INTRODUCTION
It is well known that angiogenesis is critical in the transition from premalignant to malignant lesions. Consequently, early detection and diagnosis based on morphologic changes to the microvessels are crucial. Conventional endoscopic diagnosis using white light is based on subtle morphological changes such as superficially elevated, flat, or depressed lesions and minimal changes in color. However, these findings are difficult to recognize, especially for inexperienced endoscopists. As a result, the diagnosis may be inaccurate or a superficial cancer in the gastrointestinal (GI) tract may be overlooked.
New imaging techniques which utilize the properties of light-tissue interaction have recently been developed to enhance early diagnosis of GI tract neoplasia. Endoscopic autofluorescence imaging (AFI) produces real-time pseudocolor images based on the detection of natural tissue fluorescence generated from endogenous fluorophores (collagen, nicotinamide, adenine dinucleotide, flavin and porphyrins) through emission induced by excitation light. The system can visualize lesions, including malignancies, by differences in tissue fluorescence properties and can reveal early stage cancers not detectable by conventional white light endoscopy (WLE)[1,2]. Magnifying endoscopy with narrow band imaging (ME-NBI) represents a real-time endoscopic imaging technique which enhances visualization of the surface texture and the vascular network of the mucosa with the aim of improving tissue characterization and differentiation[3].
AUTOFLUORESCENCE IMAGING
The principle of autofluorescence diagnosis is based on the interaction between light with a specific wavelength and tissue fluorophores. When tissues are exposed to short wavelength light, endogenous fluorophores (collagen, nicotinamide, adenine dinucleotide, flavin and porphyrins) are excited, leading to the emission of fluorescent light of a longer wavelength (i.e. autofluorescence)[4]. Normal, inflamed and neoplastic tissue have different autofluorescence characteristics that may thus enable their differentiation. Thus, normal tissue is pseudocolored as green, blood vessels as dark green, while hypertrophic fundic mucosa of the stomach and dysplastic/neoplastic areas appear as magenta. During AFI, a suspected neoplasia (AFI-positive lesion) is defined as any area that is different in color from the surrounding mucosa, and which has a defined circumferential margin[5].
Autofluorescence is abnormal in neoplastic tissues due to several mechanisms: (1) increase in the nuclear-cytoplasmic ratio, which consequently determines decreased autofluorescence as nuclei show no autofluorescence as compared with cytoplasm; (2) loss of collagen, submucosal collagen is the strongest fluorophore which disappears due to thickening of the mucosa; and (3) neovascularization, inducing increased hemoglobin concentration which absorbs autofluorescence light[6].
Several published studies showed an increased sensitivity for the detection of high-grade dysplasia and early cancer in the GI tract when autofluorescence techniques were used[1,2,5-9]. Kara et al[7] showed the effectiveness of AFI in identifying high-grade dysplasia and early cancer in patients with Barrett′s esophagus. Compared with WLE and random 4-quadrant biopsies, AFI increased the detection of high-grade dysplasia and esophageal adenocarcinoma by an additional 6 out of 60 patients, representing an increase of 10%, from 23% to 33%. False-positive lesions were determined by the presence of acute inflammation. On the other hand, Kara et al[8] showed that fluorescence imaging with light-induced fluorescence endoscopy (LIFE) using a fiber-optic endoscope was no better than standard WLE for the detection of high-grade dysplasia and early cancer in a randomized crossover study of patients with Barrett′s esophagus. Another study tested the diagnostic performance of AFI for early gastric neoplasms, thus Ohkawa et al[9] concluded that LIFE is highly sensitive (sensitivity 96.4%) but not very specific (specificity 49.1%), since 50.9% of benign lesions were also identified as having abnormal fluorescence images. Using the latest technology incorporated in AFI systems, Kato et al[5] obtained similar results, with approximately 25% of superficial elevated neoplasia diagnosed only by AFI and missed by WLE. In principle, a superficial elevated neoplasm of a similar hue to the surrounding mucosa might be overlooked during WLE, but it can be revealed by AFI if the elevated neoplasm appears as magenta within a normal looking green mucosa (Figure 1, Figure 2, Figure 3, Figure 4).
