Published online Jan 7, 2011. doi: 10.3748/wjg.v17.i1.118
Revised: September 20, 2010
Accepted: September 27, 2010
Published online: January 7, 2011
AIM: To identify the risk factors and three single nucleotide polymorphisms (SNPs) of NOD2/CARD15 gene in inflammatory bowel disease (IBD) of the population in Zhejiang, China.
METHODS: A case-control study was conducted using recall questionnaire to collect data on demographic, socioeconomic, lifestyle characteristics and dietary behaviors from 136 determined IBD patients and 136 paired healthy controls. COX regression method was used to screen the statistically significant risk factors for IBD. The polymorphisms of NOD2/CARD15 gene Arg702Trp, Gly908Arg and Leu1007fsinsC were genotyped and further compared between 60 patients with IBD and 60 healthy controls by polymerase chain reaction and restriction fragment length polymorphism.
RESULTS: IBD occurred primarily in young and middle-aged people. The mean age for IBD patients was 42.6 years. The ratio of males to females was 1.23:1. COX regression indicated a higher statistical significance in milk, fried food and stress compared with the other postulated risk factors for IBD. None of the patients with IBD and healthy controls had heterozygous or homozygous SNPs variants.
CONCLUSION: Milk, fried food and stress are associated with increased risk of IBD. The common variants in NOD2/CARD15 gene are not associated with IBD in China’s Zhejiang population.
- Citation: Wang ZW, Ji F, Teng WJ, Yuan XG, Ye XM. Risk factors and gene polymorphisms of inflammatory bowel disease in population of Zhejiang, China. World J Gastroenterol 2011; 17(1): 118-122
- URL: https://www.wjgnet.com/1007-9327/full/v17/i1/118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i1.118
Crohn’s disease (CD) and ulcerative colitis (UC) are the two main types of idiopathic inflammatory bowel disease (IBD) whose etiology is multifactorial and still vague. Currently, the development of IBD is considered to have a close relationship with immunology, genetics, environment and infection. The incidence of IBD in Western populations increased during the past few decades with an estimated incidence of 0.35%-1.00% for CD and 0.10%-1.00% for UC[1]. NOD2/CARD15 is the first verified predisposing gene of CD where three NOD2 variants Arg702Trp, Gly908Arg and Leu1007fsinsC were found to be associated with CD in the Caucasian populations[2,3]. Nevertheless, these single nucleotide polymorphisms (SNPs) were not found to predispose to CD in Japanese and Hong Kong populations[4,5], leaving controversies on their exact role in CD. The number of patients with IBD has been increasing in China, but only a few studies have investigated the risk factors in IBD. Moreover, association between NOD2 gene and the development of IBD has seldom been evaluated in the Chinese population[6]. Therefore, the purpose of this study was to identify the risk factors by case-control studies and determine whether the NOD2 variants are associated with IBD in the population of Zhejiang, China.
One hundred and thirty six patients with IBD and 136 age and sex-matched healthy controls were recruited from the First Affiliated Hospital of Zhejiang University, Jinhua Central Hospital, Ningbo Medical Treatment Center, Lihuili Hospital and Taizhou Hospital of Zhejiang Province between January 2005 and December 2008. The age of IBD patients (84 UC and 52 CD) ranged from 18 to 85 years. Written informed consent was obtained from all the cases and controls. Blood samples were collected from 60 patients (32 UC and 28 CD) and 60 healthy controls randomly. IBD was diagnosed based on the clinical, radiographic, endoscopic and histologic criteria.
Each subject received a questionnaire to obtain demographic data. The questionnaire also contained items specifically related to IBD: education background, heredity, occupation condition (occupation classification and stress), habitat condition during the past 5 years (drinking water and toilet), infection, appendectomy, measles, oral contraceptive use, estrogen replacement, dietary habits (vegetarian diet or carnivorous diet), smoking history, tea drinking, and alcohol, milk, fried food and spicy food intake. All questionnaires were checked for completeness, and doubtful responses from both patients and healthy controls were confirmed upon return of the questionnaire.
