Published online Mar 7, 2010. doi: 10.3748/wjg.v16.i9.1086
Revised: December 17, 2009
Accepted: December 24, 2009
Published online: March 7, 2010
AIM: To investigate the expression of vascular endothelial cell growth factor (VEGF) and its receptors Fms-like tyrosine kinase 1 (FLT-1) and fetal liver kinase 1 (FLK-1) in colorectal carcinoma (CRC), and the blocking effects of small interfering RNAs (siRNAs) on VEGF expression in human colorectal cancer HCT116 cells.
METHODS: Immunohistochemical staining for VEGF, FLT-1 and FLK-1 proteins was performed in 82 cases of CRC and 14 normal colorectal mucosae. A siRNA targeting VEGF was synthesized and transfected into HCT116 cells using lipofectamine 2000. Immunocytochemical staining and Western blotting analyses were performed to detect the expression of VEGF protein. The suppressive effect of the siRNA on cell proliferation was detected using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide (MTT) assay. Cellular apoptosis was detected using flow cytometry (FCM).
RESULTS: The expression of VEGF, FLT-1 and FLK-1 in tumor tissues was significantly higher than that in normal tissues (P = 0.008, P = 0.000, P = 0.000). The expression of VEGF was positively correlated with both lymph node metastasis and clinical stage (P = 0.009 and P = 0.025, respectively). Immunocytochemistry showed that the expression of VEGF was weakly positive and Western blotting indicated a significant reduction in VEGF-siRNA cell protein levels. VEGF-siRNA cell growth inhibition was assessed by the MTT assay, and the tumor cell proliferation rate was significantly different at 24, 48, and 72 h after transfection. FCM results showed that the VEGF-siRNA group had an apparent aneuploid peak.
CONCLUSION: VEGF, FLT-1 and FLK-1 are associated with colorectal carcinogenesis. siRNA silencing of the VEGF gene suppresses proliferation, and induces apoptosis in HCT116 cells. The results suggest that VEGF may be a new gene therapy target for colorectal cancer.
- Citation: Yin Y, Cao LY, Wu WQ, Li H, Jiang Y, Zhang HF. Blocking effects of siRNA on VEGF expression in human colorectal cancer cells. World J Gastroenterol 2010; 16(9): 1086-1092
- URL: https://www.wjgnet.com/1007-9327/full/v16/i9/1086.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i9.1086
Colorectal carcinoma (CRC) is one of the most common malignant diseases in China and has a poor survival rate. The carcinogenesis and development of CRC is a complex process attributable to numerous factors. Angiogenesis plays an important role in tumor growth. Neovascularization is regulated by multiple factors, but the most important one is vascular endothelial cell growth factor (VEGF), which exerts its effects of increasing microvascular growth and permeability via specific receptors[1]. Fms-like tyrosine kinase 1 (FLT-1) and fetal liver kinase 1 (FLK-1) are the most important among these receptors. At present, the main treatment for CRC is excision of the primary tumor, followed by radiotherapy and chemotherapy. However, in many cases, this still leaves problems such as metastatic lesions and subsequent cancer recurrence. Recently, following the development of modern molecular biology, in-depth researches have been conducted in developing new strategy of CRC treatment at a genetic level. In particular, RNA interference (RNAi) technology has the potential to become an effective treatment for tumors[2], and has established a new area of clinical therapy for CRC[3]. In this study, immunohistochemical staining using a streptavidin-peroxidase method for the VEGF, FLT-1, and FLK-1 proteins was performed on 82 CRC and 14 normal colorectal mucosal samples in an attempt to explore their roles in CRC. Moreover, a VEGF-targeting small interfering RNA (siRNA) was transferred into human colorectal cancer HCT116 cells to explore its effects on proliferation and apoptosis.
Eighty-two CRC specimens and matched adjacent normal mucosae were obtained from the Department of Pathology, First Affiliated Hospital, Anhui Medical University between 2007 and 2008. None of the patients had been treated with radiotherapy or chemotherapy before surgery. Samples were taken from areas of the tumor tissue with no evidence of hemorrhage or putrescence. Samples of matched normal mucosa were collected from the surgical cutting edge, which was approximately 3-5 cm away from the cancerous lesion. The clinical diagnosis of all 82 patients was confirmed by histological examination after surgery. The colon cancer cell line, HCT116, was obtained from the Chinese Academy of Sciences.
