Published online Mar 7, 2010. doi: 10.3748/wjg.v16.i9.1165
Revised: October 30, 2009
Accepted: November 7, 2009
Published online: March 7, 2010
We report three cases of ileocolic mucormycosis in adult immunocompromised patients presenting as acute abdomen. All patients underwent laparotomy but two of them died from multiorgan failure before the diagnoses were confirmed. The diagnosis of gastrointestinal mucormycosis is rarely suspected, and antemortem diagnosis is made in only 25%-50% of cases. These cases illustrate the difficulty encountered by surgeons in managing acute abdomen in neutropenic patients with hematological malignancy. The management of colonic mucormycosis in the published literature is also reviewed.
- Citation: Lo OSH, Law WL. Ileocolonic mucormycosis in adult immunocompromised patients: A surgeon’s perspective. World J Gastroenterol 2010; 16(9): 1165-1170
- URL: https://www.wjgnet.com/1007-9327/full/v16/i9/1165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i9.1165
Neutropenic patients can present with acute abdominal catastrophes of various causes and surgical intervention is required. However, surgery is usually associated with a high mortality and morbidity in the immunocompromised host. With the rising incidence of invasive fungal infection in patients with hematological malignancy and transplant recipients, mucormycosis has been reported to be the third most common cause of fungal infection after candidiasis and aspergillosis[1,2]. Mucormycosis refers to several different diseases caused by Mucorales[3,4]. These rare infections usually only occur in individuals with impaired immunity, having associations with diabetes mellitus, hematological or solid-organ malignancies, transplantation, neutropenia and steroid therapy. The diagnosis of mucormycosis is rarely suspected and antemortem diagnosis is made in only 25%-50% of cases[5].
