Published online Mar 28, 2011. doi: 10.3748/wjg.v17.i12.1594
Revised: February 14, 2011
Accepted: February 21, 2011
Published online: March 28, 2011
AIM: To investigate the effect of Simotang (Decoction of Four Powered Drugs) on gastrointestinal motility, motilin and cholecystokinin expression in chronically stressed mice.
METHODS: Forty mice were randomly divided into control group, stress group (model group), mosapride group and Simotang group, 10 in each group. A variety of unpredictable stimulations were used to induce chronic stress in mice. Then, the mice were treated with distilled water, mosapride or Simotang for 7 d. Gastric emptying and intestinal propulsion function were detected. Serum level of motilin was measured by enzyme-linked immunosorbent assay. Expression of cholecystokinin (CCK) in intestine, spinal cord and brain of mice was detected by immunohistochemistry and semi-quantitative reverse transcription polymerase chain reaction, respectively.
RESULTS: Simotang improved the gastric emptying and intestinal propulsion in chronically stressed mice. Furthermore, the serum motilin level was significantly higher and the expression levels of CCK-positive cells and genes were significantly lower in intestine, spinal cord and brain of Simotang group than in those of model group (P < 0.05). No significant difference was found in serum motilin level and expression levels of CCK-positive cells and genes between the mosapride and Simotang groups.
CONCLUSION: Simotang enhances the gastrointestinal motility in chronically stressed mice by regulating the serum motilin level and the expression of cholecystokinin.
- Citation: Cai GX, Liu BY, Yi J, Chen XM, Liu FL. Simotang enhances gastrointestinal motility, motilin and cholecystokinin expression in chronically stressed mice. World J Gastroenterol 2011; 17(12): 1594-1599
- URL: https://www.wjgnet.com/1007-9327/full/v17/i12/1594.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i12.1594
Functional dyspepsia (FD), a common functional gastrointestinal disorder, is steadily becoming a public health problem. Its prevalence in the United States is 15%[1], 11% in the general Italian population[2], and 12%-25% in China[3].
The pathogenesis of FD remains unknown, but is likely to involve many factors. In recent years, gastrointestinal motor dysfunction has been considered as the main pathogenesis of FD[4]. Gastrointestinal motility is modulated by many hormones and brain-gut peptides. Motilin, a polypeptide hormone containing 22 amino acids, is secreted by endocrine M cells and regulates gastrointestinal motility by increasing the migrating myoelectric complex[5,6]. cholecystokinin (CCK), a brain-gut peptide, is widely distributed in the gastrointestinal tract and central/peripheral nervous system, and regulates the gastrointestinal motility and food intake[7,8].
Simotang (Decoction of Four Powered Drugs) is a classical formula that has been used in treatment of gastrointestinal disorders for hundreds of years[9] and approved as an oral liquid drug by the Chinese National Food and Drug Administration in the 1980s. It has been shown that Simotang can affect the gastrointestinal motility in cold-restraint stressed mice[10]. Some monomer constituents, such as arecoline[11] and hesperidin[12] detected in Simotang, can affect gastrointestinal function. However, the mechanism of Simotang underlying gastrointestinal motility is still unknown. In the present study, we investigated the effect of Simotang on gastrointestinal motility in chronically stressed mice and measured the serum motilin levels, expression of CCK-positive cells and genes in intestine, spinal cord and brain of chronically stressed mice.
Simotang, composed of Fructus aurantii, Radix linderae, Radix aucklandiae, and Semen arecae (specification: 10 mL/division), was purchased from Hunan Hansen Pharmaceutical Company, Ltd (Yiyang, Hunan Province, China). Mosapride citrate was purchased from Chengdu Kanghong Pharmaceutical Company, Ltd (Chengdu, Sichuan Province, China), and dissolved in distilled water to a final concentration of 0.5 mg/mL.
