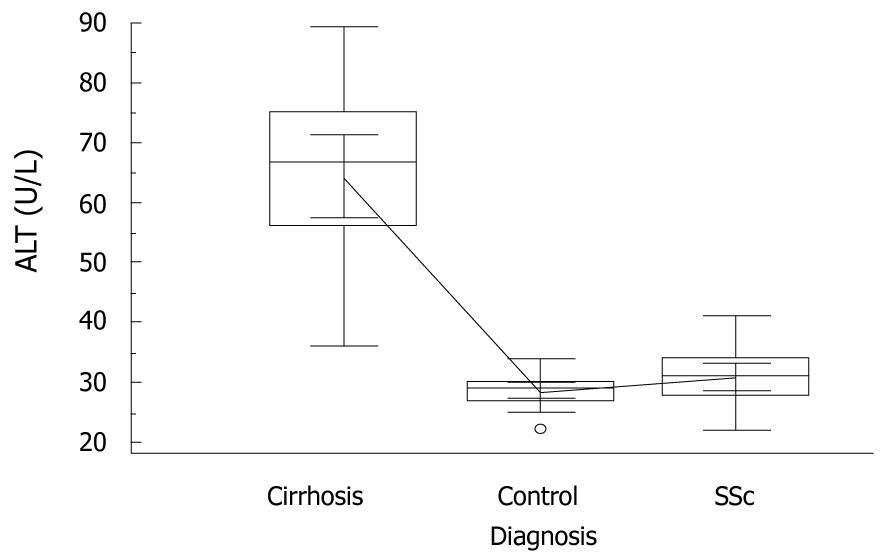
Median ALT concentrations of cirrhotic patients were greater than those of controls and SSc patients, i.e. 66.5 (36-89) U/L vs 29 (22-34) U/L and 31 (22-41) U/L, respectively, P = 0.005, Kruskal-Wallis with post-hoc analysis (Figure 1).
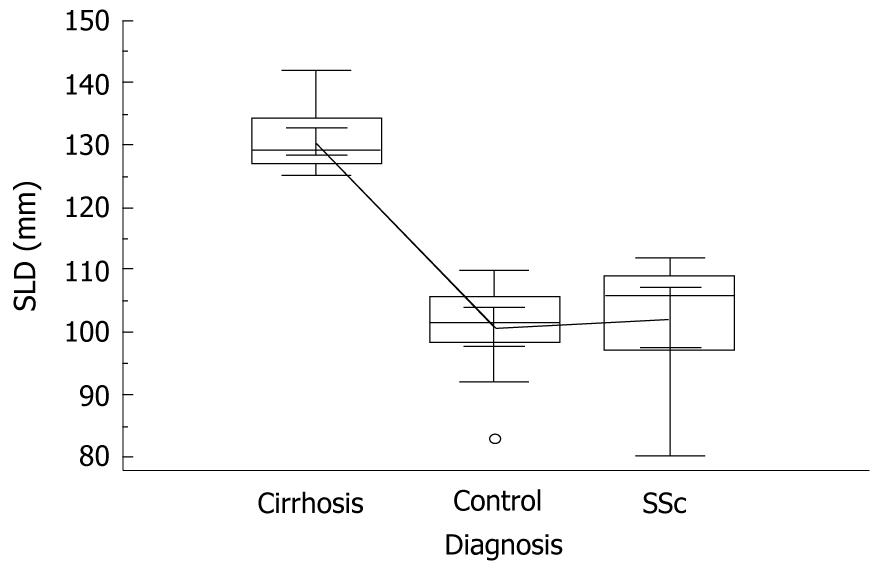
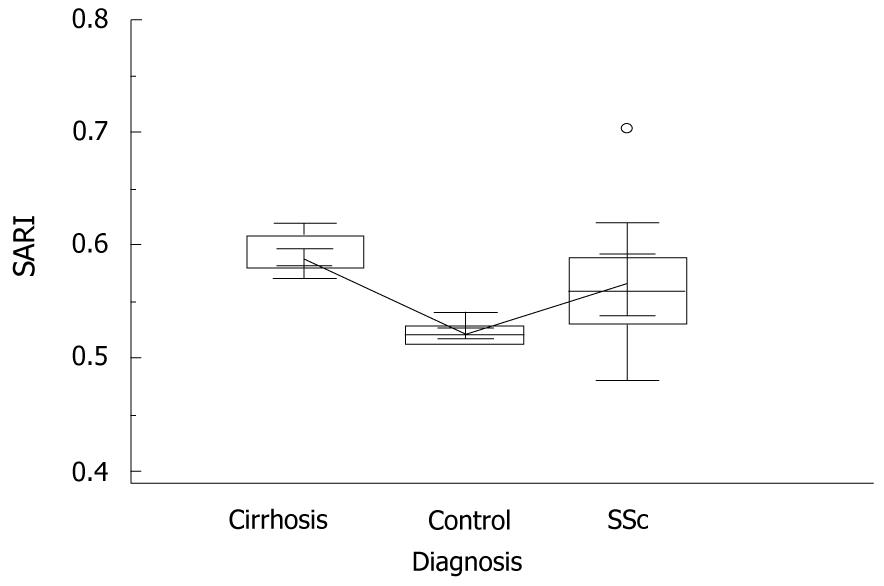
In cirrhotic individuals, SLD showed values superior to those of control subjects and SSc patients, i.e. 130.4 mm vs 100.9 mm and 102.3 mm, respectively, P = 0.0001, ANOVA with Student-Newman-Keuls test (Figure 2). In contrast, SARI determinations in cirrhotics, although significantly higher than those found in controls and SSc patients, showed some degree of overlap with SSc patients, i.e. 0.59 vs 0.52 and 0.57, respectively, P = 0.04, ANOVA with Student-Newman-Keuls test (Figure 3). Successively, we failed to find much higher values of SARI in the SSc subjects suffering from PAH, this being present in only three out of 17 individuals (18%), at least at the time of observation.
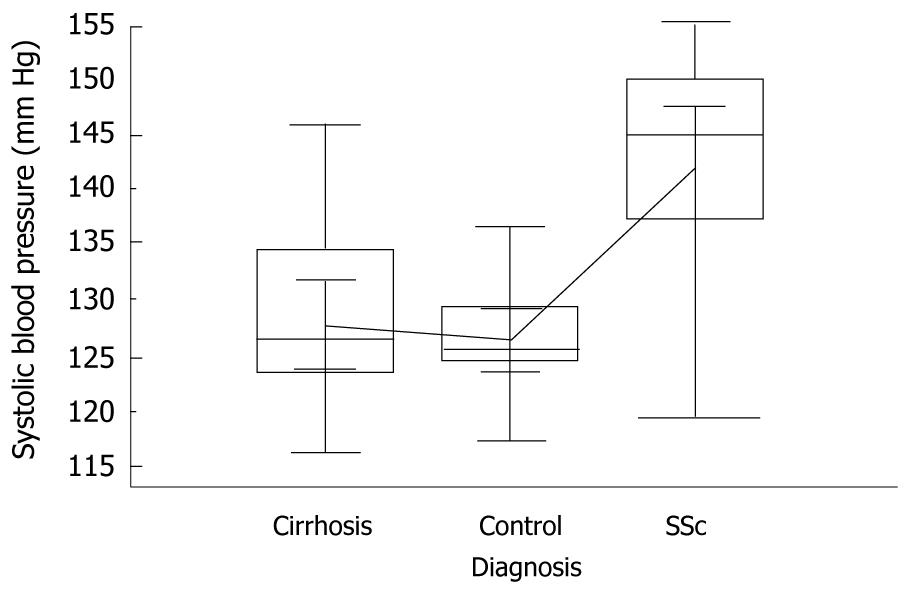
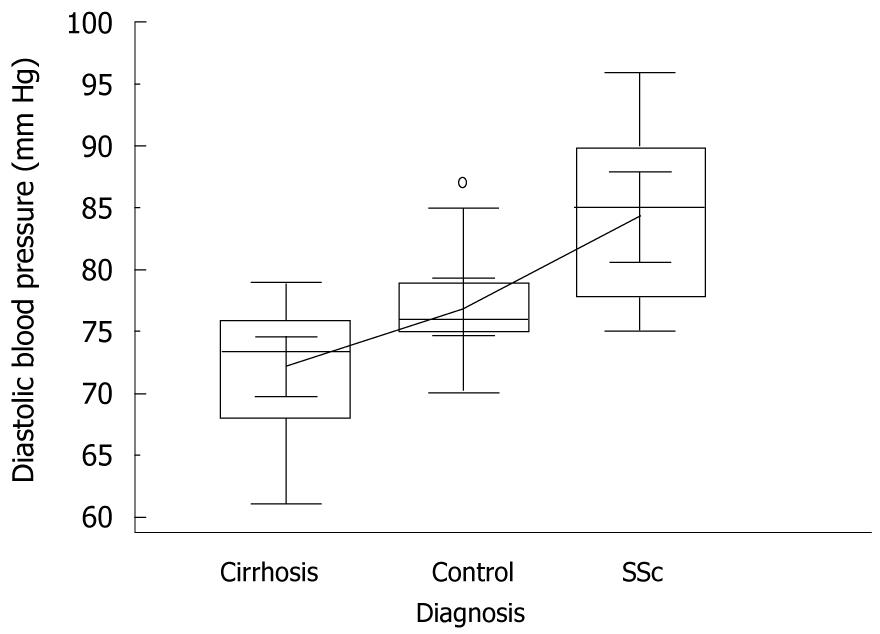