The mouse anti-human VEGF monoclonal antibody (clone VG1), and rabbit anti-human FLT-1 and FLK-1 polyclonal antibodies(clone RAB-0525, clone RAB-0522) were purchased from Maixin Co. (China). Immunohistochemistry was performed as follows: Sections were deparaffinized and rehydrated for 3 × 3 min in xylene; 3 × 2 min in 100% ethanol; 2 min in 95% ethanol, 2 min in 75% ethanol and, finally, 2 × 1 min in distilled water. Antigens were recovered by heating in a microwave oven for 15 min, and the sections were washed for 3 × 5 min with phosphate buffered solution (PBS). Endogenous peroxidase activity was blocked by soaking the slides in a solution of 3% hydrogen peroxide for 15 min at room temperature (RT), followed by washing for 3 × 5 min with PBS. Non-immune animal blood serum (50 μL) was added to each section for 15 min at RT, followed by primary antibody (50 μL). Slides were incubated overnight at 4°C in a humidified chamber, then washed for 3 × 10 min in PBS. Biotin-labeled secondary antibody (50 μL) was added to each section and kept for 15 min at 37°C, followed by 3 × 5 min washes with PBS. Samples were incubated for 15 min at RT and washed for 3 × 5 min in PBS, before addition of 100 L freshly prepared 3,3-diaminobenzidine (DAB) for approximately 5-20 min. The reaction was stopped by washing in cold water. Slides were counterstained with hematoxylin followed by a sealing procedure using neural gum. A known-positive section served as the positive control, and sections in which the primary antibody was omitted served as negative controls. VEGF, FLT-1 and FLK-1 protein expressions were observed in the cytoplasm and graded according to Volm’s standard[4]: (1) Counting the percentage of positive cells within the total number in each high power field ( no positive cells = grade 0, less than 25% positive = grade 1, 25%-50% = grade 2, > 50% = grade 3); and (2) The intensity of staining: no positively stained cells = grade 0, weak staining = grade 1, moderate staining = grade 2, strong staining = grade 3. Aggregate analysis of the results from (1) and (2): grade 0 = negative (-), grade 1-2 = weakly positive (+), grade 3-4 = moderately positive (++), grade 3-4 = strongly positive (+++). The intensity of the staining was estimated independently by two pathologists.
The sequence of the VEGF-targeting siRNA was as follows: Sense 5'-GGAGUACCCUGAUGAGAUCdTdT-3', Antisense 5'-GAUCUCAUCAGGGUACUCCdTdT-3'. All siRNAs were purchased from Shanghai ShineGene Molecular Biotechnology Co., Ltd. Each siRNA was resuspended in water and the stock solutions (20 μmol/L) were stored at 4°C until use.
The colon cancer cell line, HCT116, was grown as a monolayer in RPMI-1640 (Hyclone) containing 10% fetal bovine serum. Cells were maintained at 37°C in a humidified 5% CO2 incubator.
siRNA transfection was done according to the protocol supplied by Invitrogen. Briefly, 1 × 105 cells were seeded into six-well plates containing antibiotic-free medium and incubated overnight. For each well, 5 μL siRNA was mixed with 125 μL OPTI-MEM I. The mixture was then combined with a solution of 5 μL lipofectamine in 125 μL OPTI-MEM I and, after a 20-minute incubation at RT, the mixture was applied to the cells in a final volume of Opti-MEM I, making the final concentration of 100 nmol/L for each siRNA. After incubation for 6 h at 37°C, RPMI-1640 supplemented with serum was added. Cells were then cultured for an additional 24 h at 37°C before analysis.
After 48 h transfection, HCT116 cells were fixed in cold methanol/acetone (1:1) at -20°C for 20 min and washed twice in PBS. The fixed cells were treated with 3% hydrogen peroxide for 5 min to eliminate endogenous peroxidase activity. After blocking with 1.5% horse serum for 20 min, the cells were incubated with primary anti-Survivin antibody (Santa Cruz) for 30 min at RT, or overnight at 4°C. The cells were then incubated with biotinylated goat anti-mouse IgG (Dako), followed by peroxidase-labeled streptavidin solution (Dako) for 20 min at RT. Antibodies were visualized using DAB (Sigma). All cells were counterstained with hematoxylin and the experiments were repeated three times. Negative control slides omitting the primary antibody were included for each staining.