Forty male adult ICR mice weighing 15-22 g were provided by Hunan Slac Jingda Laboratory Animal Company, Ltd (Changsha, China) and randomly divided into control group, stress group (model group), mosapride group and Simotang group. Mice in mosapride and Simotang groups were fed with mosapride citrate (30 mg/kg) or Simotang (1.2 g/kg) consecutively for 7 d from day 21 after stress. Mice in control and model groups received the same volume of distilled water. All animals were housed in a 12 h light and dark cycle (7 Am), in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Mice in model, mosapride and Simotang groups were exposed to chronic stress. A mouse model of chronic stress was induced as previously reported[13]. In brief, mice were randomly exposed to different stressors daily from day 1 to day 21 as follows: high-speed agitation for 1 min, food deprivation for 48 h, water deprivation for 24 h, tail pinching (2 cm apart from the end of the tail) for 1 min, hung upside-down for 5 min, heat stimulation at 45°C for 5 min, day and night reversal, and cold stimulation at 4°C for 5 min. Each stressor was repeated twice during the 21 d stress procedure.
Gastrointestinal motility was evaluated by testing gastric emptying and intestinal propulsion. Six days after drug or water administration, mice were fasted for 24 h and then administered the last drug or water. One hour later, each mouse administered 0.4 mL semi-solid paste. Twenty minutes later, the mice were sacrificed with 10% chloral hydrate (10.0 mL/kg), and the rate of semi-solid paste in the stomach and the rate of semisolid paste propulsion in the small intestine were measured. The gastric emptying and intestinal propulsion rates in 20 min were calculated according to the following equations[14]: gastric emptying rate (%) = (semi-solid paste quality-gastric residue quality)/semi-solid paste quality, and intestinal propulsion rate (%) = advanced length of black semi-solid paste/total length of the small intestine × 100%.