Mean systolic blood pressure was significantly higher in SSc patients than in cirrhotics and controls, i.e. 142 mmHg vs 128.2 mmHg and 127 mmHg, respectively, P = 0.005, ANOVA with Student-Newman-Keuls test (Figure 4). Mean diastolic blood pressure behaved similarly, i.e. 84 mmHg vs 72.2 mmHg and 76.9 mmHg, respectively, P = 0.005, ANOVA with Student-Newman-Keuls test (Figure 5).
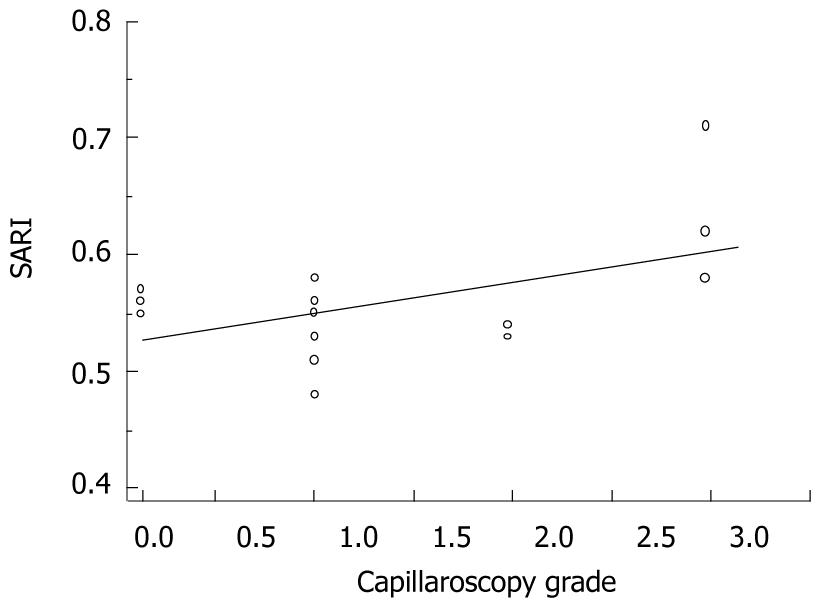
There was a substantially good association between SARI measurements and NFC grades, i.e. Spearman’s rho = 0.51, P = 0.04 (Figure 6).
