Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1410
Revised: December 9, 2010
Accepted: December 16, 2010
Published online: March 21, 2011
Non-cirrhotic portal hypertension (PHT) accounts for about 20% of all PHT cases, portal vein thrombosis (PVT) resulting in cavernous transformation being the most common cause. All known complications of PHT may be encountered in patients with chronic PVT. However, the effect of this entity on the biliary tree and pancreatic duct has not yet been fully established. Additionally, a dispute remains regarding the nomenclature of common bile duct abnormalities which occur as a result of chronic PVT. Although many clinical reports have focused on biliary abnormalities, only a few have evaluated both the biliary and pancreatic ductal systems. In this review the relevant literature evaluating the effect of PVT on both ductal systems is discussed, and findings are considered with reference to results of a prominent center in Turkey, from which the term “portal ductopathy” has been put forth to replace “portal biliopathy”.
- Citation: Bayraktar Y. Portal ductopathy: Clinical importance and nomenclature. World J Gastroenterol 2011; 17(11): 1410-1415
- URL: https://www.wjgnet.com/1007-9327/full/v17/i11/1410.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i11.1410
Although liver cirrhosis is a major cause of portal hypertension (PHT), in 20% of cases PHT is classified as non-cirrhotic, occurring as a result of portal vein thrombosis (PVT), congenital hepatic fibrosis, idiopathic PHT and other rare disorders. The portal vein, which is 12 mm in diameter, carries blood from intra-abdominal organs to the liver at a rate of approximately 1200 mL/min. Thrombotic occlusion of the portal vein, whatever the cause, is rapidly followed by compensatory mechanisms such as attempts at re-canalization and the development of new collaterals around the occluded portal vein, bile ducts and gall bladder, aimed at reestablishing portal blood flow to the liver. The portal vein is eventually replaced by a “cavernoma” after what is now known as portal vein cavernous transformation (PVCT). Splenomegaly, esophageal and gastric varices, portal gastropathy, and rarely ascites, are well recognized and extensively studied complications of PHT due to PVCT. However, the effects of PVCT on the biliary tree and pancreatic duct are as yet to be unequivocally identified. Furthermore, a dispute remains regarding the nomenclature of common bile duct (CBD) abnormalities which occur as a result of PVCT. Till today, many of the published case series have described biliary abnormalities resulting from PVT, but only a few have focused on both duct systems, the biliary and pancreatic[1,2].
In a prospective study published in 1992, abnormalities of the biliary tree in patients with PVCT which resulted in an appearance mimicking cholangiocellular carcinoma on endoscopic retrograde cholangiopancreatography (ERCP) were described[3]. Meanwhile the descriptive terms “pseudosclerosing cholangitis”[4] and “portal biliopathy” have also been introduced into the literature[5]. Till today, the issue of proper nomenclature of this phenomenon has not been sufficiently discussed, and there is a dire need for clarification.
Since the introduction of the term “pseudocholangiocellular carcinoma sign” to describe radiologic abnormalities mimicking cholangiocarcinoma caused by the compression of bile ducts by the thrombosed portal vein and its collaterals[3], several different terms have been coined, including “portal biliopathy”[6], “cholangiopathy associated with portal hypertension”[7], and “portal cavernoma-associated cholangiopathy”[8]. Finally, Dhiman et al[9] proposed the term “portal hypertensive biliopathy” to refer to abnormalities of the biliary tree, cystic duct and gall bladder in patients with PHT.
It would seem that “pseudo sclerosing cholangitis” and “portal biliopathy” do not appropriately represent or define abnormalities of the biliary system which occur as a result of PHT. Biliary strictures in patient with PVCT are smooth rather than irregular, making the term “pseudosclerosing cholangitis” an erroneous description[4]. “Portal biliopathy” is also a misnomer as it implies abnormal content of bile, which has never been reported before in any of the studies describing abnormalities of the biliary tree. Although in cases of PVCT jaundice is a common clinical finding, bile composition is considered to be normal. Additionally, the term “biliopathy,” suggests that the pathology is limited to the biliary tree, whereas PVCT has been shown to also affect the pancreatic ducts in most patients. Moreover, ERCP findings of cholangiocarcinomas rarely resemble those associated with PVCT, which also renders the term “pseudo-cholangiocarcinoma sign” inadequate.