Intestine, spinal cord and brain were rapidly removed from the mice and immediately fixed with 4% paraformaldehyde for immunohistochemistry. Specimens were cut into 15-μm thick sections with a cryostat and mounted onto silane-coated slides, and dried at 37°C for 60 min. The sections were stored at -70°C. Samples were quickly removed from an environment with a low temperature and immediately stored in liquid nitrogen for RT-PCR.
Serum was collected from the heart of mice after anesthesia, and serum motilin level was measured by ELISA according to its manufacturer’s protocol (R&D, USA).
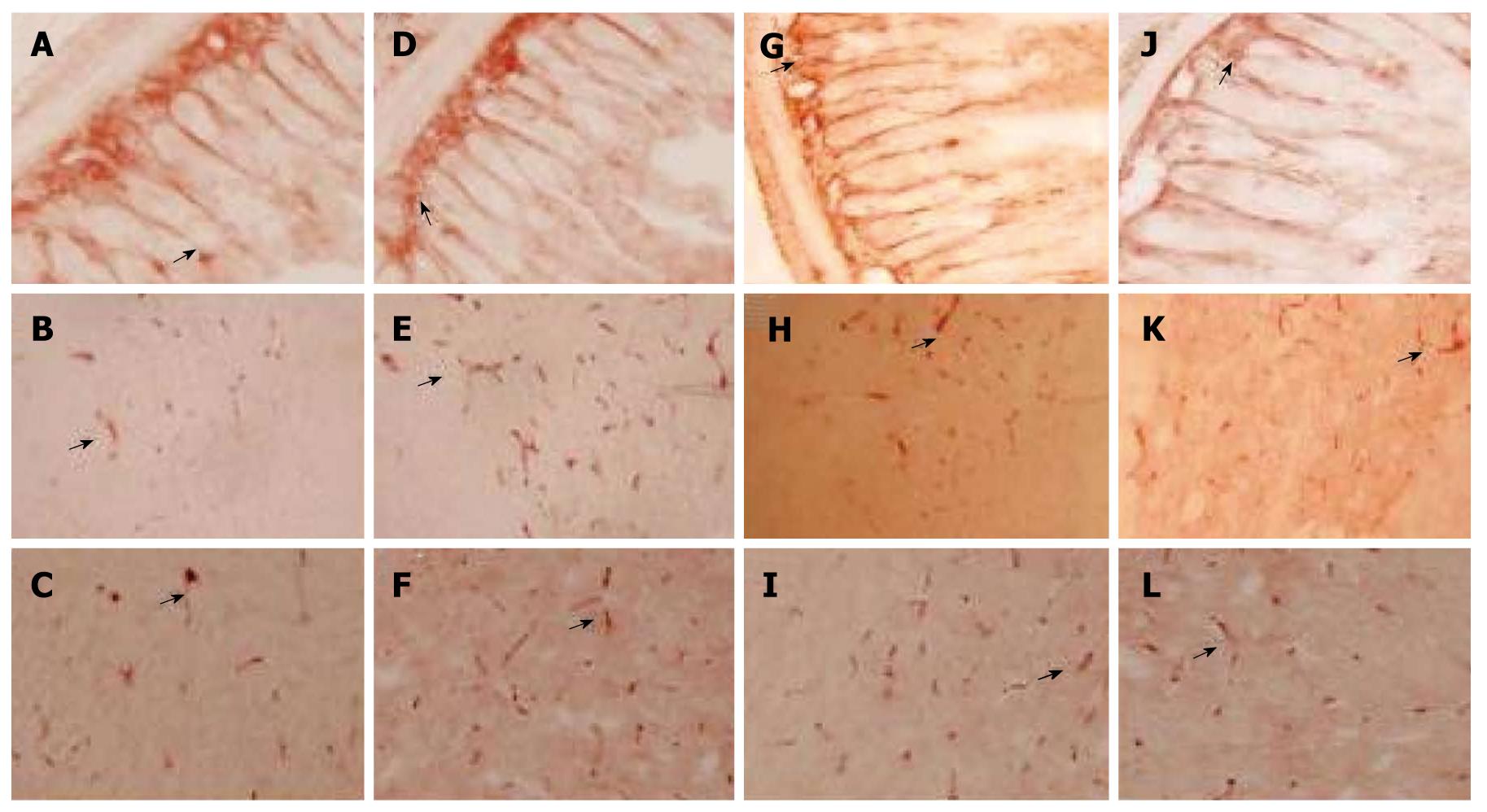
The sections were incubated with 10 μL normal goat serum at room temperature for 10 min, and treated with rat anti-CCK diluted at 1/100 (Boster Biological Technology, Wuhan, China) at room temperature for 60 min to detect CCK-positive cells in intestine, spinal cord and brain of the mice. The sections were then incubated with biotinylated secondary broad IgG antibody (Zymed Laboratories, CA, USA) for 30 min, reacted with streptavidin peroxidase and aminoethyl carbazole (Zymed Laboratories CA, USA), counterstained with aminoethyl carbazole and observed under an Olympus BX51 microscope (Olympus, Tokyo, Japan). CCK-positive cells were counted under a light microscope (200 × magnification) using the Olympus MicroImage 4.0 software (BX51, Olympus). Ten 200 × microscopic fields per sample were randomly selected from each group, and the cell counts per mm2 from 10 fields were averaged.