Diastolic blood pressure values correlated well with SARI results (i.e. Pearson’s r = 0.57, P = 0.01); meanwhile, systolic blood pressure did not (i.e. Pearson’s r = 0.25, P = 0.05).
SARI data were not correlated with SLD measurements, both in cirrhotics and SSc patients (i.e. Pearson’s r = -0.20, P = 0.43 and Pearson’s r = 0.19, P = 0.45, respectively). At univariate analysis, ATA positivity did not predict PAH presence, OR 1.5, 95% CI: 0.1 to 20.7, P = 0.7.
The intra-observer and inter-observer errors at US and DUS, measured as concordance correlation coefficients, were found more than acceptable, i.e. ρc = 0.89 and 0.88, respectively.
The non-parametric percentile methods of TE and SLD yielded the following normal ranges, i.e. 2.4-6.1 kPa and 83-110 mm, respectively.
To provide a brief synopsis of key findings, we stress the following: (1) an enhanced resistivity of the splenic artery was found in patients suffering from SSc; and (2) they did not demonstrate splenomegaly as well as liver fibrosis or any other form of liver damage.
Our data agree with the body of present knowledge that provides evidence for the idea that, in addition to inflammatory infiltrates and an accumulation of extracellular matrix proteins, vascular changes are a hallmark in the pathogenesis of SSc. Consistent with the systemic vascular damage (the vasoactive endothelin-1 system?) we find important evidence, i.e. the strict association between SARI values and the severity of features at NFC and between the same DUS parameter and diastolic blood pressure values. Surprisingly, SARI values were somewhat overlapping with those found in patients suffering from liver cirrhosis.
Considering possible discrepancies of our findings, such as why SARI does not keep up with increased spleen volume in SSc, we hypothesize that high values of this parameter are due to an intrinsic spleen vascular damage and not to portal hypertension as canonically present in cases of marked liver fibrosis following chronic liver injury. This divergence requires a distinct involvement of the two organs, i.e. spleen and liver in SSc; the former characterized by a vascular change and the latter apparently not damaged. However, what is the significance of the splenic artery in determining the increased spleen volume in the cirrhotic patients group? It is believed that passive venous congestion is the major cause of splenomegaly in advanced chronic liver disease, even though blood hyper-afflux plays an important part. Our data do support the concept of venous congestion. In fact, there was no correlation between spleen size and SARI measurements in cirrhotics, shedding further light on the mechanisms of portal hypertension in these patients with a quite compensated form.
Returning to the possible deposition of connective tissue in liver, beyond the reliance on a preliminary impression that has basis in the fragmentary published data, hepatic involvement in SSc is generally considered rare. As previously emphasized, treatment with potentially hepatotoxic drugs, coincident viral chronic hepatitis or NAFLD have usually been implicated as the main causes of liver disease in patients with connective tissue diseases. However, even after careful exclusion of these etiologies, the question remains whether to classify the patient as having a primary liver disease with associated autoimmune disorder or as having liver disease as a manifestation of generalized connective tissue disease. Now, when addressing the hepatic entanglement in our cohort of SSc patients, the absence of liver damage had been sufficiently documented, at least until the study period, even if it could not be rejected with certainty, due to the lack of histology. Obviously, if we take into consideration that nearly all the hepatic co-morbidities were excluded, we would end up in reinforcing the hypothesis that the vascular changes of the spleen are not linked to a concomitant chronic liver disease. It is therefore natural to ask whether the normal volume of spleen could play a role in explaining the absence of a liver disease. First of all, it is necessary to stress that a light-moderate chronic injury could not always be linked to an increased spleen volume. On the other hand, normal spleen size excludes an idiopathic portal hypertension complicating SSc[26].