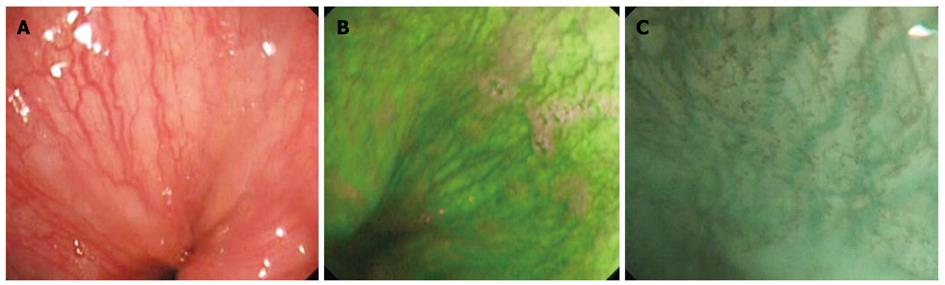
Figure 1 Normal esophageal mucosa.
A: Normal vascular pattern above the gastroesophageal (GE) junction visualized in white light endoscopy; B: Autofluorescence imaging of the normal mucosa and vascular pattern above the GE junction; C: Magnifying endoscopy with narrow band imaging depicting the submucosal vessels in cyan and intrapapillary capillary loops in brown.
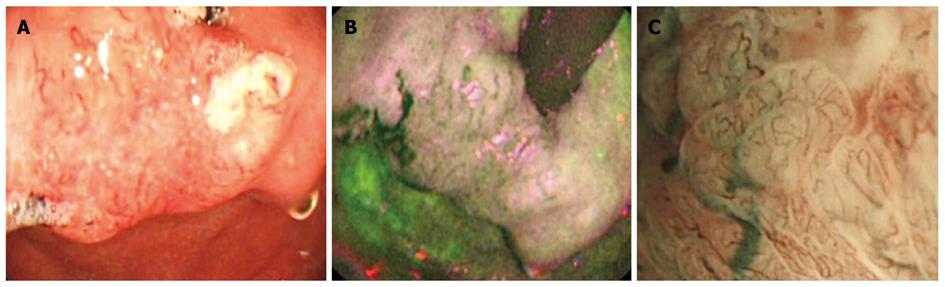
Figure 2 Esophageal squamous cell carcinoma invading the gastroesophageal junction.
A: Elevated irregular mucosa with abnormal vascular pattern, difficult to see in white light endoscopy in retroflexion, immediately below the gastroesophageal junction; B: Autofluorescence imaging showing the lesion extension in magenta, with surrounding green normal mucosa; C: Magnifying endoscopy with narrow band imaging showing irregular, thick and distorted mucosal vessels characteristic for tumor angiogenesis.
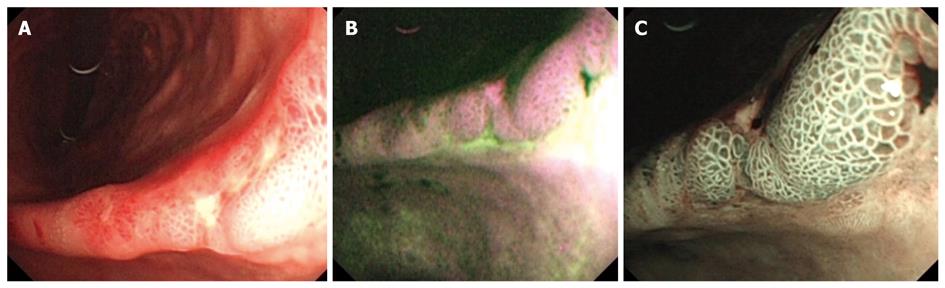
Figure 3 Early gastric adenocarcinoma at the level of the gastric angle.