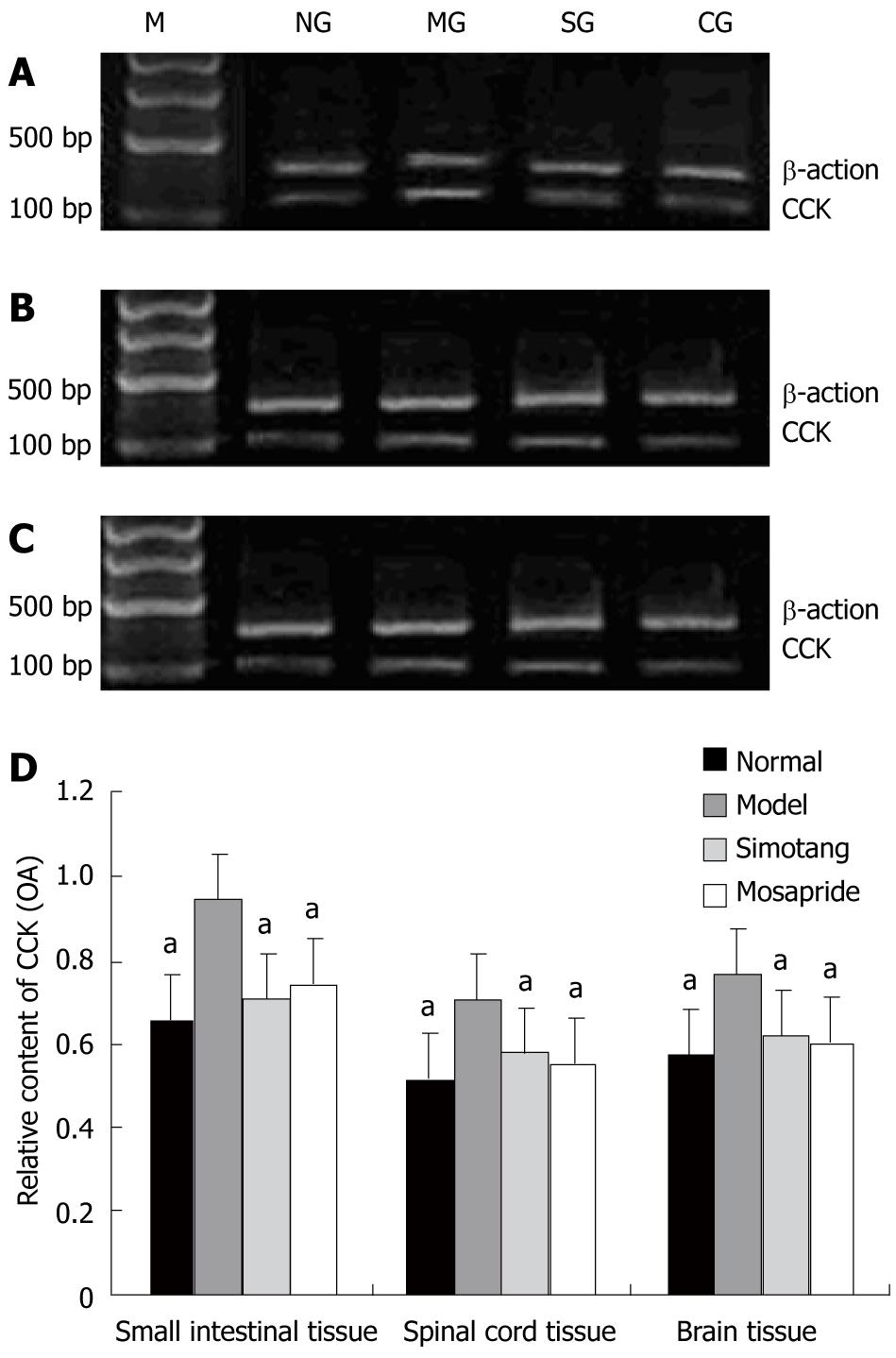
Total RNA was extracted using Trizol reagent (Invitrogen, CA, USA) according to its manufacturer’s instructions. RNA concentration was measured by 1.5% agarose gel electrophoresis. RT-PCR was performed using a TaKaRa RNA PCR kit (TaKaRa Biotechnology Co., Ltd., Dalian, China) and the sequences of primers used are 5'-TCCGTGCTTCTGCTAATA-3' for CCK forward primer and 5'-CAGCCATCACTGTCTTCC-3' (242 bp) for reverse primer, 5'-AGGGAAATCGTCGTGGAC-3' for β-actin forward primer and 5'-TGGAAGGTGGACAGTGAGG-3' (443 bp) for reverse primer. The PCR products were electrophoresed on 1.5% agarose gels and analyzed using the GIS-1000 digital gel image analysis system (Tanon Science & Technology Co., Ltd., Shanghai, China). Expression levels of CCK mRNA were normalized to those of β-actin mRNA. The experiments were performed at least in triplicate.