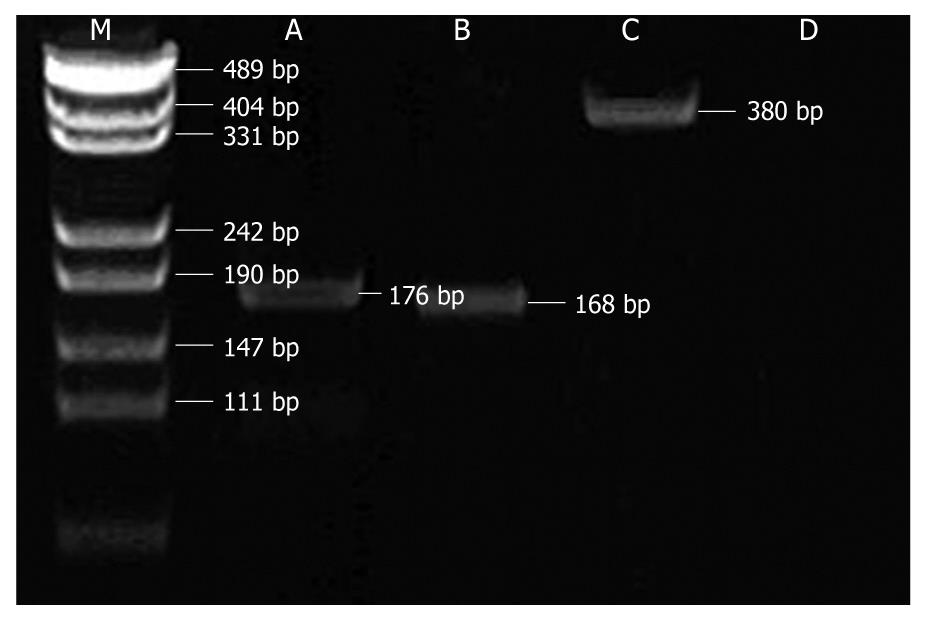
DNA was isolated from peripheral blood using the Genomic DNA Isolation Kit (Sangon, Shanghai, China). All polymerase chain reaction (PCR) assays were performed in a 25 μL volume reaction. Three single nucleotide polymorphisms (SNPs) of NOD2/CARD15 gene were amplified by specific primers (Table 1)[7] under the following conditions: an initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturing at 94°C for 20 s, annealing at 60°C (Arg702Trp), 55°C (Gly908Arg) or 58°C (Leu1007fsinsC) for 30 s and extension at 72°C for 1 min, and final incubation at 72°C for 7 min. PCR products were electrophoresed in a 2% agarose gel and visualized by ethidium bromide staining.
| SNPs | Primers | Length of products (bp) |
| Arg702Trp | Forward5’CTTCCTGGCAGGGCTGTTGTC3’ | 176 |
| Reverse5’CATGCACGCTCTTGGCCTCAC3’ | ||
| Gly908Arg | Forward 5’AAGTCTGTAATGTAAAGCCAC3’ | 380 |
| Reverse 5’CCCAGCTCCTCCCTCTTC3’ | ||
| Leu1007fsinsC | Forward 5’CCTGCAGTCTCTTTAACTGG3’ | 168 |
| Reverse 5’CTTACCAGACTTCCAGGATG3’ |
Genotyping for Arg702Trp, Gly908Arg and Leu1007fsinsC was performed using polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP): a 10 μL aliquot of the product was digested with an appropriate restriction enzyme. The tubes were incubated at 37°C for 4 h, and then transferred to 65°C for 20 min. After digestion, fragment sizes for carriers of the polymorphic alleles decreased, resulting in the presence of different fragments (Table 2). Products were electrophoresed in a 15% polyacrylamide gel and visualized by silver nitrate staining.
| SNPs | Polymorphic alleles | Restriction enzymes | Wild-type alleles (bp) | Mutant alleles (bp) |
| Arg702Trp | C2104T | MspI | 76 + 54 + 24 + 22 | 130 + 24 + 22 |
| Gly908Arg | G2722C | HhaI | 380 | 242 + 138 |
| Leu1007fsinsC | 3020insC | NlaIV | 168 | 128 + 40 |
Semi-quantitative data were analyzed using COX regression to calculate relative risk (RR) and their 95% confidence interval. Frequencies and susceptibilities of mutations among CD, UC and controls were compared based on χ2 or Fisher exact test. All data were analyzed in SPSS (version 13.0), where P value of 0.05 or less was considered statistically significant in all cases.