Anti-VEGF and anti-β-actin antibodies were obtained from Zhongshan Goldenbridge Biotechnology Co., Ltd. (Santa Cruz). For Western blotting analysis, cells were harvested 48 h after transfection, washed with cold PBS, and lysed with phenylmethanesulfonyl fluoride lysis buffer. After centrifugation at 12 000 r/min for 30 min, the supernatant was analyzed for protein content using the bicinchoninic acid reagent. Total protein (50 μg) was electrophoresed on a 10% SDS-PAGE gel, and transferred onto a polyvinylidene fluoride membrane. The membranes were blocked with PBS buffer containing 5% fat-free milk and 0.1% Tween20 for 30 min at RT, and then incubated with primary anti-VEGF antibody for at least 1 h at RT, or overnight at 4°C. Finally, the membranes were washed three times with PBS containing 0.1% Tween20, incubated with peroxidase-conjugated secondary antibodies, and developed using the ECL reagent (Pierce).
Cells were grown in 96-well plates (1 × 103 cells/well) and then transfected with VEGF-targeting siRNA. Control cells were treated with DMEM containing 0.1% dimethyl sulfoxide (DMSO). At 24, 48, and 72 h following siRNA transfection, 20 μL of MTT was added to each well to a final concentration of 0.5%. After a 4-h incubation at 37°C in the dark, 150 μL DMSO was added to each well for 10 min to dissolve the formazan crystals. The absorbance was measured using an ELISA reader (EXL800, USA) at 490 nm. All experiments were repeated three times. The viability of the siRNA-transfected cells was expressed as a percentage of population growth plus the standard error of the mean relative to that of untransfected control cells. Cell death caused by siRNA-transfection was calculated as a percentage of inhibition as follows: % inhibition = (1 - mean experimental absorbance/mean control absorbance) × 100.
At 48 h after transfection, samples (1 × 106 cells) were fixed in 70% (v/v) ethanol, washed, and RNase (0.25 mg/mL) and Tween20 [0.1% (v/v)] were added for 30 min at RT. The cells were then stained with propidium iodide and analyzed using a flow cytometer (EPICS XL; Beckman). A sub-G0-G1 peak was detected on the DNA plots using the Cell Quest software. The peak indicates the apoptosis rate of the cells.