Data are expressed as mean ± SD. Statistical analysis was performed by one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Whether Simotang affects gastrointestinal motility in chronically stressed mice was observed by evaluating their gastric emptying and intestinal propulsion rates. The gastric emptying and intestinal propulsion rates were significantly lower in model group than in control group (P < 0.01, Table 1), suggesting that chronic stress leads to gastrointestinal motility disorder in chronically stressed mice. The gastric emptying and intestinal propulsion rates were significantly higher in Simotang or mosapride group than in model group (P < 0.01, Table 1).
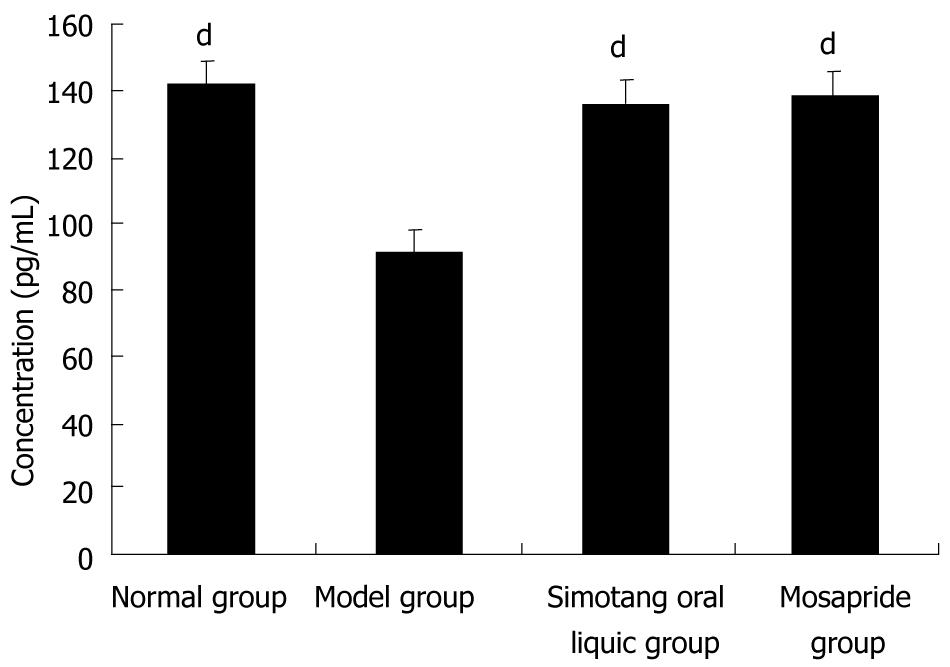
To determine the potential mechanism of Simotang underlying gastrointestinal motility, the serum motilin levels were measured in chronically stressed mice, showing that the serum motilin levels were significantly lower in model group than in control group and significantly higher after treatment with Simotang or mosapride (P < 0.01, Figure 1).