The pancreatic ducts of patients with PVCT have been thoroughly evaluated at Hacettepe University for the past two decades (since 1992), where 78 patients with PVCT have undergone ERCP procedures. Seventy of the 78 (90%) patients had involvement of the biliary tree, 54 of whom (70%) had both biliary and pancreatic duct involvement. Considering that PVCT results in “morphological” abnormalities in both ductal systems, it would be expected the nomenclature should reflect the ductal changes observed in these patients instead of misleading physicians to associate this entity with changes in biliary content. The term “portal ductopathy” may provide a more satisfactory means of depicting abnormalities seen in the biliary and pancreatic duct systems in patients with PVT.
PVT was first described by Balfour et al[10] in 1964. The re-canalization of the thrombosed portal vein at the hepatic hilum leads to this clinical and radiological condition. Ohnishi et al[11] demonstrated that after complete obstruction, the “venous rescue” begins immediately and is completed in about 5 wk. The newly formed small collaterals are mostly observed around the intrahepatic and extrahepatic biliary tract, cystic duct and around the gall bladder. There are two venous plexuses of the bile ducts and gall bladder; the so-called epicholedochal venous plexus of Saint[12], and the paracholedochal veins of Petren[13]. Saint’s plexus, which forms a fine reticular web located on the outer surface of the CBD and hepatic ducts, becomes dilated and causes fine irregularities in the biliary tract[12-14]. Petren’s plexus, on the other hand, runs parallel to the CBD and is connected to the gastric, pancreaticoduodenal and portal veins and to the liver directly. When the portal vein is occluded by a thrombus or tumor, both plexuses become dilated and cause extrinsic compression of the CBD. External compression and protrusion of these newly formed vessels on the biliary tree have been shown to be responsible for portal ductopathy in the biliary tree. However, the reasons behind changes to the pancreatic duct are yet to be elucidated. Extension of newly formed vessels towards the pancreas may be implicated, although this remains largely speculative.
In spite of the well established role of newly developed vessels around the bile system in the development of biliary abnormalities (more appropriately called portal ductopathy), most studies have overlooked other important factors. Ischemia, fibrosis, direct compression of the thrombosed vessels and excessive connective tissue formation around the biliary system have a major impact on the formation of biliary abnormalities. These factors contribute to the formation of a “frozen portal hilum” so that even if the PHT is relieved by any effective means, the cholestasis usually does not improve[15-17]. The entire process mimics the reaction of wound healing in which neovascularization, collagen formation and tissue turnover occur and re-cycle for a long period of time. Duration of PVT does not seem to have an effect on the extent of the radiological appearance of the ductal abnormalities. Biliary strictures leading to complete biliary obstruction may be caused by ischemia or by encasement within a solid tumor-like cavernoma[18]. The mechanism of ischemia causing bile duct changes in patients with PVCT remains unexplained. Venous damage due to portal thrombosis results in ischemic necrosis of bile ducts by compressing the vascular supply at the level of capillaries and the arterioles[9], resulting in biliary strictures and cholangiectasis[19]. Segmental strictures and dilatations seen on ERCP may involve both intra- and extra-hepatic bile ducts, morphologically very similar to those seen in ischemic cholangiopathy after liver transplantation[20].
In a prospective study[21], the biliary tree, either intra- or extra-hepatic, was found to be affected in almost all patients with known PVCT. Additionally, pancreatic duct abnormalities were apparent in a large proportion of this patient group. Use of the term “portal double ductopathy” was suggested to describe involvement of both systems. Involvement of any of the ductal systems individually would be referred to as either “portal biliary ductopathy” or “portal pancreatic ductopathy”.
