Published online Jan 7, 2011. doi: 10.3748/wjg.v17.i1.49
Revised: September 29, 2010
Accepted: October 6, 2010
Published online: January 7, 2011
Visualization of tumor angiogenesis can facilitate non-invasive evaluation of tumor vascular characteristics to supplement the conventional diagnostic imaging goals of depicting tumor location, size, and morphology. Hybrid imaging techniques combine anatomic [ultrasound, computed tomography (CT), and/or magnetic resonance imaging (MRI)] and molecular (single photon emission CT and positron emission tomography) imaging modalities. One example is real-time virtual sonography, which combines ultrasound (grayscale, colour Doppler, or dynamic contrast harmonic imaging) with contrast-enhanced CT/MRI. The benefits of fusion imaging include an increased diagnostic confidence, direct comparison of the lesions using different imaging modalities, more precise monitoring of interventional procedures, and reduced radiation exposure.
- Citation: Sandulescu DL, Dumitrescu D, Rogoveanu I, Saftoiu A. Hybrid ultrasound imaging techniques (fusion imaging). World J Gastroenterol 2011; 17(1): 49-52
- URL: https://www.wjgnet.com/1007-9327/full/v17/i1/49.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i1.49
Conventional cross-sectional imaging techniques [ultrasound, computed tomography (CT), magnetic resonance (MR), etc.] have important roles in noninvasive diagnosis, and in tumor treatment strategies. The techniques employed have different working principles, consequently complementing each other with respect to the information obtained. The combination (fusion) of two imaging techniques was developed in recent years, defining the so-called “hybrid techniques” or “fusion imaging”. Combinations of anatomical imaging techniques (ultrasound with CT or MR imaging), as well as associations between anatomical (CT or MR imaging) and molecular (SPECT or PET) imaging modalities are currently used in clinical practice.