A total of 272 subjects were enrolled in the study who all completed the questionnaires. The mean age for IBD patients was 42.6 years and the ratio of males to females was 1.23:1. The result showed that milk, fried food intake and stress were risk factors for IBD in both univariate and multivariate logistic regression analysis (Tables 3 and 4). The number of cases of infection, appendectomy, oral contraceptive use, and estrogen replacement was too small for statistical analysis, so the data of these variables were not shown in the tables.
| Variables | χ2 | P | RR | 95% CI | |
| Lower bound | Upper bound | ||||
| Habitat condition during the past 5 yr | 1.192 | 0.274 | 0.653 | 0.303 | 1.404 |
| Educational background | 1.250 | 0.265 | 0.724 | 0.284 | 1.528 |
| Occupation classification | 0.894 | 0.293 | 0.615 | 0.314 | 1.412 |
| Alcoholic drinking | 0.987 | 0.361 | 0.712 | 0.194 | 1.512 |
| Cigarette smoking | 1.215 | 0.194 | 0.843 | 0.247 | 1.384 |
| Tea drinking | 1.523 | 0.165 | 0.631 | 0.315 | 1.423 |
| Stress | 18.452 | < 0.001 | 1.295 | 1.151 | 1.457 |
| Milk intake | 25.425 | < 0.001 | 1.279 | 1.162 | 1.407 |
| Fried food intake | 24.378 | < 0.002 | 1.286 | 1.154 | 1.417 |
| Variables | χ2 | P | RR | 95% CI | |
| Lower bound | Upper bound | ||||
| Milk intake | 10.713 | 0.0011 | 1.243 | 1.091 | 1.415 |
| Fried food intake | 14.267 | 0.0002 | 1.238 | 1.108 | 1.383 |
| Stress | 13.377 | 0.0003 | 1.241 | 1.102 | 1.394 |
Electropherogram of the amplified DNA fragments is shown in Figure 1. PCR-PFLP analyses showed that Arg702Trp, Gly908Arg and Leu1007fsinsC alleles of 60 IBD patients and 60 healthy controls were all wild type. None of the patients with IBD and healthy controls had heterozygous or homozygous SNPs variants.
The etiology and pathogenesis of IBD have been and continue to be intensely investigated. Accumulating evidences strongly suggest that it is mediated immunologically and that the inflammatory process is influenced by environmental and host factors. In this study, we found a higher prevalence of IBD in young and middle-aged people which may be associated with the strong gastrointestinal immune function and intense immune response. The prevalence of CD was higher in males (36 cases) than in females (16 cases), but the prevalence of UC was similar among males (39 cases) and females (45 cases), which is consistent with other studies.
Many environmental factors may be involved in the pathogenesis of IBD, including: dietary habits, cigarette smoking, appendectomy, oral contraceptive use, infection and so on. However, the epidemiologic evidence for an etiologic role of these potential risk factors in IBD is inconsistent. In this study, there was a strong evidence for an increased risk of developing IBD associated with stress, milk intake and fried food. Stress increases gut muscle tone and intestinal transit time. Several studies reported that people with functional gastrointestinal disorders had significantly more behavioral and emotional symptoms than healthy people[8]. UC patients are most likely to experience the problems like mood and anxiety[9]. Some hormones released during stress (e.g. corticotrophin-releasing factor) can promote intestinal inflammation and alter visceral sensitivity when released locally in the gut[10]. Epidemiological studies showed that the prevalence of IBD varies among populations. The immigrants had a different incidence of IBD within a generation. It has been suggested that different food antigens play a significant role. The present study confirms an increased risk of developing IBD associated with milk intake. IgE-mediated allergy to cow’s milk proteins is common in the children and adults who experience repeated gastrointestinal symptoms, and often is the first manifestation of food allergy. Cow’s milk protein allergy may induce the abnormal immune response of digestive tract that increases the relative risk of IBD. In epidemiologic studies, high-temperature cooking methods have been associated with the formation of carcinogenic substances such as heterocyclic amines, acrylamide and polycyclic aromatic hydrocarbons[11]. These products have been associated with endothelial dysfunction and inappropriate immune responses, which results in the development of IBD and intestinal cancer.