A 42-year-old lady received chemotherapy for NK cell lymphoma and, concomitant with disease progression, she presented with ascites and pleural effusion. She complained of abdominal pain and fever in February 2009. Physical examination showed generalized tenderness and the aspirated peritoneal fluid was turbid. Laparotomy was undertaken for suspected perforated viscus. On laparotomy, the ascitic fluid was grossly turbid. The cecum was grossly dilated but no perforation was identified. The rest of the laparotomy was grossly normal except for the inflamed omentum which was densely adhered to the pelvic cavity. Peritoneal lavage with saline was the only procedure performed. Postoperatively, Tazocin, amikacin and voriconazole were given as Klebsiella, Escherichia coli and Candida albicans were identified from the peritoneal fluid. However, the patient’s condition rapidly deteriorated and she died two weeks after operation. The autopsy showed extensive involvement of fungal elements inside the abdominal cavity. Also, branching septate fungi were isolated in one of the peritoneal fluid cultures after the patient succumbed and later confirmed to be Rhizopus species.
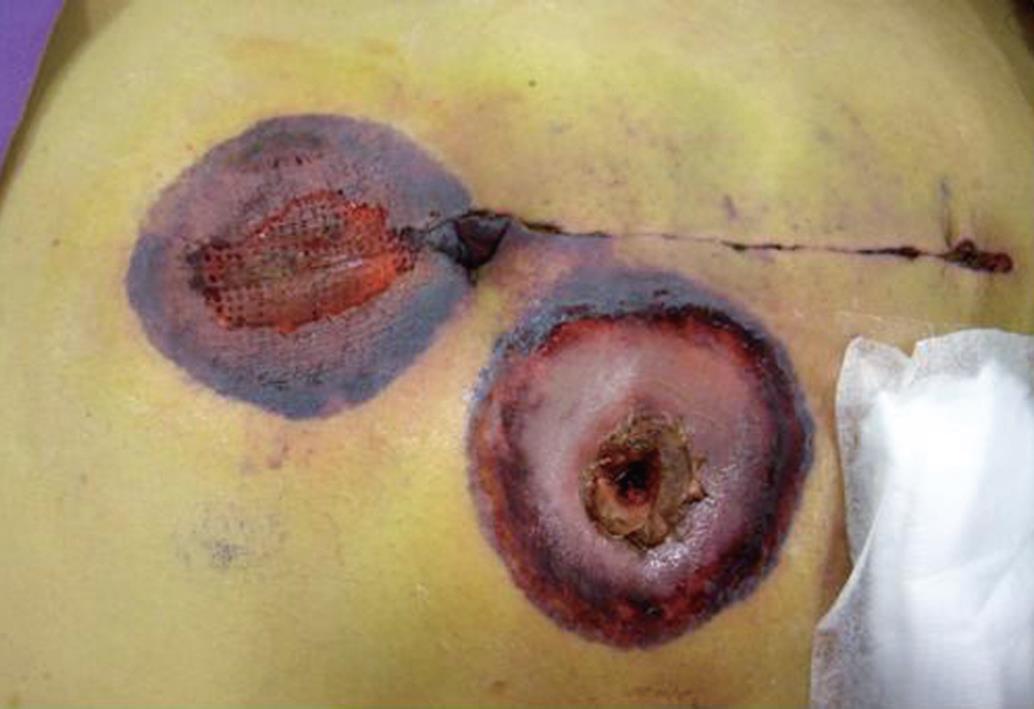
A 57-year-old man had relapsed diffuse large B-cell lymphoma and underwent palliative chemotherapy and radiotherapy. He presented with peritonitis in September 2008. An urgent computer tomography (CT) scan of the abdomen showed free intraperitoneal gas; emergency laparotomy was undertaken and two 1-cm perforations with indurated edges were identified over the cecum. Ileocecal resection and end ileostomy were performed in view of gross peritoneal contamination. Histological examination showed lymphoma involving the colonic wall and causing the perforations. Broad-spectrum antibiotics (meropenem and vancomycin) and prophylactic fluconazole were given in view of multiple bacteria shown in peritoneal fluid cultures. A well-demarcated gangrenous skin necrosis appeared over the peristomal area and at the upper midline wound on day 8 (Figure 1). Finally, the patient died of multiorgan failure two days later. Subsequent wound swab culture revealed Rhizopus species. No autopsy was performed because of refusal by the family.