One example of fusion imaging is real-time virtual sonography (RVS), a technique that enables the display of an ultrasound B mode image and CT or MR images in real-time[1]. The system includes a magnetic positioning sensor fixed on the convex-shaped probe of the ultrasound scanner, for the creation of images with identical cross-sections in real-time. This is done according to the position and the angle of the probe in relation to previously acquired CT and MR volume data. To be compatible with the RVS module, CT examination must meet certain requirements: (1) the volume data must be archived in the DICOM format; (2) the slice thickness should be 3 mm or less and image reconstruction the same; and (3) the CT scan area must include the xiphoid process.
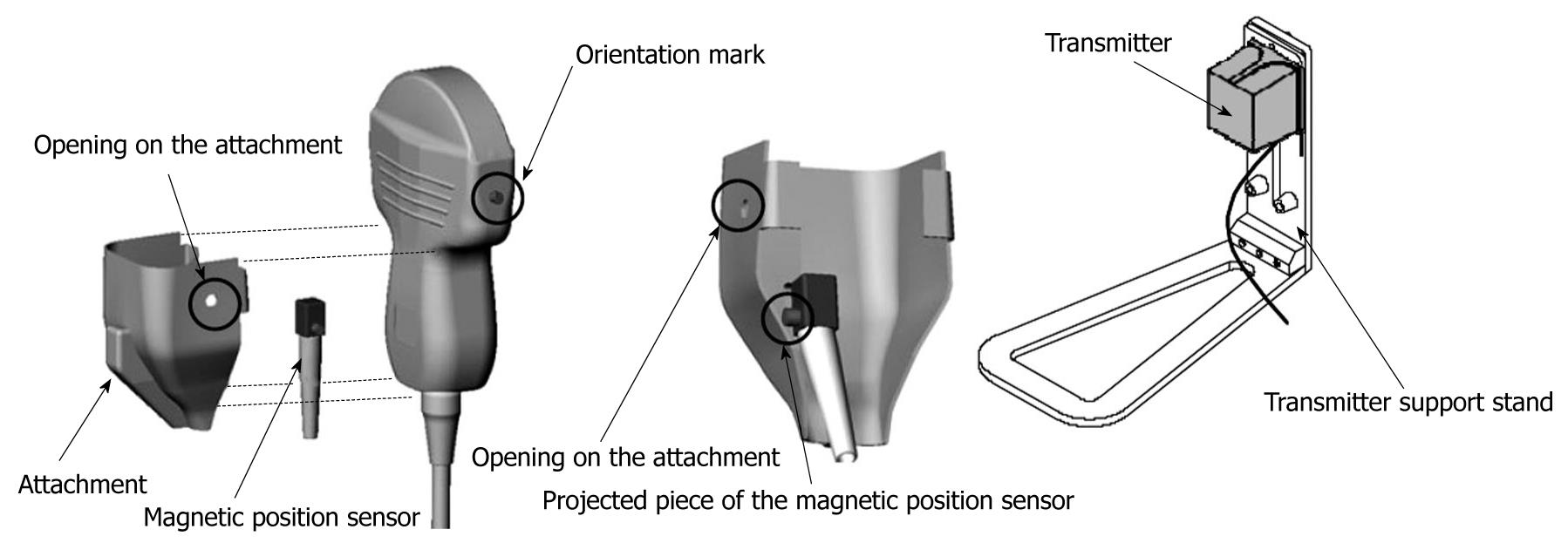
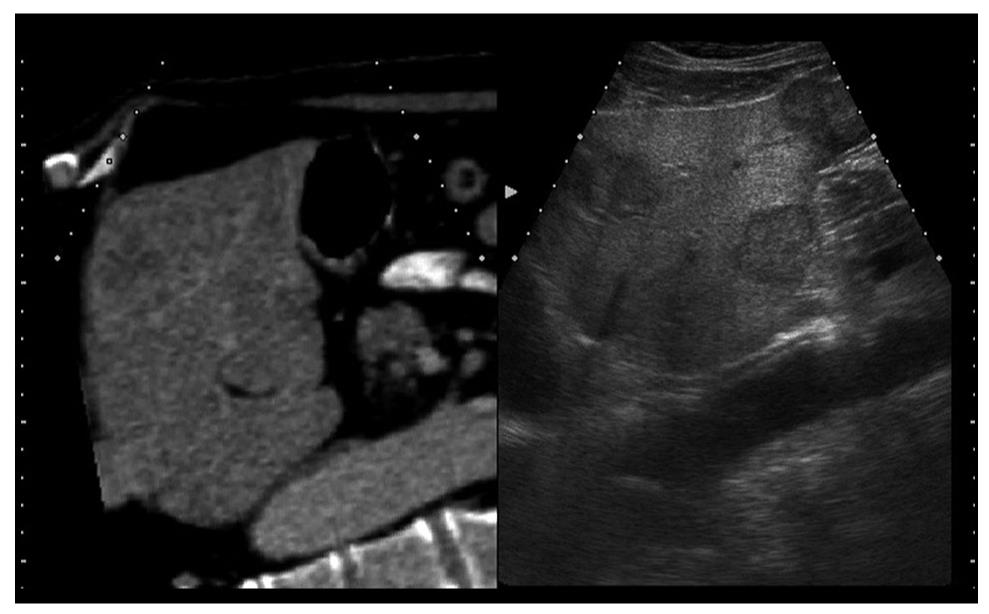
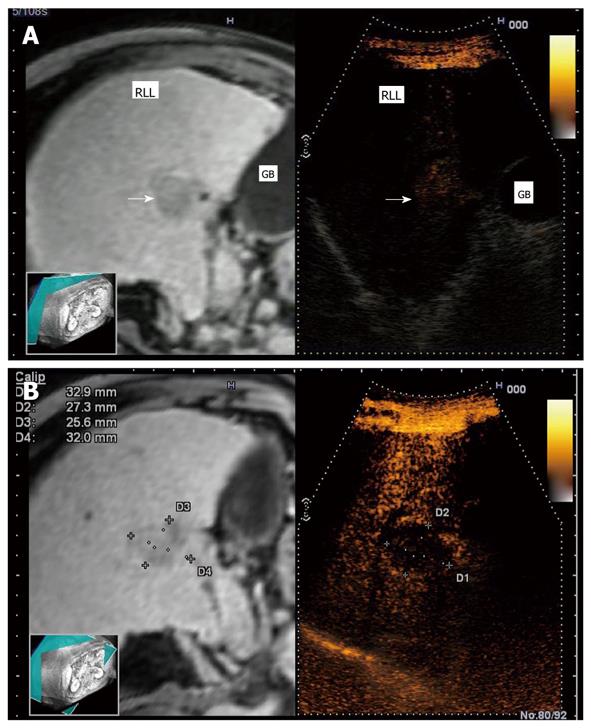
To display virtual images, it is necessary to transfer the CT or MR data to the ultrasound machine[2]. The magnetic positioning sensor unit is carefully assembled, based on the position of orientation marks. It uses a distal attachment, which helps install the magnetic positioning sensor on the probe, as shown in Figure 1. The magnetic sensor detects the changes in location, direction, and rotation of the probe during normal ultrasound scanning of the patient. The transmitter (the instrument that produces the magnetic waves) for the magnetic positioning sensor unit is installed on the left flank of the patient. Using a probe equipped with the magnetic sensor, a sagittal section of the left hepatic lobe is then captured (Figure 2). The xiphoid process is usually chosen as a reference anatomical point. In case of a mismatch between the ultrasound and the virtual image (CT or MR), it is possible to readjust and correct the mismatch during the exam. The adjustment is made possible by freezing the CT/MR images on a section with clearly visible anatomical landmarks, for example the portal vein bifurcation or the right kidney in a longitudinal view, followed by identification of a similar ultrasound image and continuation of examination[3]. The workstation monitor displays two images: the ultrasound real-time image and the virtual reconstructed CT/MR image (Figure 3).