The statistical software package SPSS 13.0 was used. Data were analyzed using either the χ2 test or Fisher’s exact test. Non-parametric Spearman rank correlation analysis was used for analysis of ranked data. The rates were compared using Z tests. A P value < 0.05 was regarded as significant.
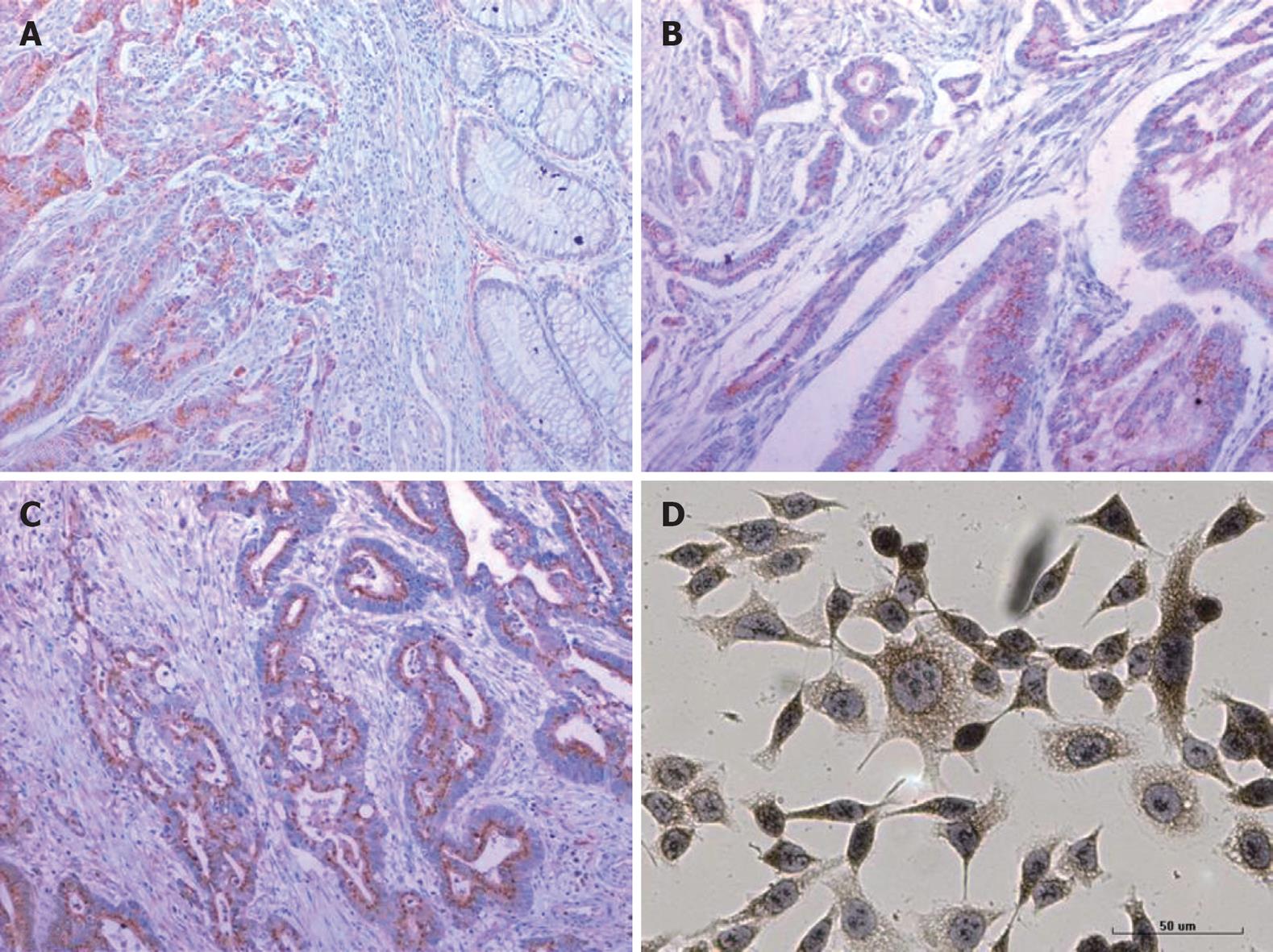
Of the 82 CRC cases examined, 57 (69.5%) expressed VEGF protein. Among them, three cases (5.2%) were weakly positive (+), 30 cases (52.6%) were moderately positive (++), and 24 cases (42.1%) were strongly positive (+++). Of the 14 normal mucosa cases, only 4 (28.6%) were weakly positive for VEGF protein expression. These results confirmed that VEGF protein was present at significantly higher levels in CRC cases than in normal mucosa (P < 0.01). Also, FLT-1 (82.9%) and FLK-1 (81.7%) protein expression was significantly higher in CRC cases than in normal mucosa (P < 0.01) (Figure 1 and Table 1). We also found a positive correlation between FLT-1 and FLK-1 expression (r = 0.457, P < 0.05) in the CRC samples.
The positive rate for VEGF protein in the lymph node-positive group (86.7%) was significantly higher than that in the lymph node-negative group (59.6%, P < 0.01), and there were significant differences between the stage A, B, and C + D groups (57.9%, 60.6%, and 86.7%, respectively, P < 0.05). The expression of VEGF protein was not correlated with age, gender, depth of tumor invasion, or degree of differentiation (P > 0.05). Moreover, the expression of the FLT-1 and FLK-1 proteins was not correlated with any clinical pathological features of CRC (P > 0.05, Table 2).
| n | VEGF | FLT-1 | FLK-1 | ||||
| Negative | Positive | Negative | Positive | Negative | Positive | ||
| Age (yr) | |||||||
| < 60 | 41 | 14 | 27 (65.9) | 10 | 31 (75.6) | 9 | 32 (78.0) |
| ≥ 60 | 41 | 11 | 30 (73.2) | 4 | 37 (90.2) | 6 | 35 (85.4) |
| Gender | |||||||