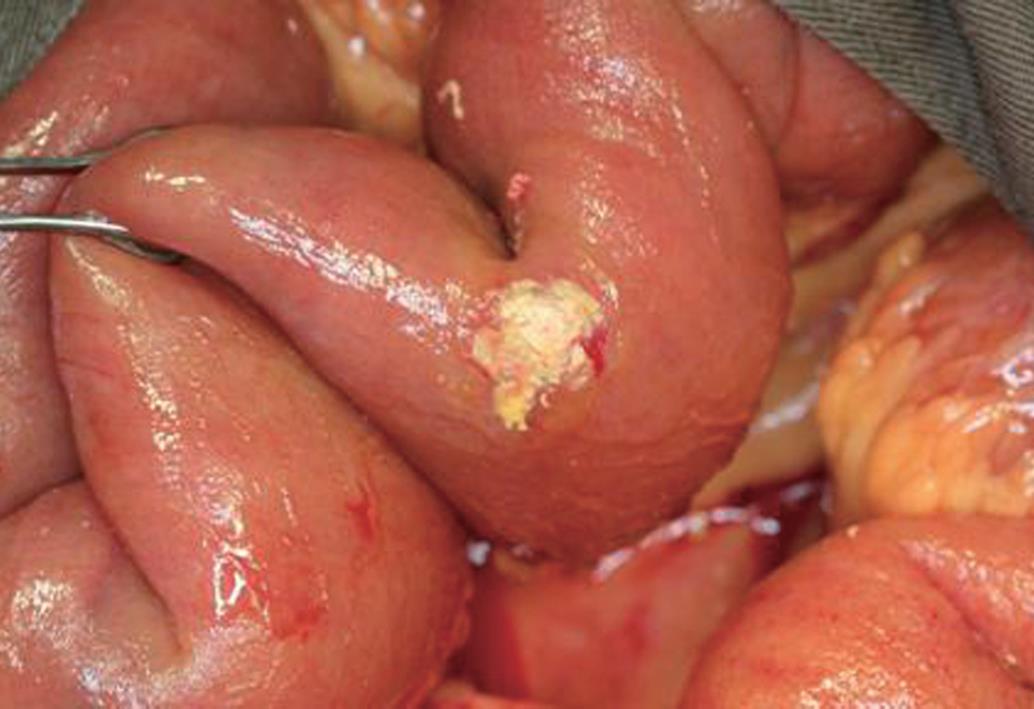
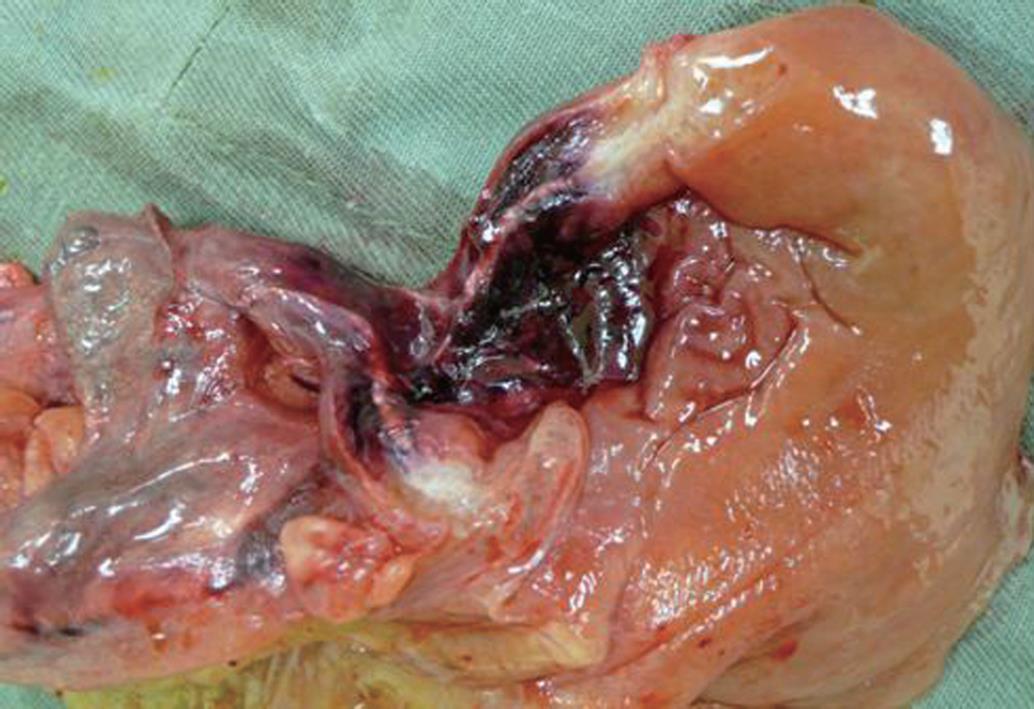
A 38-year-old male, with persistent precursor B cell acute lymphoblastic leukemia, developed abdominal pain and fever on the eighth day after receiving clofarabine therapy in February 2009. An urgent abdominal CT scan showed features suggestive of appendiceal abscess with suspected pneumoperitoneum. He underwent emergency laparotomy and was found to have gangrenous appendicitis with localized retrocecal abscess. Ileocecal resection and primary anastomosis were performed. Postoperatively, liposomal amphotericin, caspofungin and posaconazole were started immediately as the microbiologist identified Rhizopus species in the specimen. Granulocyte colony-stimulating factor was also prescribed for severe neutropenia. The patient recovered well and was transferred back to the general ward. One week later, he still had persistent abdominal distension and developed deep venous thrombosis of the right lower limb. A abdominal CT scan showed that there was no contrast opacification at the right external iliac vessels and impaired contrast enhancement at the terminal ileum. Exploratory laparotomy was undertaken on day 12 after the first operation and multiple hardened fungal colonies were noted over the omentum and small bowel (Figure 2). An inflammatory mass compressed the right iliac vessels, causing decreased venous blood flow. A 20-cm segment of small bowel proximal to the previous ileocolic anastomosis densely adhered to the previous operative site and was ischemic. Small bowel resection, end ileostomy and omentectomy were performed. Multiple patches of full-thickness bowel ischemia were seen over the colonic wall (Figure 3) and mucormycosis was confirmed on histological examination. Though extensive surgical debridement and maximal medical support were administered, the patient died four days after the second laparotomy.