The founding principles of combining the ultrasound image and the CT/MR images were based on several observations. In recent years, frequent imaging investigations led to the discovery of small focal liver lesions, which can be treated locally by ultrasound-guided radiofrequency ablation (RFA) or other ablation techniques. However, ultrasound exams are cannot always identify isoechoic tumors, tumors recurring locally in areas treated with lipiodol following transarterial chemoembolization (TACE), or tumors recurring in areas treated previously by RFA or percutaneous ethanol injection (PEI) procedures[4]. In addition, in ultrasound examinations, there are a few dead angles and it is difficult to examine the whole liver, especially in obese patients. Nodules that are poorly identified on ultrasound are clearly visible on CT/MR. However, interventional treatment is easier to perform under ultrasound-guidance, while the exposure to increased doses of radiation is also avoided. Thus, real-time virtual sonography combines the imaging advantages of both techniques. Several studies have already proved the feasibility of the RVS module, especially for percutaneous RFA of poorly visible or unidentifiable focal liver lesions during B-mode sonography[5-7].
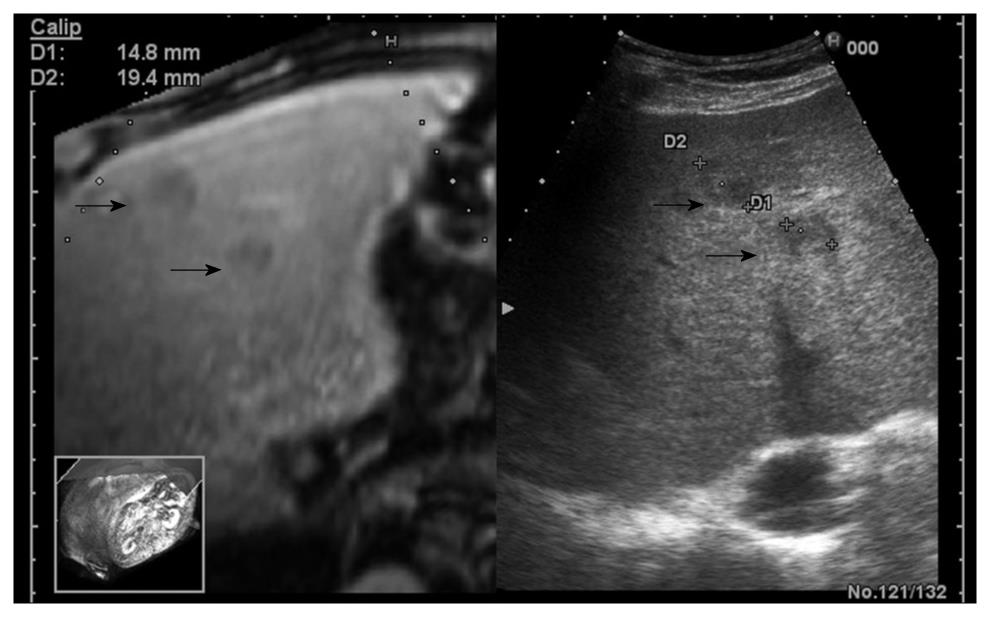
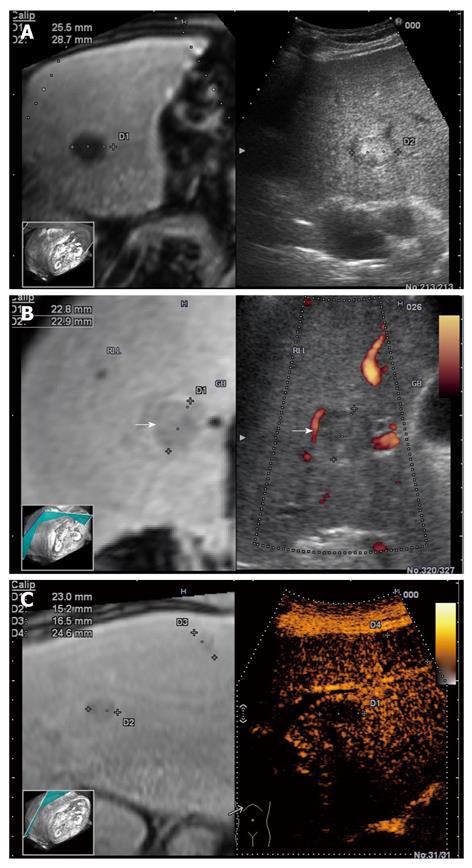
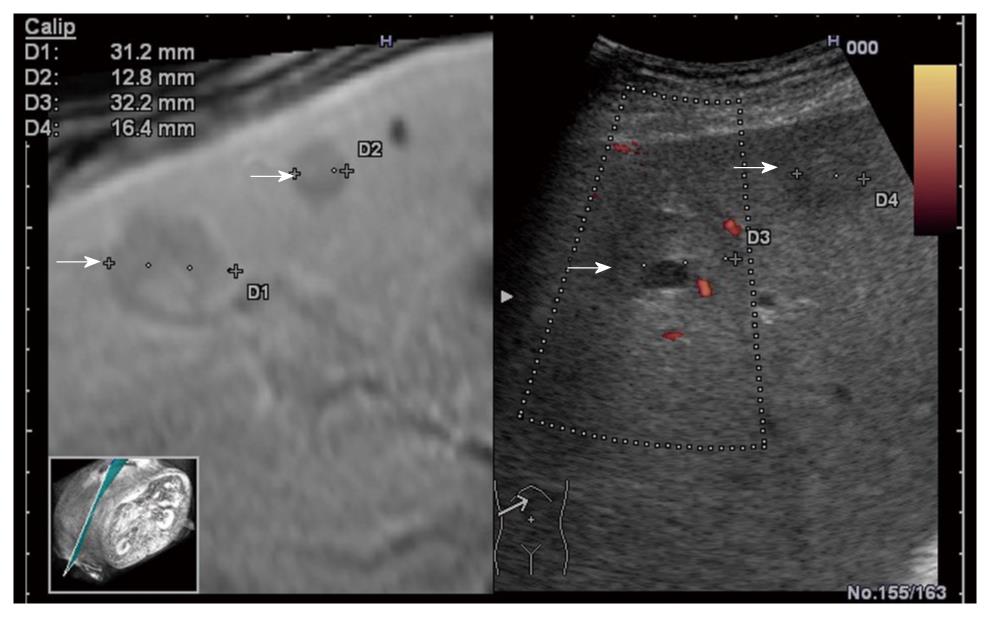
The RVS module is compatible with B-mode (Figure 4A), color Doppler imaging (CDI) mode (Figure 4B), and dynamic contrast harmonic imaging (D-CHI) mode (Figure 4C)[8]. Therefore, RVS might have important clinical applications in the assessment of tumor angiogenesis. Hepatocellular carcinoma, a hypervascular tumor mainly supplied by hepatic