It is well known that PHT, whatever its etiology, results in many complications such as ascites, portal gastropathy, esophageal and gastric varices, hypersplenism and severe coagulopathy, which pose a great challenge for clinicians in daily medical practice. Although PVT has been associated with many biliary abnormalities, the majority of cases are asymptomatic and only a small percentage of this patient population develop signs and symptoms of biliary obstruction, presenting with jaundice, pruritus, fever and abdominal pain.
Cholestasis, one of the main clinical features of portal biliary ductopathy, may be explained by the mass effect of enlarged collaterals or chronic thrombus compressing on the intra- and/or extra-hepatic biliary lumen. Ensuing ischemia and fibrosis may also be implicated. In some cases compression may be so severe as to result in secondary biliary cirrhosis due to longstanding severe cholestasis. Fortunately this complication is rare; one which at Hacettepe University has been encountered in only 2 cases, both of whom eventually underwent liver transplantation. Both patients are still under follow-up and are healthy, productive members of society. Mild jaundice seen in these cases because of incomplete obstruction of CBD is not uncommon, usually leading to unnecessary investigative tests towards the cause of the direct hyperbilirubinemia. Cholestatic enzymes are generally elevated in parallel with bilirubin levels.
PVCT has been reported to result in an increase in the frequency of biliary stone disease and related complications. Regardless of age, sex and underlying etiology, an association between PVCT and an increased incidence of biliary tree diseases has consistently been reported in the literature[4,6,7,15,22]. In such cases, direct bilirubin levels are quite elevated. Stone formation is facilitated by the chronic but incomplete obstruction caused by the above-mentioned factors. It is necessary to stress that incomplete obstruction at multiple levels of the intra- and extra-hepatic biliary system may exist simultaneously. The occurrence of fever and abdominal pain during follow-up of a patient with biliary stones associated with PVCT should raise a suspicion of cholangitis.
According to Dhiman et al[9], choledocal varices were observed in 7.5% of cases with PVCT. It is important to note that these varices may bleed severely, thus complicating the clinical picture. Additionally, endoscopic procedures such as stenting and stone extraction may also result in bleeding from these otherwise silent varices[23-25]. Endoscopic ultrasonography (EUS) with Doppler is a particularly useful technique in diagnosing bile duct varices and differentiating them from bile duct stones. Great care should be taken when undertaking interventional procedures such as stone extraction and sphincterotomy, as even gentle balloon dilatation may lead to bleeding from these small varices. Of note, such patients may already have thrombocytopenia and some degree of coagulopathy because of splenomegaly and tissue congestion caused by PHT. Liver function tests are typically normal in patients with PVT in the absence of an underlying disorder such as polycythemia vera or Behçet’s disease. However, according to unpublished data at Hacettepe University, most patients have prolonged prothrombin times compared to healthy controls, without having any other signs of compromised liver function, an entity which as yet remains unexplained.
Chronic congestion due to PHT affects almost all intra-abdominal organs, including the pancreas. The effects of PVCT on pancreatic parenchyma and duct have not been fully established. In the only study to investigate pancreatic exocrine function in this patient group, Egesel et al[21] demonstrated that the pancreatic ducts of PVCT patients tended to be smaller than normal controls. Additionally, in 15 of the 18 patients with chronic PVT who had pancreatic atrophy, they found that urinary excretion of para-aminobenzoic acid was significantly less than in control subjects. Moreover, the authors have attributed some uncertain symptoms in these patients, such as abdominal discomfort, abdominal pain and anorexia, to latent pancreatic insufficiency shown by bentiromide test. As the pancreas has a tremendous capacity to manipulate the body needs, the manifestation of symptoms occurs after a latent period, requiring significant pancreatic parenchymal pathology. More sensitive tests are needed to clarify the extent of exocrine and endocrine dysfunction of the pancreas associated with this disorder.
