Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.909
Revised: December 16, 2009
Accepted: December 23, 2009
Published online: February 21, 2010
Attention has recently been focused on biliary papillary tumors as the novel disease entity intraductal papillary neoplasm of the bile duct (IPNB), which consists of papillary proliferation of dysplastic biliary epithelium. As even benign papillary tumors are considered as premalignant, some investigators recommend aggressive surgical therapy for IPNB, although no guidelines are available to manage this disease. Few reports have described long-term follow-up of patients with benign IPNB without radical resection. If patients with IPNB who are treated only with endoscopic procedures are noted, clinical profiles and alternative therapies other than resection may be recommended. We report the case of a patient who experienced repetitive cholangitis for 10 years and was finally diagnosed with IPNB. Radical resection could not be recommended because of the age of the patient, therefore, endoscopic sphincterotomy was performed. Although an endoscopic retrograde biliary drainage catheter was placed several times for repetitive cholangitis, the patient has done well during follow-up. Our case may offer insights into the natural course and management decisions for the novel disease entity of IPNB.
- Citation: Tsuchida K, Yamagata M, Saifuku Y, Ichikawa D, Kanke K, Murohisa T, Tamano M, Iijima M, Nemoto Y, Shimoda W, Komori T, Fukui H, Ichikawa K, Sugaya H, Miyachi K, Fujimori T, Hiraishi H. Successful endoscopic procedures for intraductal papillary neoplasm of the bile duct: A case report. World J Gastroenterol 2010; 16(7): 909-913
- URL: https://www.wjgnet.com/1007-9327/full/v16/i7/909.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.909
Recently, the novel disease entity of intraductal papillary neoplasm of the bile duct (IPNB) has been proposed to include biliary papillomatosis, which comprises multiple biliary papillomas composed of papillary proliferation of the dysplastic biliary epithelium, and papillary cholangiocarcinoma[1]. IPNB is thought to represent the biliary counterpart of pancreatic intraductal papillary mucinous neoplasm (IPMN-P) and is thus considered premalignant[1,2]. The prognosis of patients with IPNB is good when curative surgery is performed, therefore, aggressive resection is recommended as the first choice[3-5]. However, little information is available regarding the prognosis of IPNB without curative surgery, as few patients with IPNB who have been followed for several years without surgery have been reported. Here, we present a patient with IPNB who was followed for 10 years without surgical resection, and has done well during the follow-up period. This case indicates a natural course of the novel entity IPNB and alternative therapies for this disease.
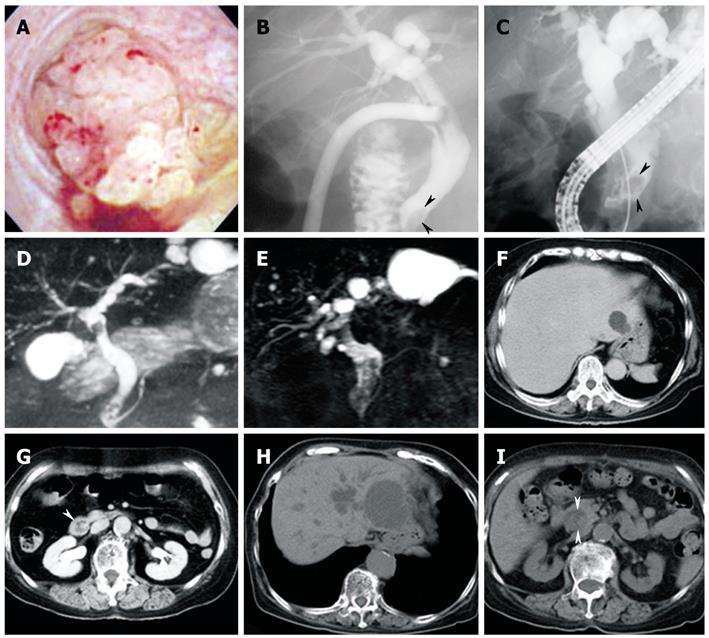
A previously healthy 76-year-old woman developed acute cholecystitis caused by gallbladder stones, and cholecystectomy was performed in 1999. Cholangioscopic examination during surgery revealed a bile duct tumor with papillary proliferation protruding into the common bile duct (Figure 1A). The tumor was followed without resection, and a biopsy specimen showed tubular adenoma packed with small glandular or papillary components.