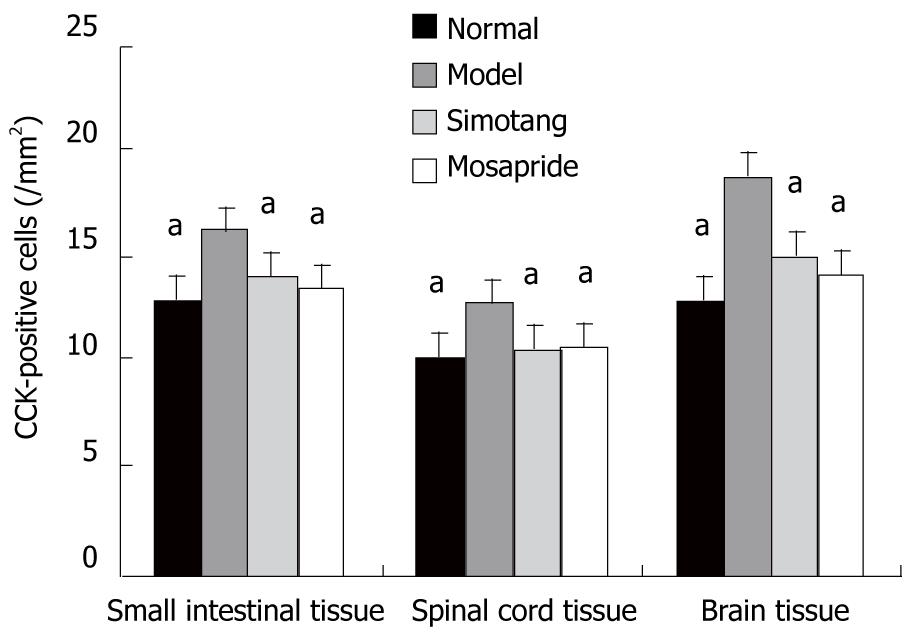
The expression level of CCK-positive cells in control group is shown in Figure 2A-C. The expression level of CCK-positive cell protein was significantly higher in model group than in control group (P < 0.01, Figure 2D-F) and remarkably lower in Simotang group (Figure 2G-I) and mosapride group (Figure 2J-L) than in model group (P < 0.01, Figure 3).
The expression level of CCK mRNA was significantly higher in model group than in control group and significantly lower in Simotang and mosapride groups than in model group after treatment with Simotang (P <0.01, Figure 4).
FD, a meal-related and pain-predominant symptom in the absence of organic disease according to the Rome III criteria[15], is considered hazardous to health because of its high prevalence and recurrence rates. Although its etiology and pathogenesis have not been clearly identified, a wide variety of pathogenetic mechanisms are involved. For example, gastrointestinal motor abnormality is thought to be an important mechanism underlying FD[16]. It has been reported that 20%-40% of FD patients have delayed gastric emptying and altered duodenojejunal motility[17,18]. With the development of neural gastroenterology in recent years, it has been shown that gastrointestinal motility is modulated by the central nervous system, autonomic nervous system and enteric nervous system (brain-gut axis) through hormones or brain-gut peptides, and that external stimuli can affect the motility or sensation of the gastrointestinal tract through the brain-gut axis[19]. Psychological factors are considered another important etiology of FD, and FD is currently considered to be a biopsychosocial disorder[20]. The proportion of psychosocial factors, such as depression and anxiety, is higher in FD patients than in healthy people, and psychosocial factors also affect gastric motility[21]. Stressed animals are often used as a model of FD. In the present study, the gastrointestinal motility was lower in chronically stressed mice than in unstressed control mice, which is consistent with the reported findings[22].
Motilin, a hormone secreted by endocrine cells in the duodenal mucosa, interacts directly with its receptor, induces smooth muscle contraction and improves peristalsis in the small intestine[23,24]. Its concentration is closely related with gastric emptying[25]. Although the role of motilin in the pathogenesis of FD has not yet been determined, the plasma motilin concentration is lower in FD patients than in healthy people[6]. FD patients have a similar proximal gastric motor response to motilin as healthy volunteers[24]. In the present study, the serum motilin level was significantly lower in stressed mice than in unstressed control mice and significantly higher after treatment with Simotang.