Three other studies have demonstrated significant changes in the pancreatic ducts of patients with PVCT[1,2,21]. In a report published in 2008[1], 31 of 36 (86.1%) patients with PVT had luminal narrowing throughout the pancreatic duct, local atrophy at the head of pancreas with moderate dilatation behind the narrowed segment and other unclassified pancreatic duct abnormalities. Since 2008, 22 more patients with PVCT due to portal thrombosis have been evaluated at Hacettepe University, 16 of whom (72%) had pancreatic duct abnormalities demonstrated by ERCP. In total, 78 patients with PVCT have been seen since 1992, all of whom underwent ERCP as part of a work-up for unexplained elevations in ALP, GGT and direct bilirubin levels. Approximately 70% of patients had pancreatic abnormalities. Although the clinical significance of these ductal abnormalities has not been well delineated, as stated above, partial pancreatic insufficiency and some other patient complaints such as abdominal pain and dyspepsia may be explained by chronic congestion due to PHT. It is possible that PVT contributes to more severe pancreatic congestion when compared to cirrhotic causes of PHT, as extension of the thrombus to the splenic vein may hinder pancreatic venous drainage. As a result, pancreatic duct and parenchyma may be more significantly affected. Further studies are needed to investigate this phenomenon.
Characteristically, the majority of patients with PVCT have a predominant cholestatic pattern of elevated liver enzymes. This biochemical finding reflects the biliary duct changes secondary to PVCT. Despite the presence of PHT manifesting as massive splenomegaly and large esophageal varices, serum albumin levels are usually within normal limits, unless massive bleeding occurs. Mild ALP and GGT elevations occur at any one time during the follow-up period of such patients. Clinicians must bear in mind that portal ductopathy may be responsible for such mild elevations, in order to avoid further unnecessary testing towards a cause of the cholestatic picture. In the presence of biliary strictures or stones, more marked elevations in cholestatic enzymes and bilirubin levels may be observed.
Although not part of the diagnostic work-up for portal ductopathy, a liver biopsy is essential in establishing whether PVT is due to cirrhotic or non-cirrhotic causes. It is necessary to perform this procedure to rule out the presence of liver cirrhosis, particularly in patients with atrophic livers with heterogeneous parenchyma on sonographic examination. Usually liver biopsies show normal or nearly normal histology, sometimes with signs of portal vein dilatation in the portal tract, or segmental luminal narrowing of bile ducts with dilated small intrahepatic bile ducts. In congenital hepatic fibrosis, where histopathological examination is vital for making a diagnosis, portal vein abnormalities mimicking PVCT are relatively more common[26,27].
Ultrasonography has traditionally been the most widely utilized modality for demonstrating biliary duct abnormalities in patients with chronic PVT. However, this technique has many shortcomings. For example, the presence of a high level of echoes in the porta hepatis may obscure the biliary system. Similarly, CBD may be hidden behind multiple collaterals demonstrating themselves as anechoic tubular and fibrotic structures. Color Doppler examination may help confirm the presence of multiple tortuous structures in the porta hepatis of patients with PVCT. The nature of these tubular structures may not be correctly identified as blood vessels initially on grayscale. Real time and Doppler sonographic findings compatible with PVCT may prompt further evaluation by splenoportography, either with digital subtraction angiography or by computed tomography to confirm the diagnosis.
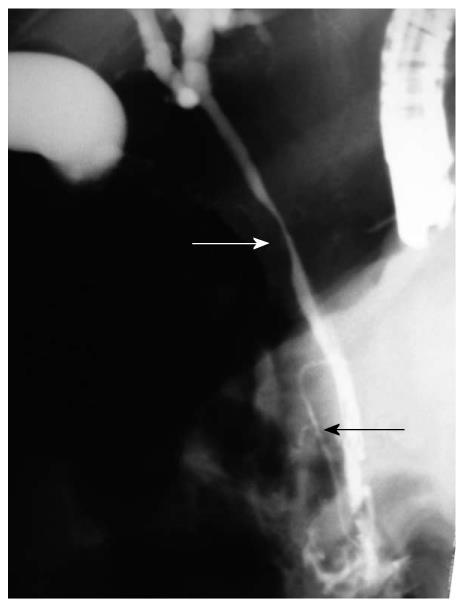
This modality has established itself as one the most important procedures in diagnosing and defining the extent of involvement of the intrahepatic and/or extrahepatic biliary tree. As PVT may occur in either or both intra- and extra-hepatic portions of the portal vein, any part of the biliary system, including the gall bladder, may be affected by this thrombotic event. The development of cavernous changes, located at the portal hilum, may still affect the left and right intrahepatic biliary channels, usually manifesting as dilatations. Changes described so far include undulation on the CBD along with narrowing and irregularity of various lengths and degree, sometimes leading to nearly complete obstruction (Figure 1), as well as segmental upstream and asymmetrical dilatation.