A: Irregular ulcer visualized in white light endoscopy (WLE); B: Autofluorescence imaging showing in magenta the neoplastic margins and a larger lesion extension, as compared with WLE; C: Magnifying endoscopy with narrow band imaging showing a modified pit pattern, with irregular and distorted vascular pattern in the center suggesting high-grade dysplasia/ early cancer, and with villous pits and light blue crest sign in the margins suggesting intestinal metaplasia.
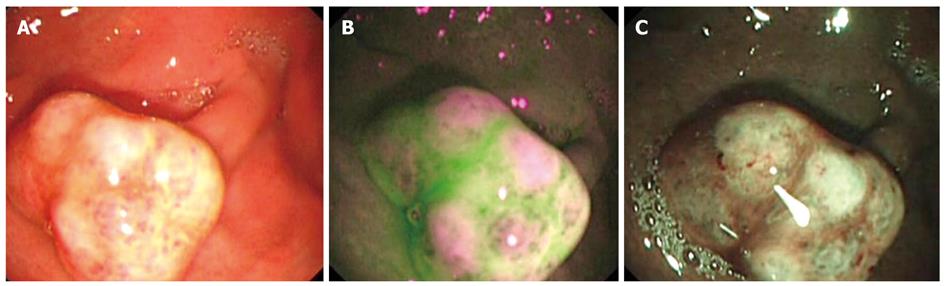
Figure 4 Gastric polyp with moderate dysplasia.
A: White light endoscopy showing a 10 mm gastric polyp; B: Autofluorescence imaging with magenta areas on the surface of the polyp, surrounded by green normal mucosa; C: Magnifying endoscopy with narrow band imaging showing a modified pit pattern of the mucosa with an increased number of capillaries.
While all these studies demonstrate the vast potential of AFI to target premalignant lesions (high grade dysplasia) and early cancers, they also reveal important limitations of this technique and set-up directions for future improvement. The large number of false-positive results with a consequent low positive predictive value implies a potential benefit from adjunct methods such as ME-NBI, optical-coherence tomography (OCT) or confocal laser endomicroscopy (CLE)[10] which would provide greater detection specificity. The use of trimodal imaging endoscopy that includes WLE, AFI and ME-NBI incorporated in one endoscopy system might improve diagnostic accuracy for high-grade dysplasia and early cancer[11-13]. ME-NBI is currently considered the technique of choice for improvement of diagnostic accuracy because it reduces the high rate of false-positive results associated with WLE and AFI. Autofluorescence consists primarily of visible light, resulting in images limited essentially to the mucosal surface. Consequently, the development of infrared techniques may provide greater tissue penetration, obtaining images with greater contrast between lesions and their surrounding regions, and allowing the visualization of vascularization in deeper lesions, including the submucosa. Some studies reported that infrared endoscopy is capable of detecting abnormal submucosal vascularization in tumor lesions, with retention of indocyanine green being correlated with the size of the submucosal vascular bed[14-18]. There is also a direct correlation between the presence of infrared fluorescence and the number of submucosal vessels. With the development of tumor invasion, there is a tendency for more abnormal blood vessels to be formed, further accompanied by an increase in fluorescence[18].
NARROW BAND IMAGING
Narrow band imaging (NBI) is an optical image technology that enhances structural mucosal patterns (pit-pattern), as well as mucosal/submucosal vessels, by employing the characteristics of the light spectrum. The technology consists of placing narrow bandpass filters in front of a conventional white-light source to obtain tissue illumination at selected narrow wavelength bands. Currently available NBI systems use 2 narrow band filters that provide tissue illumination in the blue (415 nm) and green (540 nm) spectrum of light. The superficial penetrating wavelength of 415 nm corresponds to the main peak on the absorption spectrum of hemoglobin, while the deeper penetrating wavelength of 540 nm corresponds to a secondary hemoglobin absorption peak. Capillaries in the superficial mucosal layer are emphasized by the 415 nm light and are displayed in brown, whereas deeper mucosal and submucosal vessels are made visible by the 540 nm light and are displayed in cyan (Figures 1-4). NBI performance is certainly maximized when it is combined with magnification (ME-NBI)[3,19]. This technique improves the morphological analysis of epithelial crests of the mucosa and enables a more precise analysis of the abnormal surface architecture (pit-pattern) of neoplastic lesions. However, the most important contribution is represented by the clear visualization of the vascular network in the mucosa, being especially useful in evaluation of the abnormal neoangiogenesis process in high-grade dysplasia/early cancer[20].