| Male | 49 | 13 | 36 (73.5) | 11 | 38 (77.6) | 10 | 39 (79.6) |
| Female | 33 | 12 | 21 (63.6) | 3 | 30 (90.9) | 5 | 28 (84.8) |
| Depth of tumor invasion | |||||||
| Not exceed muscular layer | 25 | 10 | 15 (60.0) | 5 | 20 (80.0) | 5 | 20 (80.0) |
| Exceed muscular layer | 57 | 15 | 42 (73.7) | 9 | 48 (84.2) | 10 | 47 (82.5) |
| Lymph node metastasis | |||||||
| Negative | 52 | 21 | 31 (59.6) | 8 | 44 (84.6) | 8 | 44 (84.6) |
| Positive | 30 | 4 | 26 (86.7)b | 6 | 24 (80.0) | 7 | 23 (76.7) |
| Degree of differentiation | |||||||
| Well | 20 | 10 | 10 (50.0) | 4 | 16 (80.0) | 5 | 15 (75.0) |
| Moderate | 41 | 10 | 31 (75.6) | 5 | 36 (87.8) | 5 | 36 (87.8) |
| Poor | 21 | 5 | 16 (76.2) | 5 | 16 (76.2) | 5 | 16 (76.2) |
| Duke’s stage | |||||||
| A | 19 | 8 | 11 (57.9)a | 4 | 15 (78.9) | 4 | 15 (78.9) |
| B | 33 | 13 | 20 (60.6) | 4 | 29 (87.9) | 4 | 29 (87.9) |
| C + D | 30 | 4 | 26 (86.7) | 6 | 24 (80.0) | 7 | 23 (86.7) |
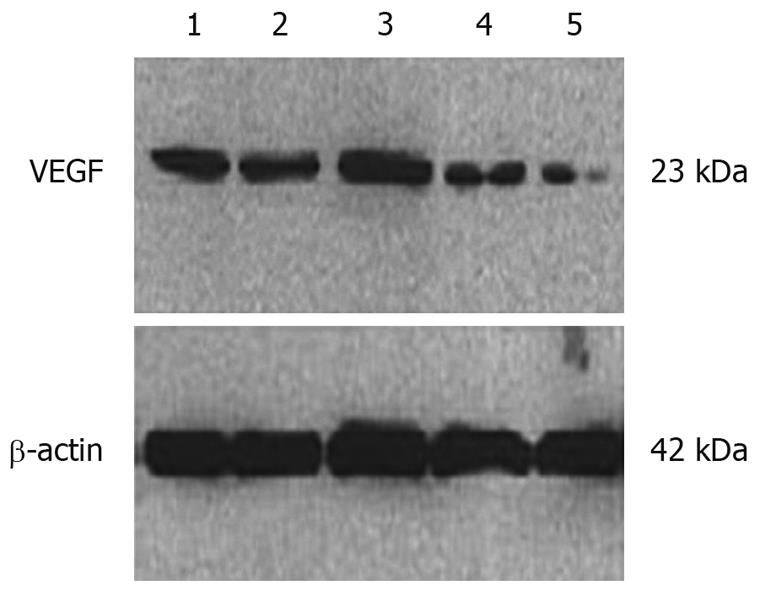
The cells positively stained by the anti-VEGF antibody accounted for 92%-100%. The normal control group, Lipofectamine 2000 control group, and mismatched control group all showed strongly positive cytoplasmic expression of VEGF (dark brown yellow), while the VEGF-siRNA group showed only weakly positive cytoplasmic expression (light brown yellow). Western blotting showed that VEGF-siRNA transfected cells expressed significantly less VEGF than the normal control, Lipofectamine 2000 control, or the mismatched control groups (Figures 1 and 2).
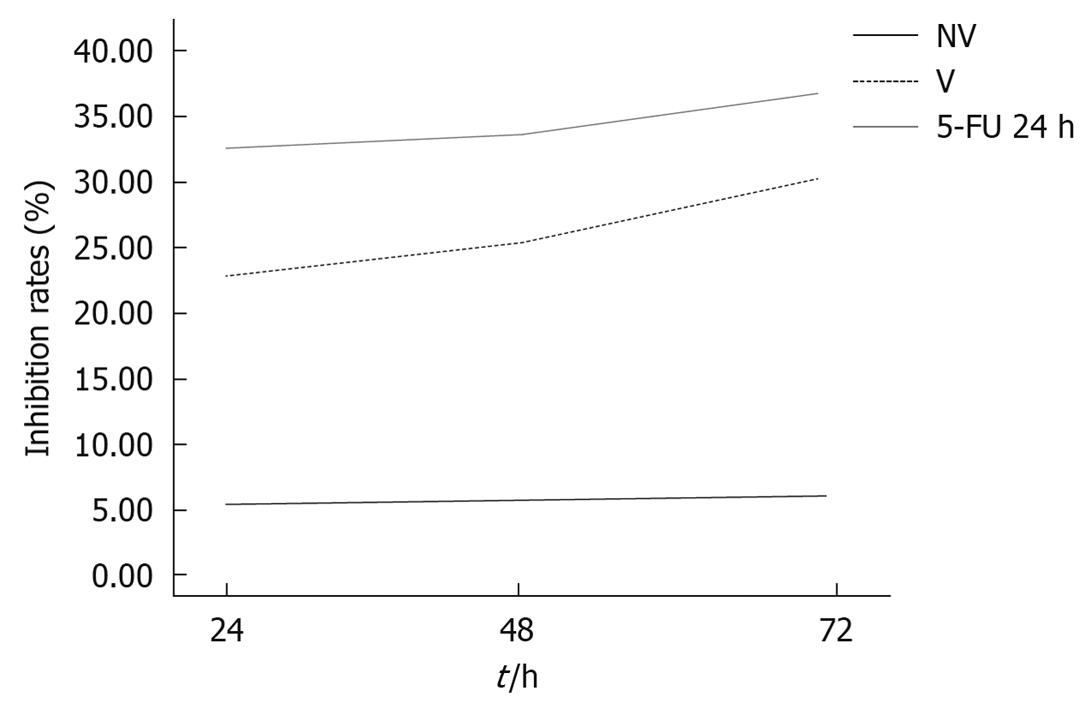
The proliferation rate of the HCT116 cell line 24, 48, and 72 h after transfection with VEGF-siRNA (22.43% ± 3.08%, 24.53% ± 1.94%, and 28.53% ± 2.00%, respectively) was significantly higher than those of the normal control group (0.00, 0.00, 0.00) and the mismatched control group (5.92% ± 0.49%, 6.10% ± 0.90%, 6.46% ± 0.60%, respectively) as assessed by the MTT assay. These effects were time-dependent. These results clearly showed that cell proliferation was markedly inhibited in the VEGF-siRNA-transfected group (Figure 3).