Mucormycosis is an opportunistic fungal infection caused by fungi of the order Mucorales[4]. However, it is often inappropriately interchanged with the term zygomycosis (infection caused by the class Zygomycetes) in medical literature. The Zygomycetes are divided into two orders: the Entomophthorales, containing very rare pathogenic species; and the Mucorales, which contain the most common human pathogens, including Rhizopus, Mucor, Absidia, and Cunninghamellaceae (Table 1)[3,4]. Differentiation among these species is based on the morphology of the asexual cycle, physiologic characteristics, and zygospore production. Nonetheless, they rarely cause disease because of the low virulence of the organisms and thus they mainly affect immunocompromised individuals. Novel immunosuppressive therapies and other advances in the understanding of disease pathology and diagnosis have contributed to patients living longer with previously debilitating medical problems and impaired immune systems.
| Class | Order | Family | Genus | Species causing human diseases |
| Zygomycetes | Mucorales | Mucoraceae | Rhizopus | R. oryzae1,2 |
| R. microsporus var. rhizopodiformis1,2 | ||||
| Rhizomucor | R. pusillus1,2 | |||
| Mucor | M. circinelloides1,2 | |||
| Absidia | A. corymbifera1,2 | |||
| Apophysomyces | A. elegans1,2 | |||
| Cunninghamellaceae | Cunninghamella | C. bertholletiae12 | ||
| Entomophthorales | Ancylistaceae | Conidiobolus | C. coronatus2 | |
| Basidiobolaceae | Basidiobolus | B. ranarum2 |
The most common agent of mucormycosis is the Rhizopus species, which is considerably more virulent than other fungi in the order. Similar to Aspergillus, the pathogenesis may be attributed to the tendency to be angioinvasive (vasculotropism) as blood vessels are the best source of oxygen, resulting in local ischemia, necrosis, and tissue infarction as well as providing the nidus for hematogenous dissemination[3]. Though being less clearly understood than for aspergillosis, several risk factors or predisposing conditions have been well described in the literature. In a large review of 929 patients with zygomycosis[6], diabetes was the most common underlying risk factor (36%), followed by malignancy (17%), solid organ transplantation (7%), desferroxamine therapy (6%), and bone marrow transplantation (5%). Most of these conditions are associated with impairment of normal leukocyte immune function. Impairment of this function or a critical decline in white cell number is associated with increased risk for invasive fungal infections. Apart from differences in environmental factors, changes in transplantation procedures, new use of immunosuppressives and the use of voriconazole for the prophylaxis of opportunistic fungal infections have been proposed as risk factors for developing zygomycosis[7,8]. Voriconazole is not active against Zygomycetes and subsequently may provide selective growth of these fungi. Breakthrough zygomycosis after voriconazole treatment has been reported but the association is still uncertain[7,9].