In contrast to obstruction of the CBD where both intrahepatic and extrahepatic bile ducts are proportionately dilated above the level of the obstruction, the most consistent radiologic finding that has been encountered at Hacettepe University is that the CBD tended to be narrower than the intrahepatic biliary ducts. In other words, the left or right hepatic ducts were usually dilated either alone or in combination with a dilated common hepatic duct.
Irregularities on the gallbladder wall may be seen, most probably because of intraluminal varices in a few cases. These varices may also be present in the CBD, manifesting themselves as filling defects, although rarely leading to bleeding, or so-called hemobilia.
There are mainly three types of pancreatic duct abnormalities: (1) Diffuse pancreatic duct abnormality in which the whole duct is narrowed with kinking, distortion of the normal anatomic pathway, thumb-printing type of compression and with local luminal irregularities. The whole pancreatic duct is atrophic (Figure 1); (2) Proximal pancreatic duct abnormality in which the narrow part of the duct is limited to the level of head of pancreas, in contrast to the normal pancreatic duct in which the widest caliber is at the head of pancreas. Interestingly, the distal segment of these ducts appeared relatively dilated compared to the head region; and (3) unclassified abnormalities in which multiple small ducts connect to each other (different from pancreas divisum). Indentation, displacement and angulations occur.
Although splenoportography is invasive with several reported complications, we have been utilizing this procedure in our hospital for a long time for the diagnosis of PVCT without the occurrence of any adverse effects. This technique is best for providing a clear image of the PVCT as well as other collaterals; however it does not evaluate the biliary system. Nowadays the use of CT portography as a less invasive imaging modality may be preferred.
This procedure which helps to diagnose portal vein obstruction is particularly useful in identifying the presence of cavernous transformation, as well as for evaluating the dimension of bile duct abnormalities. Recently, CT portography has been introduced as an alternative to conventional angiographic splenoportography.
Not only is this technique non–invasive, but it is also more informative in that it allows for clear visualization of portal vein collaterals when confirming the presence of a cavernoma. However, in some cases ERCP is superior with regards to evaluation of the bile duct system. With the advent of high-resolution magnetic resonance (MR), MR cholangiography (MRCP) may eventually replace ERCP as the modality of choice for examining abnormalities of the intra-hepatic bile ducts, as it offers the advantage of being less invasive with fewer associated complications. If available, MRCP with MR portographic evaluation should follow real time or Doppler ultrasonography when investigating the bile duct system and portal vein.
After conventional ultrasonography and Doppler ultrasound, the advent of EUS has been particularly useful in identifying CBD varices and/or bile duct stones, both of which may be the primary cause of biliary obstruction in patients with PVCT[28]. Recognizing stones and differentiating them from varices is important as any intervention in the form of balloon dilatation or stone extraction may result in severe bleeding. In patients where an obstructive clinical picture is predominant, EUS with Doppler should be performed to properly identify the cause of the obstruction, whether it is due to bile duct varices, stones, strictures or a tumor. In this perspective, EUS is a quite useful, even inevitable procedure.
Most patients with portal ductopathy who are asymptomatic do not require any treatment. When symptoms due to stone formation, obstructive jaundice and cholangitis occur, treatment should be adjusted individually according to patient characteristics. Naturally, the occurrence of PVT warrants investigation into the cause, whether there is an underlying myeloproliferative disorder, deficiency of anticoagulant proteins, or an autoimmune disease. In the presence of an underlying thrombophilic condition, anticoagulant treatment may be indicated. On a different note, a proportion of patients may first present with variceal bleeding from the upper GI tract. Although the management of variceal bleeding is beyond the scope of this paper, it is important to stress that bleeding from gastric and esophageal varices may prove very challenging.