Commenting on drawbacks of the present study and the methods used to compensate for the main ones, we pinpoint that, although liver biopsy remains the ‘best standard’ to assess liver fibrosis presence, limitations are considerable, including patient discomfort and rare, but serious, complications such as bleeding or pneumothorax and a mortality rate of 1/10 000 to 1/12 000. Moreover, only 1/50 000 of the liver volume is investigated, resulting in sampling error[27]. Furthermore, this tool, representing an ‘instant snapshot’ of a dynamic process, does not reflect a long-lasting assessment. For this reason, other authorities employ substitute ‘gold standards’ as relate to the natural history of the disease. Alternative attempts to diagnose and follow-up the collagen deposition in liver range from routine biochemistry (AST/platelets calculated ratio, APRI) to surrogate fibrosis markers in serum[28], hepatic clearance tests, various imaging techniques and, more recently, the use of non-invasive TE. The reported values of this tool give a good correlation with various markers of fibrosis and increase proportionally with the progression of the hepatic fibrosis stage[29]. There are no evidence-based data justifying biopsy as a first line estimate of liver fibrosis. Health authorities in some countries have already approved validated biomarkers as the first line procedure for the staging of liver fibrosis[30]. However, HVPG should have been employed in our SSc cohort to exclude increased portal hypertension, but this is based on an invasive technique and X-ray exposure; thus, heavy ethical restrictions did not permit its use in our patients without evidence of serious liver chronic diseases. In this respect, DUS examination is gaining widespread consensus in order to provide patients, suspected of having portal hypertension, with important diagnostic/prognostic information. Moreover, it is important to pinpoint the lack of data from TE in cirrhotic patients; but this tool, as previously emphasized, is very good at excluding advanced chronic disease, not at confirming its presence. Final flaws could be the small sample size due to the fact that this study was carried out using “strict” inclusion criteria and the fact that SSc is a heterogeneous disease; clinical presentations are highly variable among the patients. This may result in a wide range of SARI in patients with SSc. We should have analyzed which disease subset (diffuse vs limited cutaneous SSc, short vs long disease duration, or the presence vs absence of individual organ involvement) was associated with high SARI in SSc, although the number of patients enrolled was too small to perform sub-analysis.
In conclusion, as we have revealed spleen vascular changes, the crucial future research direction should zero in on other vascular areas, such as retinal artery[31] or renal artery[32], by means of the simple and reliable tool that is DUS, to evaluate to what extent vasculopathy is represented in SSc. If these data are confirmed and expanded in a larger population, physicians will be advised to investigate the spleen circulation at the “earliest possible time” in the progression of disease to provide another window on systemic vasculopathy of these patients. How significant a role can this clinical study play? The clinical implications of this work summarized in a straightforward and circumspect manner are that our data support the possibility of a new tool (SARI) to evaluate this ongoing process in SSc, mainly before and after disease-modifying treatment.
Systemic sclerosis is a disease characterized by a complex interplay of inflammation, fibrosis and vascular damage. In fact, the arterial microvessels of systemic sclerosis display rarefaction and mural thickening, including medial smooth muscle and intimal cell hyperplasia.
There are some a priori reasons, mainly indirect causations, for predicting a reasonable incidence of hepatic fibrosis in patients with systemic sclerosis. The hepatic mesenchymal tissue would be expected to share enhanced synthesis and/or deposition of collagen.
The key findings in this study were the following: (1) an enhanced resistivity of the splenic artery was found in patients suffering from systemic sclerosis; and (2) they did not demonstrate splenomegaly as well as liver fibrosis or any other form of liver damage.