Mucormycosis can manifest as different clinical forms, namely rhinocerebral, pulmonary, cutaneous, gastrointestinal, central nervous system, and disseminated/miscellaneous. Gastrointestinal mucormycosis is the rarest form and constitutes only 7% of all mucormycosis cases[6,10]. It most commonly involves the stomach (57.5%), followed by colon (32.3%) and ileum (6.9%)[10]. This rare and opportunistic infection has been reported in neonates, probably due to their immature immunity, presenting as necrotizing enterocolitis. In published literature, less than 20 cases of gastrointestinal mucormycosis with colon involvement in adult patients have been published in the last two decades (Table 2) [10-22]. It also occurs in those with severe malnutrition and intrinsic abnormalities of the gastrointestinal tract, including amoebic colitis, typhoid, pellagra and kwashiorkor[10]. The infection may arise from ingestion of fungal spores on food or contaminated sputum. Actually, the three patients we have described here were also reported in an outbreak of intestinal infection in our hospital[23]. They had sole intestinal involvement, likely due to the intake of contaminated allopurinol tablets and commercially packaged food items[23].
| Age (yr)/Sex | Morbidity | Location | Operation | Medications | Outcome | Ref. | Yr |
| 21/F | APL | Cecum | Colectomy | Amp B | Survived | [11] | 1985 |
| 21/F | AML | Descending | Hartmann | Amp B | Survived | [12] | 1986 |
| 54/F | ALL | Cecum | Right hemicolectomy | Amp B | Dead | [13] | 1998 |
| 42/F | SLE | Left colon + stomach | Subtotal colectomy | Amp B | Dead | [14] | 1998 |
| 35/M | Liver Tx | Cecum + liver | Primary repair | - | Dead | [15] | 1999 |
| 53/F | NHL | Sigmoid + transverse | Hartmann + right hemicolectomy | Amp B → Lip Amp + GMCSF | Survived | [16] | 2000 |
| 48/F | ALL | Ileocolic + liver | Bowel resection | Amp B → Lip Amp | Dead | [17] | 2000 |
| 33/M | Renal Tx | Right colon + esophagus | Graft nephrectomy | Amp B + GMCSF | Survived | [18] | 2001 |
| 65/M | COAD | Right colon | Right hemicolectomy | Amp B | Survived | [19] | 2004 |
| 56/F | ALL | Ileocolic | Right hemicolectomy | Lip Amp | Dead | [20] | 2005 |
| 43/F | Renal Tx | Cecum | Right hemicolectomy | Lip Amp | Survived | [21] | 2005 |
| 58/? | MS | Sigmoid | Hartmann | - | Dead | [22] | 2006 |
A typical gastrointestinal lesion consists of a dark ulcer with sharply demarcated edges and with necrosis and thrombosis in adjacent vessels. The infection can extend from the lumen of the gut and may cause obstruction, perforation or bleeding. Initial presentations may be abdominal pain and distension, fever, and diarrhea. If there is extensive bowel involvement with multiple ulcers caused by the fungal infection, it may present with gastrointestinal bleeding or even visceral perforation at late presentation[10]. Consequently, neutropenic fever can be a common presenting feature of mucormycosis, as in our patients. A high degree of clinical suspicion is needed to diagnose this rare condition. Therefore, persistent severe abdominal pain in a patient with neutropenia should alert the clinicians to the possibility of this invasive fungal infection.
Diagnosis depends on histological examination for the presence of predominantly aseptate wide hyphae with focal bulbous and non dichotomous branching occasionally at right angles[4]. Over 94% of sampled tissues also show infarction and angioinvasion on histology examination[24]. Though culture remains the predominant way in which one can identify fungal species, it is positive in only 52% of autopsy cases and only 30% of surgical specimens[25]. This is because the infection may be localized and cannot be detected in all portions of the specimen submitted for culture. Nowadays there are no reliable serologic or skin tests for mucormycosis. Recently, some have attempted to improve the diagnosis by detecting fungal nucleic acid in the serum using polymerase chain reaction (PCR) or in situ hybridization techniques[3]. As an adjuvant diagnostic tool, this can be used for confirming the presence of presumptive organisms when histology is positive and cultures are negative. It may provide some guidance in selecting appropriate antifungal therapy when the histological diagnosis is undetermined. Besides pathological and microbiological diagnosis, CT/MRI scan[26] and endoscopy examination[11,14] have been described in the literature. Endoscopic features of colonic mucormycosis have been reported as hemorrhagic, edematous mucosa with erosions, resembling endoscopic features of ischemic colitis. A smooth, mushroom-like greenish fungal mass with a small base of attachment to the bowel wall may be seen. Occasionally a black crust overlies the ulcerated area[11].