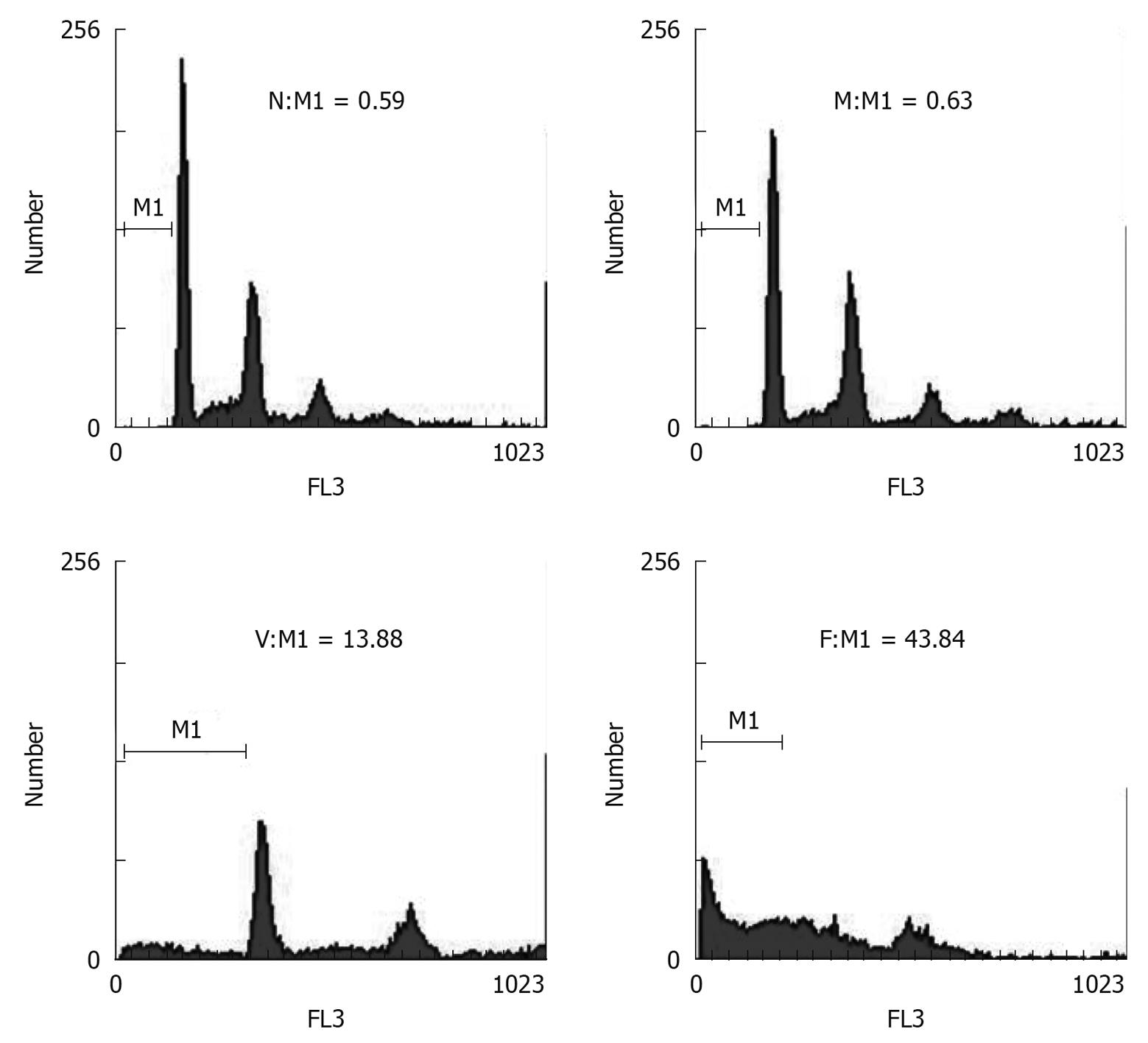
To test for apoptotic cells, we used FCM to look for an apoptotic sub-G0-G1 (M1 value) peak at 48 h after siRNA transfection. The results showed that, compared with the normal control group (M1 = 0.59), and the mismatched group (M1 = 0.63), the VEGF-siRNA group displayed an apparent aneuploid peak (M1 = 13.88) (Figure 4), suggesting the apoptosis of the cells transfected with VEGF-siRNA.