Cigarette smoking that has been reported as a risk factor for IBD in many studies might even have beneficial effects on the course of UC, but exacerbates the course of CD. The potential mechanisms involved in this dual relationship may include the effects of nicotine administration on inflammatory cytokine, changes in blood flow and gut permeability[12]. In this study, cigarette smoking was not significantly associated with the development of IBD. Studies showed that IBD patients were more likely to be white collar and urban residents with high educational background. Infection and oral contraceptive use were considered to increase the risk of developing IBD, whereas vegetarian diet, tea drinking, spicy food intake and appendectomy decreased the risk. In this study, no significant association was found between these factors and IBD.
Monozygotic twin concordance, familial predisposition, and segregation analyses have shown that genetic factors confer susceptibility to IBD. NOD2/CARD15 gene locates on chromosome 16q12, encoding a member of the Apat-1/Ced-4 superfamily of apoptosis regulator that is expressed in monocytes. NOD2/CARD15 gene is involved in the recognition of lipopolysaccharide and subsequent activation of necrosis factor-κB, and disturbs the activation of the innate immune system by bacterial antigens. NOD2/CARD15 gene mutation or deletion induces the abnormal innate immune response, which is important for immunological protection against intestinal microbes and may contribute to the development of IBD. Increased CARD15 was detected in mononuclear and epithelial cells of colon in CD patients[13,14]. Hugot et al[2] showed that a frame shift variant and two missense variants were associated with CD. This result is in accordance with the studies in different populations[3,7]. In our study, common NOD2 variants associated with increased susceptibility to CD in Caucasian populations were not verified in the Zhejiang population. It is conceivable that the NOD2 variants present in Caucasian patients are rare or nonexistent in the Zhejiang population and not detected in our limited population sample. Our results are in agreement with those studies in Asian patients[4,5]. This diversity of linkage analyses may arise from the heterogeneity of the disease and differences in genetic background of the population studied.
Human epidemiologic studies combine both parts of the typical risk assessment process into a single study by assessing the degree of exposure and the association with the risk of disease in a single study. The present study supports that intestinal environmental and genetic factors are vital for the pathogenesis of IBD. However, the heterogeneity among the small number of studies limited the ability to draw conclusions. Further studies using a larger cohort of patients with IBD are warranted to identity the risk factors and gene susceptibility to IBD.
Crohn’s disease (CD) and ulcerative colitis (UC) are the two main types of idiopathic inflammatory bowel disease (IBD) whose etiology is multifactorial and still vague. The development of IBD is considered to be closely related to immunology, genetics, environment and infection. NOD2/CARD15 is the first verified predisposing gene of CD in the Caucasian populations.
The etiology and pathogenesis of IBD have been and continue to be intensely investigated. Three NOD2 variants Arg702Trp, Gly908Arg and Leu1007fsinsC were found to be associated with CD in the Caucasian populations, but not in Japanese and Hong Kong populations. In this study, the authors identified the risk factors by case-control study and determined whether the NOD2 variants are associated with IBD in China’s Zhejiang population.
Only a few studies have investigated the risk factors of IBD in China. Moreover, the association between NOD2 gene and the development of IBD has seldom been evaluated in the Chinese population. This study demonstrated that milk, fried food and stress are associated with increased risk of IBD, and the common variants in NOD2/CARD15 gene are not associated with IBD in the Zhejiang population.