It was previously recognized that the morphological changes of an intrapapillary capillary loop (IPCL) might represent a new option for early diagnosis of squamous cell carcinoma in the esophagus[21,22]. However, evaluation of IPCLs under white light observation requires high levels of proficiency and is usually not possible during the usual clinical workup. By using the magnifying scope, the normal appearance of the IPCL is identified as red dots. NBI enables a more vivid observation of the IPCLs, increasing diagnostic accuracy, especially for inexperienced endoscopists (Figure 1C)[23]. Branching vessels which are located relatively deeper in the wall layers are observed in cyan, while IPCLs which are located in a more superficial layer, are observed as brown loops (brown dots).
Changes in the IPCL pattern include dilatation, tortuosity and/or caliber change of individual IPCL or multiple IPCLs of various shapes. According to the degree of change, these are classified into five types[23,24]: type I is associated with normal epithelium and IPCLs are observed as smooth running small-diameter capillary vessels; type II involves minimal dilatation and elongation of IPCLs, and is often equivalent to regenerative tissue or inflammation; type III assumes minimal changes in IPCLs and corresponds to borderline lesions which potentially include esophagitis and low-grade intraepithelial neoplasia; in type IV, 3 of the 4 abnormal IPCLs patterns are present and correspond to high-grade intraepithelial neoplasia; finally, type V includes all 4 abnormal IPCLs characteristics and signifies the presence of cancer. Type V is subdivided in four types, type V-1, V-2, V-3 to VN which reflect cancer infiltration depth. In type V-1, IPCLs demonstrate characteristic changes, dilatation, meandering, irregular caliber and variable form, and corresponds to m1 lesions (carcinoma in situ). As it advances to m2 and m3, destruction of IPCL advances gradually and these changes are further extended into the submucosa. Thus, in type VN, which is characteristic of sm deep invasive carcinoma, new tumor vessels appear, around 10 times larger than the irregular vessels which appear in IPCLs type V-3 (Figure 2C)[23,24].
ME-NBI is very useful for identifying superficial squamous cell carcinoma in the head and neck region. Muto et al[25,26] reported that visualization of abnormal microvessel architecture in cancerous lesions is significantly improved by NBI as compared with WLE. This finding is clinically significant because no cases of superficial cancer in the oropharynx or hypopharynx were previously reported before the advent of NBI.
Most of the studies using NBI were designed to evaluate the mucosal pattern and capillary network of patients with Barrett′s esophagus, knowing that during WLE it is difficult to identify dysplastic and early neoplastic changes. In all these studies, the accuracy of the diagnosis was higher for NBI as compared with WLE[27-34]. NBI with magnifying endoscopy thus enables visualization of the details of the mucosal surface and capillary networks without using dyes. Regular villous/gyrus-forming mucosal patterns, as well as flat mucosa with long branching blood vessels are highly predictive for specialized intestinal metaplasia without dysplasia. Irregular/disrupted mucosal pattern, an irregular vascular pattern and abnormal blood vessels are associated with high-grade intraepithelial neoplasia or early cancer. Abnormal vascularity was defined as dilated, corkscrew vessels with increased vascularity and an abnormal, nonuniform branching pattern (Figure 2C)[27,28]. All high-grade intraepithelial neoplasia have at least one abnormality, and 85% have two or more abnormalities[27]. Goda et al[29] reported that the addition of capillary pattern to fine mucosal patterns improved the diagnostic value of ME-NBI for detecting specialized intestinal metaplasia and superficial adenocarcinoma.