Angiogenesis is necessary for tumors to grow beyond a certain size, and is a prerequisite for tumor invasion and metastasis. VEGF plays a critical role in the process of angiogenesis. VEGF is a glycoprotein of 34-50 kDa, first purified from cattle hypophysis folliculo-stellate cells in 1989[5,6]. VEGF stimulates the proliferation and migration of vascular endothelial cells, and modeling of the tumor neovasculature. VEGF acts via specific receptors, including FLT-1 and FLK-1. FLT-1 is mainly found in vascular endothelial cells[7]. FLT-1 has a stronger affinity for VEGF than FLK-1, but is unable to activate the proliferation signal in vascular endothelial cells, so probably acts as a down-regulator of angiogenesis triggered by VEGF[8]. By binding and activating FLK-1, VEGF stimulates the proliferation of endothelial cells, increases vascular permeability and induces neovascularization[2,6]. Therefore, FLK-1 plays a more important role in tumor angiogenesis. Our study shows that the expression of VEGF, FLT-1 and FLK-1 were all significantly higher in CRC than in normal tissues (P < 0.01). The expression of VEGF was positively correlated with lymph node metastasis and with clinical stages of disease (P < 0.05). This indicates that VEGF, FLT-1 and FLK-1 are all associated with colorectal carcinogenesis.
One technique that has been used recently for the rapid and efficient identification of gene function is RNAi[9]. This refers to a group of related post-transcriptional gene silencing mechanisms in which the terminal effector is a short antisense RNA[10]. The use of RNAi in mammalian cells involves the transfection of an annealed 21-mer comprising sense and antisense RNA oligonucleotides (siRNAs) that correspond to a portion of the genes of interest. These RNAs then bind specifically to the cellular RNA and activate a process that leads to the degradation of the mRNA, and a subsequent 80%-90% decrease in the levels of the corresponding protein. Thus, RNAi is an important technique that specifically down-regulates the expression of cellular genes and has opened the door to the therapeutic use of siRNAs. In our study, we used RNAi technology to transfect a siRNA targeted against VEGF into HCT116 cells using Lipofectamine 2000. Immunocytochemistry and Western blotting both showed that the expression of the VEGF protein was significantly reduced. Our results indicate that VEGF-siRNA can actively suppress VEGF protein expression and inhibit cell proliferation, all consistent with current literature[11,12].
The appearance of the tumor cell proliferation is an important indicator of its biological function. In our study, blocking the VEGF expression using RNAi resulted in significant inhibition of cell growth in a time-dependent manner. A recent study showed that VEGF is closely associated with apoptosis. Castro-Rivera et al[13] found that VEGF165 could suppress apoptosis in lung cancer and breast cancer cells. Pidgeon et al[14] found that VEGF could up-regulate bcl-2 levels in breast cancer cells, thus inhibiting apoptosis in both tumor cells and vascular endothelial cells. Our results show that the rate of apoptosis in the VEGF-siRNA group was significantly higher than that in the normal control group. This suggests that siRNA can effectively silence the VEGF gene and promote apoptosis in CRC cells.
In summary, our study suggests that VEGF is associated with colorectal carcinogenesis. Blocking VEGF expression using RNAi technology significantly reduces VEGF protein levels, suppresses the proliferation of HCT116 cells, and induces apoptosis. Our data indicate that siRNA may have therapeutic potential for inhibiting gene expression, and that VEGF is an attractive therapeutic target for the treatment of colon cancer.