NOD2 variants present in Caucasian patients may be rare or nonexistent in the Zhejiang population. Milk, fried food and stress are the potential risk factors for IBD.
NOD2/CARD15 gene locates on chromosome 16q12, encoding a member of the Apat-1/Ced-4 superfamily of apoptosis regulator that is expressed in monocytes. NOD2/CARD15 gene is involved in the recognition of lipopolysaccharide and subsequent activation of necrosis factor-κB, and disturbs the activation of the innate immune system by bacterial antigens. NOD2/CARD15 gene mutation or deletion can induce the abnormal innate immune response.
This is an interesting paper looking at 136 IBD patients and paired healthy controls from the Zhejiang population comparing risk factors, and SNP analysis of NOD2/CARD15 in 60 patients and paired controls. No patients had variants in the NOD2/CARD15 gene by the methods used. Milk, fried food and stress were cited at potential risk factors. This work adds to the existing literature on NOD2, and supports the finding of lack of association with IBD in oriental populations.
Peer reviewer: Dr. John B Schofield, MB, BS, MRCP, FRCP, Department of Cellular Pathology, Preston Hall, Maidstone, Kent, ME20 7NH, United Kingdom
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 1. | Chinese Medical Association Digestion Branch. Guidelines for diagnosis and management of inflammatory bowel disease. Zhonghua Xiaohua Zazhi. 2001;21:236-239. |
| 2. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599-603. |
| 3. | Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, Frenzel H, King K, Hasselmeyer A, MacPherson AJ. Association between insertion mutation in NOD2 gene and Crohn’s disease in German and British populations. Lancet. 2001;357:1925-1928. |
| 4. | Inoue N, Tamura K, Kinouchi Y, Fukuda Y, Takahashi S, Ogura Y, Inohara N, Núñez G, Kishi Y, Koike Y. Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology. 2002;123:86-91. |
| 5. | Leong RW, Armuzzi A, Ahmad T, Wong ML, Tse P, Jewell DP, Sung JJ. NOD2/CARD15 gene polymorphisms and Crohn’s disease in the Chinese population. Aliment Pharmacol Ther. 2003;17:1465-1470. |
| 6. | Ouyang Q. Research strategies of inflammatory bowel disease. Zhonghua Xiaohua Zazhi. 2005;25:385-386. |
| 7. | Tukel T, Shalata A, Present D, Rachmilewitz D, Mayer L, Grant D, Risch N, Desnick RJ. Crohn disease: frequency and nature of CARD15 mutations in Ashkenazi and Sephardi/Oriental Jewish families. Am J Hum Genet. 2004;74:623-636. |
| 8. | Wang L, Xia XZ, Jiang SD. Psychological analysis of functional gastrointestinal diseases. Tiedao Yixue. 2002;30:31-32. |
| 9. | Liu FQ, Chu GW, Li ZH, Li P, Zhang RQ. Psychological factors and ulcerative colitis. Zhongguo Jiankang Xinlixue Zazhi. 2001;9:307-308. |
| 10. | Larauche M, Kiank C, Tache Y. Corticotropin releasing factor signaling in colon and ileum: regulation by stress and pathophysiological implications. J Physiol Pharmacol. 2009;60 Suppl 7:33-46. |
| 11. | Terry PD, Lagergren J, Wolk A, Steineck G, Nyrén O. Dietary intake of heterocyclic amines and cancers of the esophagus and gastric cardia. Cancer Epidemiol Biomarkers Prev. 2003;12:940-944. |
| 12. | Karban A, Eliakim R. Effect of smoking on inflammatory bowel disease: Is it disease or organ specific? World J Gastroenterol. 2007;13:2150-2152. |
| 13. | Berrebi D, Maudinas R, Hugot JP, Chamaillard M, Chareyre F, De Lagausie P, Yang C, Desreumaux P, Giovannini M, Cézard JP. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn’s disease colon. Gut. 2003;52:840-846. |
| 14. | Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nuñez G, Keshav S. Crohn’s disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47-57. |