When the patient was admitted for acute cholangitis in 2004, the papillary tumor in the bile duct appeared unchanged. The patient recovered well until she became symptomatic again in September 2007, for which an endoscopic retrograde biliary drainage (ERBD) catheter (FLEXIMA™ Biliary Stent System, Boston Scientific Co., Natick, MA, USA) was placed. In December 2007, the patient developed recurrent cholangitis because of slippage of the ERBD catheter, which was re-inserted into the bile duct. In early January 2008, the ERBD catheter slipped, and the patient was seen for abdominal pain and fever. Laboratory test results included: alkaline phosphatase, 498 U/L (normal: 104-338 U/L); γ-glutamyltranspeptidase, 122 U/L (normal: 18-66 U/L); aspartate aminotransferase, 58 U/L (normal: 10-37 U/L); alanine aminotransferase, 71 U/L (normal: 3-34 U/L); carbohydrate antigen 19-9, 414 U/mL (normal: 0-37 U/mL); white blood cell count, 11 400/mm3 (normal: 4000-9000/mm3); and C-reactive protein, 17.3 mg/dL (normal: 0-0.3 mg/dL).
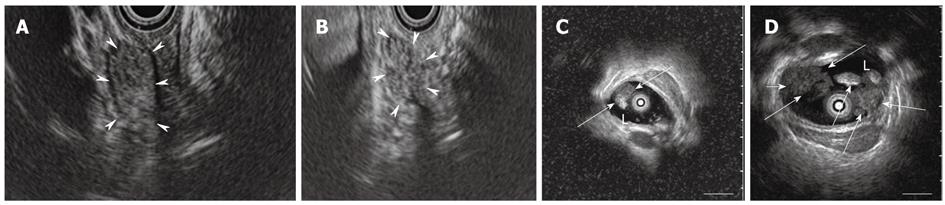
Endoscopic retrograde cholangiography (ERC) revealed extrahepatic bile duct dilatation, which was more marked than that seen in 1999, and the presence of intraductal polypoid and amorphous filling defects (Figure 1B and C). Magnetic resonance cholangiopancreatography (MRCP) showed cystic lesions connected to the intrahepatic bile duct in the left liver. Cystic lesions were enlarged compared with those in 1999 (Figure 1D and E). Abdominal computed tomography (CT) showed both intrahepatic and extrahepatic bile ducts to be dilated and the presence of a 20-mm mass in the distal common bile duct. In addition, the cystic lesion in the left liver was enlarged compared to that in 1999 (Figure 1F-I). Contrast-enhanced harmonic endoscopic ultrasound (CEH-EUS) with Sonazoid® (Daiichi-Sankyo, Tokyo, Japan) showed flow signals inside the whole mass, which revealed the papillary structure (Figure 2A and B). CEH-EUS for biliary diseases was approved by the ethical committee of Dokkyo Medical University, and written informed consent was obtained from the patient before the examination. Intraductal ultrasonography (IDUS) showed a 10-mm papillary mass in the middle bile duct (Figure 2C), and multilobular lesions in the distal bile duct (Figure 2D). Duodenoscopy demonstrated a 10-mm discolored mass on the ampulla of Vater. A biopsy specimen from the discolored mass showed adenoma.