Colorectal carcinoma (CRC) is one of the most common malignant diseases in China and has a poor survival rate. Angiogenesis plays an important role in the growth of tumors. Neovascularization is regulated by multiple factors, the most important one is vascular endothelial cell growth factor (VEGF). VEGF acts to increase microvascular growth and permeability through binding to specific receptors, including Fms-like tyrosine kinase 1 (FLT-1) and fetal liver kinase 1 (FLK-1). Recently, RNA interference (RNAi) technology has been regarded as an effective tool that can be used against tumors, and has established a new domain for CRC clinical therapy.
VEGF plays an important role in tumor angiogenesis through binding to vascular endothelial growth factor receptors, including FLT-1 and FLK-1. RNAi refers to a group of related post-transcriptional gene silencing mechanisms in which the terminal effector is a short antisense RNA. RNAi is an important method for specifically down-regulating the expression of cellular genes, and has opened the door to the therapeutic use of small interfering RNAs (siRNAs).
In this study, immunohistochemical staining for the VEGF, FLT-1, and FLK-1 proteins was performed in CRC samples to explore their roles in carcinogenesis. Also, a VEGF-targeting siRNA was transfected into human colorectal cancer HCT116 cells using Lipofectamine 2000 to investigate the effect of VEGF-targeted siRNA on the proliferation and apoptosis of HCT116 cells.
As indicated in this study, siRNA may have therapeutic potential for inhibiting the expression of specific genes, and VEGF is an attractive therapeutic target.
In this manuscript, the authors demonstrate the relationship between the expression of VEGF and its receptors, FLT-1 and FLK-1, in CRC. The series of experiments was well planned and well performed, and the manuscript well written.
Peer reviewer: Kotaro Miyake, MD, PhD, Department of Surgery, Institute of Health Biosciences, The University of Tokushima Graduate School, 3-18-15 Kuramoto, Tokushima 770-8503, Japan
S- Editor Tian L L- Editor Ma JY E- Editor Zheng XM
| 1. | Hartenbach EM, Olson TA, Goswitz JJ, Mohanraj D, Twiggs LB, Carson LF, Ramakrishnan S. Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Lett. 1997;121:169-175. |
| 2. | Molhoek KR, Griesemann H, Shu J, Gershenwald JE, Brautigan DL, Slingluff CL Jr. Human melanoma cytolysis by combined inhibition of mammalian target of rapamycin and vascular endothelial growth factor/vascular endothelial growth factor receptor-2. Cancer Res. 2008;68:4392-4397. |
| 3. | Wang S, Liu H, Ren L, Pan Y, Zhang Y. Inhibiting colorectal carcinoma growth and metastasis by blocking the expression of VEGF using RNA interference. Neoplasia. 2008;10:399-407. |
| 4. | Volm M, Koomägi R, Mattern J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int J Cancer. 1997;74:64-68. |
| 5. | Kumar H, Heer K, Lee PW, Duthie GS, MacDonald AW, Greenman J, Kerin MJ, Monson JR. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res. 1998;4:1279-1285. |
| 6. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. |
| 7. | Shin JW, Huggenberger R, Detmar M. Transcriptional profiling of VEGF-A and VEGF-C target genes in lymphatic endothelium reveals endothelial-specific molecule-1 as a novel mediator of lymphangiogenesis. Blood. 2008;112:2318-2326. |
| 8. | Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568-581. |
| 9. | Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci. 2001;114:4557-4565. |
| 11. | Wannenes F, Ciafré SA, Niola F, Frajese G, Farace MG. Vector-based RNA interference against vascular endothelial growth factor-A significantly limits vascularization and growth of prostate cancer in vivo. Cancer Gene Ther. 2005;12:926-934. |
| 12. | Murata M, Takanami T, Shimizu S, Kubota Y, Horiuchi S, Habano W, Ma JX, Sato S. Inhibition of ocular angiogenesis by diced small interfering RNAs (siRNAs) specific to vascular endothelial growth factor (VEGF). Curr Eye Res. 2006;31:171-180. |
| 13. | Castro-Rivera E, Ran S, Thorpe P, Minna JD. Semaphorin 3B (SEMA3B) induces apoptosis in lung and breast cancer, whereas VEGF165 antagonizes this effect. Proc Natl Acad Sci USA. 2004;101:11432-11437. |
| 14. | Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer. 2001;85:273-278. |