CCK is not only a gastrointestinal hormone, but also a neurotransmitter which is widely distributed in both the enteric and central nervous systems[7,8]. As an established brain-gut peptide, CCK transfers signals between the gut and central nervous system, plays an important role in regulation of gastrointestinal function, and is linked to the etiology of stress-related anxiety disorders. CCK not only inhibits gastric motility and emptying[26], but also is related to food intake and satiety[27]. It has been shown that the CCK levels are higher in FD patients than in controls[28]. Exogenous CCK can suppress the appetite and gastric emptying in healthy volunteers[29,30]. In the present study, the CCK protein and mRNA levels were increased not only in the small intestine but also in the spinal cord and brain of chronically stressed mice, and the CCK expression level was higher in the brain than in the small intestine and spinal cord of chronically stressed mice. Furthermore, the CCK expression level was higher in the small intestine, spinal cord and brain of chronically stressed mice after treatment with Simotang.
In conclusion, Simotang affects the gastrointestinal motility in chronically stressed mice by regulating the serum motilin level and expression of CCK, which provides a pharmacological basis for its clinical application in treatment of functional gastrointestinal disorders.
Simotang has been used in treatment of gastrointestinal disorders for hundreds of years but its mechanism is unknown. Dysfunction of the brain-gut axis is thought to be involved in the pathogenesis of functional dyspepsia.
As one of the brain-gut peptides, cholecystokinin (CCK) not only affects gastric motility and emptying, but also plays an important role in the pathogenesis of Functional dyspepsia (FD). However, how CCK is regulated by Simotang still remains unknown. In the current study, the authors demonstrated that chronic stress could inhibit gastric emptying and intestinal propulsion by investigating the mechanism of Simotang underlying gastrointestinal motility.
The results of this study show that Simotang can improve gastric emptying and intestinal propulsion in chronically stressed mice by regulating the serum motilin level and expression of cholecystokinin in the small intestine, spinal cord and brain of chronically stressed mice.
By understanding the mechanism of Simotang underlying FD and providing a pharmacological basis for Simotang, it can be used in treatment of functional gastrointestinal disorders.
The authors investigated the effect of Simotang on the expression of motilin and cholecystokinin, showing that Simotang can improve gastric emptying and intestinal propulsion in chronically stressed mice by regulating the expression of motilin and cholecystokinin, thus providing a pharmacological basis for the clinical application of Simotang in treatment of functional gastrointestinal disorders.
Peer reviewer: Jing-Bo Zhao, Associate Professor, Mech-Sense, Research House, AalborgHospital, Adr. Skovvej 15, Aalborg 9000, Denmark
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
| 1. | Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210-2216. |
| 2. | Zagari RM, Law GR, Fuccio L, Cennamo V, Gilthorpe MS, Forman D, Bazzoli F. Epidemiology of functional dyspepsia and subgroups in the Italian general population: an endoscopic study. Gastroenterology. 2010;138:1302-1311. |
| 3. | Chen MH, Zhong BH, Li CJ, Peng XZ, Hu PJ. An epidemiological study on dyspepsia in Guangdong area. Zhongguo Neike Zazhi. 1998;37:312-314. |
| 4. | Leung MW, Wong BP, Chao NS, Chung KW, Kwok WK, Liu KK. Electrogastrography in the management of pediatric functional dyspepsia and motility disorder. J Pediatr Surg. 2006;41:2069-2072. |
| 5. | Satoh M, Itoh Z. [Motilin and motilin receptor]. Nippon Rinsho. 1996;54:1092-1096. |