arteries, shows a typical pattern in contrast-enhanced ultrasound: arterial hypervascularization (Figure 5A) with washout in the portal venous during the late phase (Figure 5B)[9]. Real-time virtual ultrasound can reveal the contrast-enhanced ultrasound image in a similar way to contrast-enhanced CT/MR, although the mechanism of action is clearly different[8]. Likewise, liver metastases that are poorly visible in 2D ultrasound can also be discovered in real-time virtual ultrasound, especially if contrast-enhancement is used (Figure 6). Although, the combined use of contrast-enhanced images has not yet been described in terms of accuracy for the positive and differential diagnosis of small focal liver lesions, RVS might combine the advantages of both methods (ultrasound and CT/MR).
For oncological patients, the tumor response to chemotherapy is conventionally assessed by RECIST and WHO size criteria, obtained during CT/MR follow-up[10]. Anti-angiogenic treatments induce lesion necrosis with no change in volume of the initial tumor; therefore, the use of size criteria appear inappropriate. Several studies have shown that the use of microbubble contrast agents can detect changes in vascularization, by calculation of maximal perfusion parameters, such as peak intensity, time to peak intensity, area under the curve, and slope coefficient of wash-in[11-14]. Thus, the simultaneous use of real-time virtual sonography with dynamic contrast-enhanced ultrasound can be also employed. The method might allow the quantification of tumor perfusion for early assessment and quantitative monitoring of the efficacy of antiangiogenic agents, based on changes in vascularity, even before morphological changes become apparent[10].