Having revealed spleen vascular changes, the crucial future research directions should zero in on other vascular areas, such as retinal artery or renal artery, by means of the simple and reliable tool that is doppler ultraSound, to evaluate to what extent vasculopathy is represented in systemic sclerosis. If these data are confirmed and expanded in a larger population, physicians will be advised to investigate the spleen circulation at the “earliest possible time” in the progression of disease to provide another window on systemic vasculopathy of these patients.
The authors investigated the spleen vascular involvement and the presence of liver fibrosis in patients with established SSc. In order to proceed as described above, they have included in the present study seventeen patients with SSc compared with eighteen patients suffering from hepatitis C virus-related liver cirrhosis and eighteen non elderly subjects as a control group. As findings, the authors relate an enhanced resistivity of the splenic artery in patients suffering from SSc, who did not demonstrate splenomegaly as well as liver fibrosis or any other form of liver damage.
Peer reviewer: Damiao Carlos Moraes Santos, DCM, PhD, Bio-Manguinhos, Fundacao Oswaldo Cruz; Avenida Brasil 4365 - Manguinhos; Rio de Janeiro, 21040360, Brazil
S- Editor Sun H L- Editor Logan S E- Editor Ma WH
| 1. | Fleming JN, Shulman HM, Nash RA, Johnson PY, Wight TN, Gown A, Schwartz SM. Cutaneous chronic graft-versus-host disease does not have the abnormal endothelial phenotype or vascular rarefaction characteristic of systemic sclerosis. PLoS One. 2009;4:e6203. |
| 2. | Shi-wen X, Kennedy L, Renzoni EA, Bou-Gharios G, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum. 2007;56:4189-4194. |
| 3. | Fujii H, Hasegawa M, Takehara K, Mukaida N, Sato S. Abnormal expression of intracellular cytokines and chemokine receptors in peripheral blood T lymphocytes from patients with systemic sclerosis. Clin Exp Immunol. 2002;130:548-556. |
| 4. | White B. Immunopathogenesis of systemic sclerosis. Rheum Dis Clin North Am. 1996;22:695-708. |
| 5. | Fineschi S, Goffin L, Rezzonico R, Cozzi F, Dayer JM, Meroni PL, Chizzolini C. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum. 2008;58:3913-3923. |
| 6. | Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol. 2008;20:713-719. |
| 7. | Tarantino G, Conca P, Pasanisi F, Ariello M, Mastrolia M, Arena A, Tarantino M, Scopacasa F, Vecchione R. Could inflammatory markers help diagnose nonalcoholic steatohepatitis? Eur J Gastroenterol Hepatol. 2009;21:504-511. |
| 8. | Hamza SM, Kaufman S. Role of spleen in integrated control of splanchnic vascular tone: physiology and pathophysiology. Can J Physiol Pharmacol. 2009;87:1-7. |
| 9. | Abraham S, Begum S, Isenberg D. Hepatic manifestations of autoimmune rheumatic diseases. Ann Rheum Dis. 2004;63:123-129. |
| 10. | Takagi K, Nishio S, Akimoto K, Yoshino T, Kawai S. A case of systemic sclerosis complicated by idiopathic portal hypertension: case report and literature review. Mod Rheumatol. 2006;16:183-187. |
| 11. | Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. Am J Gastroenterol. 2007;102:2589-2600. |
| 12. | Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511-1517. |
| 13. | Malik R, Afdhal N. Stiffness and impedance: the new liver biomarkers. Clin Gastroenterol Hepatol. 2007;5:1144-1146. |
| 14. | Singal AK, Ahmad M, Soloway RD. Duplex Doppler ultrasound examination of the portal venous system: an emerging novel technique for the estimation of portal vein pressure. Dig Dis Sci. 2010;55:1230-1240. |