As mentioned before, PVCT results in a pathological condition involving ductular organs; CBD and pancreatic duct. Since bile composition is not an issue, all efforts should instead focus on the management of strictures, stones or sludge in the biliary tree. In cases such as these, endoscopic papillotomy and, if indicated, stone extraction and balloon dilatation, are the treatment modalities of choice. In some cases, concomitant presence of severe biliary stricture and biliary stones may be observed[14]. This poses a challenge for the endoscopist, since after performing a sphincterotomy followed by stricture dilatation, great care should be taken while extracting any stone, since underlying thrombocytopenia due to hypersplenism and the presence of small or large varices in the peri-ampullary area and inside the CBD result in the risk of severe bleeding, further complicating an already complex and delicate clinical condition. In fact, some cases may even require the use of a mechanical lithotripter to crush large stones into small pieces.
Liver transplantation should be reserved for patients who develop secondary biliary cirrhosis or severe liver failure. At Hacettepe University, only 2 female patients developed secondary biliary cirrhosis due to biliary strictures and stone formation, both of whom underwent successful liver transplantation. To date, both patients are under follow-up with no significant restrictions in their daily activities.
Several disorders result in thrombosis of the portal vein which eventually undergoes cavernous transformation. The intra- and extra-hepatic bile ducts are affected by these changes in almost all cases, with relatively less frequent involvement of the pancreatic duct. Although several terms such as “portal biliopathy” and “pseudocholangiocarcinoma sign” have been postulated to describe these changes, to allay any doubts regarding abnormalities in bile composition, use of the term “portal ductopathy” may be more appropriate. Involvement of both duct systems may be further described as “portal double ductopathy”. Clinical implications of portal ductopathy consist of cholestasis, stone formation, and consequently cholangitis.
Peer reviewer: Gianpiero Gravante, BSc (Hons), MBBS (Hons), Clinical Fellow in Upper Gastrointestinal Surgery, Frenchay Hospital, North Bristol NHS Trust, Flat 8 Room 25, Frenchay Park Road, Bristol, BS16 1LE, United Kingdom
S- Editor Sun H L- Editor Logan S E- Editor Zheng XM
| 1. | Bayraktar Y, Harmanci O, Ersoy O, Aydinli M, Balkanci F. "Portal double ductopathy sign" in patients with portal vein cavernous transformation. Hepatogastroenterology. 2008;55:1193-1200. |
| 2. | Bayraktar Y, Balkanci F, Bayraktar M, Koseoglu T, Serap Arslan, Uzunalimoglu B, Van Thiel DH, Kaythan B. Cavernous transformation of the portal vein is associated with pancreatic duct atrophy. Hepatogastroenterology. 1996;43:954-960. |
| 3. | Bayraktar Y, Balkanci F, Kayhan B, Ozenç A, Arslan S, Telatar H. Bile duct varices or "pseudo-cholangiocarcinoma sign" in portal hypertension due to cavernous transformation of the portal vein. Am J Gastroenterol. 1992;87:1801-1806. |
| 4. | Dilawari JB, Chawla YK. Pseudosclerosing cholangitis in extrahepatic portal venous obstruction. Gut. 1992;33:272-276. |
| 5. | Chandra R, Kapoor D, Tharakan A, Chaudhary A, Sarin SK. Portal biliopathy. J Gastroenterol Hepatol. 2001;16:1086-1092. |
| 6. | Sarin SK, Bhatia V, Makwana U. Poratal biliopathy in extrahepatic portal venous obstruction [abstract]. Indian J Gastroenterol. 1992;11 (Suppl 1): A82. |