| 6. | Jonsson BH, Hellström PM. Motilin- and neuropeptide Y-like immunoreactivity in a psychophysiological stress experiment on patients with functional dyspepsia. Integr Physiol Behav Sci. 2000;35:256-265. |
| 7. | Raybould HE, Taché Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol. 1988;255:G242-246. |
| 8. | Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144-1152. |
| 9. | Chen SP, Wang XP. Effect of Simotang oral liquid on anal exhaust in patients after abdominal gynecological operation. Chin J Integr Med. 2006;12:221-223. |
| 10. | Lin X, Cai Y, Xie Y, Yi J, Li D, Liu J, Liu B, Cai G. Effect of Simo Juice Decoction on gastrointestinal function and Gastrin in binding-cold stressed mice. Hunan Zhongyiyao Daxue Xuebao. 2009;29:19-21. |
| 11. | Xie DP, Chen LB, Liu CY, Zhang CL, Liu KJ, Wang PS. Arecoline excites the colonic smooth muscle motility via M3 receptor in rabbits. Chin J Physiol. 2004;47:89-94. |
| 12. | Fang YS, Shan DM, Liu JW, Xu W, Li CL, Wu HZ, Ji G. Effect of constituents from Fructus Aurantii Immaturus and Radix Paeoniae Alba on gastrointestinal movement. Planta Med. 2009;75:24-31. |
| 13. | D’Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861-867. |
| 14. | Chen Q. Pharmacology Research Methodology. Beijing: People’s Medical Publishing House 1996; 133. |
| 15. | Halder SL, Talley NJ. Functional Dyspepsia: A New Rome III Paradigm. Curr Treat Options Gastroenterol. 2007;10:259-272. |
| 16. | Mizuta Y, Shikuwa S, Isomoto H, Mishima R, Akazawa Y, Masuda J, Omagari K, Takeshima F, Kohno S. Recent insights into digestive motility in functional dyspepsia. J Gastroenterol. 2006;41:1025-1040. |
| 17. | Stanghellini V, Ghidini C, Maccarini MR, Paparo GF, Corinaldesi R, Barbara L. Fasting and postprandial gastrointestinal motility in ulcer and non-ulcer dyspepsia. Gut. 1992;33:184-190. |
| 18. | Troncon LE, Bennett RJ, Ahluwalia NK, Thompson DG. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut. 1994;35:327-332. |
| 19. | Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919-933. |
| 20. | Talley NJ, Herrick L, Locke GR. Antidepressants in functional dyspepsia. Expert Rev Gastroenterol Hepatol. 2010;4:5-8. |
| 21. | Hsu YC, Liou JM, Liao SC, Yang TH, Wu HT, Hsu WL, Lin HJ, Wang HP, Wu MS. Psychopathology and personality trait in subgroups of functional dyspepsia based on Rome III criteria. Am J Gastroenterol. 2009;104:2534-2542. |
| 22. | Seto K, Sasaki T, Katsunuma K, Kobayashi N, Tanaka K, Tack J. Acotiamide hydrochloride (Z-338), a novel prokinetic agent, restores delayed gastric emptying and feeding inhibition induced by restraint stress in rats. Neurogastroenterol Motil. 2008;20:1051-1059. |
| 23. | Kamerling IM, Van Haarst AD, Burggraaf J, Schoemaker HC, Biemond I, Jones R, Cohen AF, Masclee AA. Dose-related effects of motilin on proximal gastrointestinal motility. Aliment Pharmacol Ther. 2002;16:129-135. |
| 24. | Kamerling IM, Van Haarst AD, Burggraaf J, Schoemaker RC, Biemond I, Heinzerling H, Jones R, Cohen AF, Masclee AA. Motilin effects on the proximal stomach in patients with functional dyspepsia and healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2003;284:G776-G781. |
| 25. | Yang M, Fang DC, Li QW, Sun NX, Long QL, Sui JF, Gan L. Effects of gastric pacing on gastric emptying and plasma motilin. World J Gastroenterol. 2004;10:419-423. |
| 26. | Kissileff HR, Carretta JC, Geliebter A, Pi-Sunyer FX. Cholecystokinin and stomach distension combine to reduce food intake in humans. Am J Physiol Regul Integr Comp Physiol. 2003;285:R992-R998. |
| 27. | Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131-1158. |
| 28. | Chua AS, Keeling PW, Dinan TG. Role of cholecystokinin and central serotonergic receptors in functional dyspepsia. World J Gastroenterol. 2006;12:1329-1335. |
| 29. | Brennan IM, Little TJ, Feltrin KL, Smout AJ, Wishart JM, Horowitz M, Feinle-Bisset C. Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E1487-E1494. |
| 30. | Geary N. Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav. 2004;81:719-733. |