From a surgeon’s point of view, the principal of management of colonic mucormycosis in these severely immunocompromised patients largely depends on timely diagnosis, reversal of the underlying predisposing conditions, early surgical debridement, and rapid initiation of effective systemic antifungal therapy[3]. As neutropenic enterocolitis is the commonest cause in the neutropenic cancer patient, careful serial clinical observation with liberal use of CT scanning is suggested[27,28]. Contrast CT scans may show a thickened colonic wall with decreased attenuation due to edema, necrosis, or a collection of extraluminal fluid[28]. Also, the scan may detect the presence of a small amount of free gas or pneumatosis intestinalis not visible on plain films, suggestive of bowel perforation. If the initial conservative treatment is not successful, including bowel rest, hydration, and broad-spectrum antibiotics, some have advocated a more aggressive surgical intervention[29-31]. As for our patients, they were initially managed in their medical wards for chemotherapy-induced neutropenic colitis; however, our surgical team was consulted after failed conservative treatment.
In the first patient of our series, exploratory laparotomy was selected for investigating suspected bowel perforation in view of persistent peritoneal signs and turbid peritoneal fluid aspirated. However, only dilated cecum with edematous wall but no perforation were identified; the intraoperative diagnosis of neutropenic enterocolitis was compatible with this finding, so that only peritoneal lavage was performed and conservative management was continued afterwards. Although the patient’s condition was rapidly deteriorating, no surgical intervention was considered in view of multiple organ failure.
Continuing aggressive medical treatment and supportive measures after surgery for neutropenic enterocolitis have been reported, with high mortality[28]. Fungal neutropenic enterocolitis has been reported as fatal[32] as the mucormycosis is a highly angioinvasive infection, resulting in extensive thrombosis and tissue necrosis, and antifungal agents often display poor penetration at the site of infection. Removal of as much of the infected or devitalized tissue as possible while the infection is localized provides the greatest benefit. In the study by Roden et al[6], the survival rate was 57% (51/90) for those treated with surgery alone and 62% (369/596) for those treated with some form of antifungal therapy alone. However, the survival increased to 70% (328/470) for those treated with combined surgery and antifungal therapy. In our third patient, the diagnosis of mucormycosis was confirmed pathologically soon after the first operation, and he began antifungal therapy immediately. Currently, the recommended antifungal therapy for mucormycosis includes amphotericin B and its liposomal preparation which can be delivered with reduced nephrotoxicity[3]. The common prophylactic antifungal agents used in neutropenic patients (such as fluconazole and itraconazole) or even new agents (voriconazole and caspofungin) are not active against the Mucorales order in clinical and in vitro studies. An orally available broad-spectrum investigational triazole, posaconazole, seems to possess activity against this fungus and improves patients’ survival in refractory cases[33]. Furthermore, in addition to posaconazole treatment, another exploratory laparotomy with extensive small bowel resection and omentectomy was performed in our patient, in order to debride all infection foci. This approach has been reported as providing a survival advantage in the published literature[16,19,21].
Apart from early diagnosis, as mentioned before, correction of any reversible predisposing factors, such as by rectifying diabetic ketoacidosis, withdrawing desferroxamine therapy or reducing the level of immunosuppression, is an important consideration in patient management. In neutropenic patients, resolution of neutropenia is directly correlated with clinical improvement and better outcomes[34]. Without neutrophil recovery, antifungal drugs are ineffective. Cytokines, such as interferon-γ and granulocyte macrophage-colony stimulating factor (GMC-SF), can reduce the degree and duration of neutropenia and subsequent infections in these patients. Other non-medication-based interventions, such as hyperbaric oxygen therapy[35] and novel iron chelators[36], have found limited success as an adjunctive treatment.