| 7. | Malkan GH, Bhatia SJ, Bashir K, Khemani R, Abraham P, Gandhi MS, Radhakrishnan R. Cholangiopathy associated with portal hypertension: diagnostic evaluation and clinical implications. Gastrointest Endosc. 1999;49:344-348. |
| 8. | Condat B, Vilgrain V, Asselah T, O'Toole D, Rufat P, Zappa M, Moreau R, Valla D. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003;37:1302-1308. |
| 9. | Dhiman RK, Behera A, Chawla YK, Dilawari JB, Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001-1008. |
| 10. | Balfour GW, Stewart TG. Case of enlarged spleen complicated with ascites, both depending upon varicose dilatation and thrombosis of portal vein. Edinburgh Med J 1869; 14: 589-598 . . |
| 11. | Ohnishi K, Okuda K, Ohtsuki T, Nakayama T, Hiyama Y, Iwama S, Goto N, Nakajima Y, Musha N, Nakashima T. Formation of hilar collaterals or cavernous transformation after portal vein obstruction by hepatocellular carcinoma. Observations in ten patients. Gastroenterology. 1984;87:1150-1153. |
| 12. | Saint JH. The epicholedochal venous plexus and its importance as a means of identifying the common duct during operations on the extrahepatic biliary tract. Br J Surg. 1961;48:489-498. |
| 13. | Petren T. Die extrahepatischen Gallengangsvenen und ihre pathologisch-anatomische Bedeutung. Verh Anat Ges. 1932;41:139-143. |
| 14. | Dhiman RK, Puri P, Chawla Y, Minz M, Bapuraj JR, Gupta S, Nagi B, Suri S. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646-652. |
| 15. | Khuroo MS, Yattoo GN, Zargar SA, Javid G, Dar MY, Khan BA, Boda MI. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807-813. |
| 16. | Mörk H, Weber P, Schmidt H, Goerig RM, Scheurlen M. Cavernous transformation of the portal vein associated with common bile duct strictures: report of two cases. Gastrointest Endosc. 1998;47:79-83. |
| 17. | Henne-Bruns D, Kremer B, Soehendra N. [Cavernous transformation of the portal vein. A rare cause of mechanical obstructive jaundice]. Chirurg. 1989;60:704-706. |
| 18. | Condat B, Pessione F, Helene Denninger M, Hillaire S, Valla D. Recent portal or mesenteric venous thrombosis: increased recognition and frequent recanalization on anticoagulant therapy. Hepatology. 2000;32:466-470. |
| 19. | Batts KP. Ischemic cholangitis. Mayo Clin Proc. 1998;73:380-385. |
| 20. | Cameron AM, Busuttil RW. Ischemic cholangiopathy after liver transplantation. Hepatobiliary Pancreat Dis Int. 2005;4:495-501. |
| 21. | Egesel T, Unsal I, Dikmen G, Bayraktar Y. The assessment of pancreatic exocrine function by bentiromide test in patients with chronic portal vein thrombosis. Pancreas. 2002;25:355-359. |
| 22. | Nagi B, Kochhar R, Bhasin D, Singh K. Cholangiopathy in extrahepatic portal venous obstruction. Radiological appearances. Acta Radiol. 2000;41:612-615. |
| 23. | Sezgin O, Oğuz D, Altintaş E, Saritaş U, Sahin B. Endoscopic management of biliary obstruction caused by cavernous transformation of the portal vein. Gastrointest Endosc. 2003;58:602-608. |
| 24. | Tighe M, Jacobson I. Bleeding from bile duct varices: an unexpected hazard during therapeutic ERCP. Gastrointest Endosc. 1996;43:250-252. |
| 25. | Mutignani M, Shah SK, Bruni A, Perri V, Costamagna G. Endoscopic treatment of extrahepatic bile duct strictures in patients with portal biliopathy carries a high risk of haemobilia: report of 3 cases. Dig Liver Dis. 2002;34:587-591. |
| 26. | Yonem O, Bayraktar Y. Is portal vein cavernous transformation a component of congenital hepatic fibrosis? World J Gastroenterol. 2007;13:1928-1929. |
| 27. | Yönem O, Ozkayar N, Balkanci F, Harmanci O, Sökmensüer C, Ersoy O, Bayraktar Y. Is congenital hepatic fibrosis a pure liver disease? Am J Gastroenterol. 2006;101:1253-1259. |