| 15. | Hicken BL, Sharara AI, Abrams GA, Eloubeidi M, Fallon MB, Arguedas MR. Hepatic venous pressure gradient measurements to assess response to primary prophylaxis in patients with cirrhosis: a decision analytical study. Aliment Pharmacol Ther. 2003;17:145-153. |
| 16. | Vizzutti F, Arena U, Rega L, Romanelli RG, Colagrande S, Cuofano S, Moscarella S, Belli G, Marra F, Laffi G. Performance of Doppler ultrasound in the prediction of severe portal hypertension in hepatitis C virus-related chronic liver disease. Liver Int. 2007;27:1379-1388. |
| 17. | Berzigotti A, Zappoli P, Magalotti D, Tiani C, Rossi V, Zoli M. Spleen enlargement on follow-up evaluation: a noninvasive predictor of complications of portal hypertension in cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1129-1134. |
| 18. | Cutolo M, Pizzorni C, Tuccio M, Burroni A, Craviotto C, Basso M, Seriolo B, Sulli A. Nailfold videocapillaroscopic patterns and serum autoantibodies in systemic sclerosis. Rheumatology (Oxford). 2004;43:719-726. |
| 19. | Kojima H, Sakurai S, Kuriyama S, Yoshiji H, Imazu H, Uemura M, Nakatani Y, Yamao J, Fukui H. Endothelin-1 plays a major role in portal hypertension of biliary cirrhotic rats through endothelin receptor subtype B together with subtype A in vivo. J Hepatol. 2001;34:805-811. |
| 20. | Xu B, Zhu GH, Weng JF, Cai WS, Xia JT, Li SH. [The roles of caveolin-1 and endothelial nitric oxide synthase in the development of portal hypertension in rats with liver cirrhosis]. Zhonghua Gan Zangbing Zazhi. 2008;16:184-187. |
| 21. | Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581-590. |
| 22. | LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, Rowell N, Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202-205. |
| 23. | Andrade LE, Gabriel Júnior A, Assad RL, Ferrari AJ, Atra E. Panoramic nailfold capillaroscopy: a new reading method and normal range. Semin Arthritis Rheum. 1990;20:21-31. |
| 24. | Lee P, Leung FY, Alderdice C, Armstrong SK. Nailfold capillary microscopy in the connective tissue diseases: a semiquantitative assessment. J Rheumatol. 1983;10:930-938. |
| 25. | Simonneau G, Galiè N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S-12S. |
| 26. | Moschos J, Leontiadis GI, Kelly C, Henry J, Kadis S. Idiopathic portal hypertension complicating systemic sclerosis: a case report. BMC Gastroenterol. 2005;5:16. |
| 27. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. |
| 28. | Shaikh S, Memon MS, Ghani H, Baloch GH, Jaffery M, Shaikh K. Validation of three non-invasive markers in assessing the severity of liver fibrosis in chronic hepatitis C. J Coll Physicians Surg Pak. 2009;19:478-482. |
| 29. | Takemoto R, Nakamuta M, Aoyagi Y, Fujino T, Yasutake K, Koga K, Yoshimoto T, Miyahara T, Fukuizumi K, Wada Y. Validity of FibroScan values for predicting hepatic fibrosis stage in patients with chronic HCV infection. J Dig Dis. 2009;10:145-148. |
| 30. | Poynard T, Morra R, Ingiliz P, Imbert-Bismut F, Thabut D, Messous D, Munteanu M, Massard J, Benhamou Y, Ratziu V. Assessment of liver fibrosis: noninvasive means. Saudi J Gastroenterol. 2008;14:163-173. |
| 31. | Sorrentino P, Tarantino G, Conca P, Ragucci P, Perrella A. Abnormally high resistive index of central retinal artery by ultrasound color Doppler in patients with viral chronic liver disease: correlation with worsening liver staging. Ultrasound Med Biol. 2004;30:599-604. |
| 32. | Rosato E, Cianci R, Barbano B, Menghi G, Gigante A, Rossi C, Zardi EM, Amoroso A, Pisarri S, Salsano F. N-acetylcysteine infusion reduces the resistance index of renal artery in the early stage of systemic sclerosis. Acta Pharmacol Sin. 2009;30:1283-1288. |