In summary, we report three cases of ileocolic mucormycosis presenting as acute abdomen in neutropenic patients which failed to respond to combined surgical and medical treatment. Though the new antifungal agent posaconazole is effective against the Mucorales order of fungi, timely diagnosis and adequate surgical debridement are essential for the successful management of colonic mucormycosis.
Peer reviewers: Dr. Benjamin Perakath, Professor, Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India; Venkatesh Shanmugam, MBBS, MS (Gen. Surg.), Dip.NB (Gen. Surg.), FRCS (Glasg.), MD, Specialist Registrar (Trent Deanery), Royal Derby Hospital, Uttoxeter Road, Derby, DE22 3NE, United Kingdom
S- Editor Wang YR L- Editor Logan S E- Editor Lin YP
| 1. | Kara IO, Tasova Y, Uguz A, Sahin B. Mucormycosis-associated fungal infections in patients with haematologic malignancies. Int J Clin Pract. 2009;63:134-139. |
| 2. | Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Piccardi M, Corvatta L, D'Antonio D, Girmenia C, Martino P. Mucormycosis in hematologic patients. Haematologica. 2004;89:207-214. |
| 3. | Kontoyiannis DP, Lewis RE. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect Dis Clin North Am. 2006;20:581-607, vi. |
| 4. | Kontoyiannis DP; Mucormycosis. Ann Intern Med. 1980;93:93-108. |
| 5. | Nosari A, Oreste P, Montillo M, Carrafiello G, Draisci M, Muti G, Molteni A, Morra E. Mucormycosis in hematologic malignancies: an emerging fungal infection. Haematologica. 2000;85:1068-1071. |
| 6. | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653. |
| 7. | Oren I. Breakthrough zygomycosis during empirical voriconazole therapy in febrile patients with neutropenia. Clin Infect Dis. 2005;40:770-771. |
| 8. | Brown J. Zygomycosis: an emerging fungal infection. Am J Health Syst Pharm. 2005;62:2593-2596. |
| 9. | Ustun C, Farrow S, DeRemer D, Fain H, Jillella AP. Early fatal Rhizopus infection on voriconazole prophylaxis following allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:807-808. |
| 10. | Thomson SR, Bade PG, Taams M, Chrystal V. Gastrointestinal mucormycosis. Br J Surg. 1991;78:952-954. |
| 11. | Agha FP, Lee HH, Boland CR, Bradley SF. Mucormycoma of the colon: early diagnosis and successful management. AJR Am J Roentgenol. 1985;145:739-741. |
| 12. | Parra R, Arnau E, Julia A, Lopez A, Nadal A, Allende E. Survival after intestinal mucormycosis in acute myelogenous leukemia. Cancer. 1986;58:2717-2719. |
| 13. | Elnakadi I, Mehdi A, Franck S, Roger T, Larsimont D, Pector JC. Cecal infarct: report of a case. Dis Colon Rectum. 1998;41:1585-1586. |
| 14. | Hosseini M, Lee J. Gastrointestinal mucormycosis mimicking ischemic colitis in a patient with systemic lupus erythematosus. Am J Gastroenterol. 1998;93:1360-1362. |
| 15. | Mazza D, Gugenheim J, Baldini E, Mouiel J. Gastrointestinal mucormycosis and liver transplantation; a case report and review of the literature. Transpl Int. 1999;12:297-298. |
| 16. | Mir N, Edmonson R, Yeghen T, Rashid H. Gastrointestinal mucormycosis complicated by arterio-enteric fistula in a patient with non-Hodgkin's lymphoma. Clin Lab Haematol. 2000;22:41-44. |
| 17. | Suh IW, Park CS, Lee MS, Lee JH, Chang MS, Woo JH, Lee IC, Ryu JS. Hepatic and small bowel mucormycosis after chemotherapy in a patient with acute lymphocytic leukemia. J Korean Med Sci. 2000;15:351-354. |