In a recent study, Singh et al[30] validated a simplified classification of the various morphologic patterns visualized in Barrett′s esophagus in four easily distinguishable types: A, round pits with regular microvasculature (columnar mucosa without intestinal mucosa); B, villous/ridge pits with regular microvasculature (intestinal metaplasia); C, absent pits with regular microvasculature (intestinal metaplasia); D, distorted pits with irregular microvasculature (high-grade intraepithelial neoplasia). This classification showed reproducibility and repeatability, both by experienced endoscopists and for those unfamiliar with NBI, suggesting a rapid learning curve. Therefore, ME-NBI allows all endoscopists to perform targeted biopsies for specialized intestinal metaplasia and high-grade intraepithelial neoplasia with a high rate of success[30-34].
There is no evidence to prove the clinical usefulness of NBI during non-magnifying endoscopic observation for detecting abnormal pathology within the stomach and the duodenum. From a technical point of view, the mucosal image by non-magnification observation with NBI is too dark and noisy for meaningful investigation, because the lumen of the stomach is large[35]. Feasibility studies showed the potential of NBI with magnification to identify gastric intestinal metaplasia[36], predict the histologic subtypes of early gastric cancer[37], and improve margin delineation of gastric cancer for endoscopic mucosal resection[38]. Uedo et al[36] reported that a distinctive finding called light blue crests is a good indicator of histological intestinal metaplasia, which is a well-known risk factor for the development of differentiated-type gastric cancer. NBI observation of a light blue crest, defined as a fine blue-white line on the crests of the epithelial surface or gyri, correlated with the histologic diagnosis of intestinal metaplasia with 89% sensitivity and 93% specificity. The light blue crest was frequently observed in the mucosa surrounding differentiated-type early gastric cancers, and it demarcated the extent of the tumors (Figure 3C).
In a study involving 165 patients with depressed-type early gastric cancers, Nakayoshi et al[37] reported that ME-NBI is not sufficient to replace conventional histology, but is capable of predicting the histological characteristics of gastric cancer. They classified the abnormal microvascular pattern into two types. In the case of differentiated-type depressed early gastric cancer, a relatively regular fine network pattern was more likely to be observed, while for the undifferentiated-type, a irregular, twisting, or corkscrew pattern was more likely to be observed, representing a relatively low density of microvessels. However, ME-NBI may not be sufficient to replace conventional histology, but it may allow improved differentiation between benign and malignant minute lesions and may be useful for diagnosing the extent of cancerous infiltration.
MULTIBAND IMAGING
Multiband imaging (MBI) represents a digital image processing technique that enhances the appearance of mucosal surface structures by using selected wavelengths of light in reconstructed virtual images. MBI technology uses a software-driven image-processing algorithm that is based on spectral estimation methods. A standard image captured by a color charge-coupled device video endoscope is sent to a spectral estimation matrix processing circuit contained in the video processor. Here, reflectance spectra of corresponding pixels that make up the conventional image are mathematically estimated. From these spectra, it is feasible to reconstruct a virtual image of a single wavelength. Three such single-wavelength images can be selected and assigned to the red, green and blue monitor inputs, respectively, to display a composite color-enhanced MBI image in real-time[3]. There are very few published data thus far on the efficacy of MBI for detection or differentiation of GI tract lesions, although the technique seems to be superior to WLE, noninvasive and may more easily detect lesions without dye, during both routine and detailed examinations[39,40].
In conclusion, these new endoscopic techniques provide better visualization of mucosal surface microstructure and microvascular architecture and may enhance the diagnosis and characterization of mucosal lesions in the GI tract. In the near future, trimodal imaging endoscopy, which combines WLE, AFI and ME-NBI, is expected to be practiced as a standard endoscopy technique as it is quick, safe and accurate for making a precise diagnosis of GI pathology. Although there is compelling evidence that these new techniques are superior to conventional endoscopy, current clinical guidelines are still limited. Further large-scale randomized controlled trials comparing these modalities in different patient subpopulations are, of course, warranted before their endorsement in the routine practice of GI endoscopy.