RVS cannot be used in patients if CT/MR is contraindicated (patients with known contrast medium allergies, renal failure, or metallic implants). Another limitation of RVS is that the technique does not always show the best synchronization between ultrasound and CT/MR images, although the discrepancy can be adjusted with careful visualization of neighboring portal and hepatic veins in most cases[8]. The use of the RVS module does prolong the examination time; therefore, the clinical impact, in terms of improved decision making, should be further assessed. The technique adds the costs of CT/MR to the costs of the contrast-enhanced ultrasound exam; thus the cost-effectiveness of this approach must be evaluated in future studies[15].
In conclusion, hybrid ultrasound imaging techniques now play a pivotal role in diagnosis, staging, and follow-up during treatment. During local ablation of focal liver lesions hybrid ultrasound imaging allows better control of the procedure and less radiation exposure.
Peer reviewers: Giedrius Barauskas, Professor, Department of Surgery, Kaunas University of Medicine, Eiveniu str. 2, Kaunas, LT-50009, Lithuania; Naoaki Sakata, MD, PhD, Division of Hepato-Biliary Pancreatic Surgery, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8574, Japan
S- Editor Sun H L- Editor Stewart GJ E- Editor Ma WH
| 1. | Available from: http://www.hitachi-medical-systems.es/index.php?Âid=768&0; cited on 18th February 2010. |
| 2. | Real-time Virtual Sonography Unit. Instruction Manual. Hitachi Medical Corporation. 2004-2006. . |
| 3. | Sandulescu L, Saftoiu A, Dumitrescu D, Ciurea T. The role of real-time contrast-enhanced and real-time virtual sonography in the assessment of malignant liver lesions. J Gastrointestin Liver Dis. 2009;18:103-108. |
| 4. | Kawasoe H, Eguchi Y, Mizuta T, Yasutake T, Ozaki I, Shimonishi T, Miyazaki K, Tamai T, Kato A, Kudo S. Radiofrequency ablation with the real-time virtual sonography system for treating hepatocellular carcinoma difficult to detect by ultrasonography. J Clin Biochem Nutr. 2007;40:66-72. |
| 5. | Minami Y, Kudo M, Chung H, Inoue T, Takahashi S, Hatanaka K, Ueda T, Hagiwara H, Kitai S, Ueshima K. Percutaneous radiofrequency ablation of sonographically unidentifiable liver tumors. Feasibility and usefulness of a novel guiding technique with an integrated system of computed tomography and sonographic images. Oncology. 2007;72 Suppl 1:111-116. |
| 6. | Minami Y, Chung H, Kudo M, Kitai S, Takahashi S, Inoue T, Ueshima K, Shiozaki H. Radiofrequency ablation of hepatocellular carcinoma: value of virtual CT sonography with magnetic navigation. AJR Am J Roentgenol. 2008;190:W335-W341. |
| 7. | Kitada T, Murakami T, Kuzushita N, Minamitani K, Nakajo K, Osuga K, Miyoshi E, Nakamura H, Kishino B, Tamura S. Effectiveness of real-time virtual sonography-guided radiofrequency ablation treatment for patients with hepatocellular carcinomas. Hepatol Res. 2008;38:565-571. |
| 8. | Sandulescu L, Saftoiu A, Dumitrescu D, Ciurea T. Real-time contrast-enhanced and real-time virtual sonography in the assessment of benign liver lesions. J Gastrointestin Liver Dis. 2008;17:475-478. |
| 9. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. |
| 10. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D'Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. |
| 11. | De Giorgi U, Aliberti C, Benea G, Conti M, Marangolo M. Effect of angiosonography to monitor response during imatinib treatment in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2005;11:6171-6176. |
| 12. | Lassau N, Lamuraglia M, Chami L, Leclère J, Bonvalot S, Terrier P, Roche A, Le Cesne A. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006;187:1267-1273. |
| 13. | Lassau N, Chami L, Benatsou B, Peronneau P, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol. 2007;17 Suppl 6:F89-F98. |
| 14. | Lassau N, Brule A, Chami L, Benatsou B, Péronneau P, Roche A. [Evaluation of early response to antiangiogenic treatment with dynamic contrast enhanced ultrasound]. J Radiol. 2008;89:549-555. |
| 15. | Giesel FL, Delorme S, Sibbel R, Kauczor HU, Krix M. [Contrast-enhanced ultrasound for the characterization of incidental liver lesions - an economical evaluation in comparison with multi-phase computed tomography]. Ultraschall Med. 2009;30:259-268. |