| 18. | Ju JH, Park HS, Shin MJ, Yang CW, Kim YS, Choi YJ, Song HJ, Kim SW, Chung IS, Bang BK. Successful treatment of massive lower gastrointestinal bleeding caused by mixed infection of cytomegalovirus and mucormycosis in a renal transplant recipient. Am J Nephrol. 2001;21:232-236. |
| 19. | Azadeh B, McCarthy DO, Dalton A, Campbell F. Gastrointestinal zygomycosis: two case reports. Histopathology. 2004;44:298-300. |
| 20. | Karanth M, Taniere P, Barraclough J, Murray JA. A rare presentation of zygomycosis (mucormycosis) and review of the literature. J Clin Pathol. 2005;58:879-881. |
| 21. | Echo A, Hovsepian RV, Shen GK. Localized cecal zygomycosis following renal transplantation. Transpl Infect Dis. 2005;7:68-70. |
| 22. | Sakorafas GH, Tsolakides G, Grigoriades K, Bakoyiannis CN, Peros G. Colonic mucormycosis: an exceptionally rare cause of massive lower gastrointestinal bleeding. Dig Liver Dis. 2006;38:616-617. |
| 23. | Cheng VC, Chan JF, Ngan AH, To KK, Leung SY, Tsoi HW, Yam WC, Tai JW, Wong SS, Tse H. Outbreak of intestinal infection due to Rhizopus microsporus. J Clin Microbiol. 2009;47:2834-2843. |
| 24. | Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med. 2001;125:375-378. |
| 25. | Tarrand JJ, Lichterfeld M, Warraich I, Luna M, Han XY, May GS, Kontoyiannis DP. Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol. 2003;119:854-858. |
| 26. | Horger M, Hebart H, Schimmel H, Vogel M, Brodoefel H, Oechsle K, Hahn U, Mittelbronn M, Bethge W, Claussen CD. Disseminated mucormycosis in haematological patients: CT and MRI findings with pathological correlation. Br J Radiol. 2006;79:e88-e95. |
| 27. | Scott-Conner CE, Fabrega AJ. Gastrointestinal problems in the immunocompromised host. A review for surgeons. Surg Endosc. 1996;10:959-964. |
| 28. | Badgwell BD, Cormier JN, Wray CJ, Borthakur G, Qiao W, Rolston KV, Pollock RE. Challenges in surgical management of abdominal pain in the neutropenic cancer patient. Ann Surg. 2008;248:104-109. |
| 29. | Tokar B, Aydoğdu S, Paşaoğlu O, Ilhan H, Kasapoğlu E. Neutropenic enterocolitis: is it possible to break vicious circle between neutropenia and the bowel wall inflammation by surgery? Int J Colorectal Dis. 2003;18:455-458. |
| 31. | Alt B, Glass NR, Sollinger H. Neutropenic enterocolitis in adults. Review of the literature and assessment of surgical intervention. Am J Surg. 1985;149:405-408. |
| 32. | Gorschlüter M, Mey U, Strehl J, Schmitz V, Rabe C, Pauls K, Ziske C, Schmidt-Wolf IG, Glasmacher A. Invasive fungal infections in neutropenic enterocolitis: a systematic analysis of pathogens, incidence, treatment and mortality in adult patients. BMC Infect Dis. 2006;6:35. |
| 33. | van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42:e61-e65. |
| 34. | Antman KS, Griffin JD, Elias A, Socinski MA, Ryan L, Cannistra SA, Oette D, Whitley M, Frei E 3rd, Schnipper LE. Effect of recombinant human granulocyte-macrophage colony-stimulating factor on chemotherapy-induced myelosuppression. N Engl J Med. 1988;319:593-598. |
| 35. | Barratt DM, Van Meter K, Asmar P, Nolan T, Trahan C, Garcia-Covarrubias L, Metzinger SE. Hyperbaric oxygen as an adjunct in zygomycosis: randomized controlled trial in a murine model. Antimicrob Agents Chemother. 2001;45:3601-3602. |
| 36. | Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE Jr, Spellberg BJ. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649-2657. |