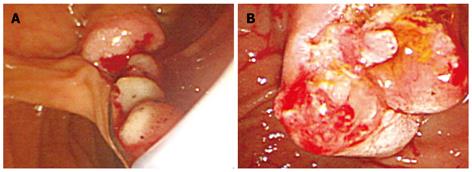
As the patient was diagnosed with cholangitis caused by obstruction of the bile duct by intraductal papillary tumor, endoscopic biliary sphincterotomy (EST) was performed (Figure 3). Radical resection was not recommended because of the age of the patient. Through the orifice of the ampulla of Vater, a soft, bead-like mass was extracted by balloon sweep and a net-type catheter.
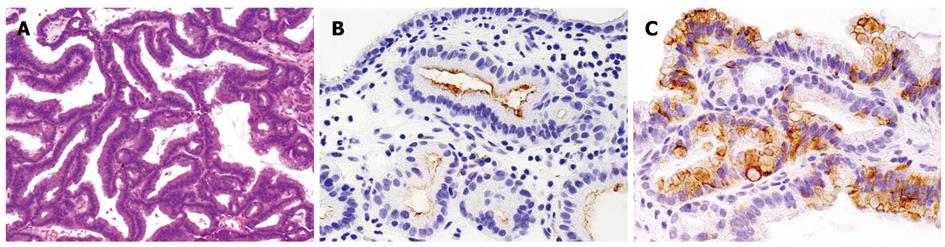
Pathological diagnosis of the extracted mass was papillary neoplasia without invasive carcinoma (Figure 4A). Immunohistochemical analysis was performed for mucin core proteins and cytokeratins (CKs). Less than 5% of adenoma cells showed positivity for MUC1 in the apical membrane (Figure 4B). Negative results were obtained for MUC2. About 40%-50% of adenoma cells showed positivity for MUC5AC in the cytoplasm (Figure 4C). Both CK7 and CK20 were negative.
Although the patient was readmitted in May 2008 and in June 2009 with signs of cholangitis, for which no special procedures and placement of ERBD catheter were respectively performed, the patient has done well since EST.
This case is compatible with the novel disease entity of IPNB[1], and biliary papillomatosis can be diagnosed as discussed below. Biliary papilloma is a rare benign neoplasm that consists of papillary proliferation of atypical biliary epithelium along with delicate fibrovascular stalks[6]. Biliary papillomatosis is defined as the presence of more than three papillomas at different sites of the biliary tree. Some cholangiocarcinomas that show mainly papillary proliferation in the bile duct are designated as papillary cholangiocarcinoma. Zen et al[1] have proposed biliary papilloma(tosis) and papillary cholangiocarcinoma with or without mucus hypersecretion as belonging to the novel tumor entity of IPNB. In this case, cholangioscopy and tissue specimens demonstrated that the tumor originated from the biliary epithelium, and various imaging modalities revealed tumors with papillary formation. Two papillary tumors in the extrahepatic bile duct detected by IDUS, and an expanding cystic lesion in the liver contiguous with the intrahepatic bile duct suggested that at least three lesions were present at different sites of the biliary tree. To summarize, this case could be diagnosed as biliary papillomatosis, although whether malignant transformation was present is unknown.
Occasional association with mucin hypersecretion is one of the pathological similarities between IPNB and IPMN-P[1]. Zen et al[1] have performed immunohistochemical analysis for mucin core proteins in patients with IPNB and compared the results with patients with IPMN-P. They have proposed the typical mucin and cytokeratin expression profile of IPNB as MUC1-negative, MUC2-positive, MUC5AC-positive, CDX2-positive, CK7-positive, CK20-positive. According to Shibahara et al[7], patients with MUC1-positive expression show poorer survival than those with MUC1-negative expression in papillary cholangiocarcinoma. In addition, the same group has reported that IPMN-P tends to show MUC1-negative and MUC2-positive expression, in contrast to invasive carcinoma, which shows MUC1-positive and MUC2-negative expression[8]. The mucin and cytokeratin expression profile in the present case was MUC1-positive, MUC2-negative, MUC5AC-positive, CK7-negative, CK20-negative, which differs from the typical profile reported by Zen et al[1]. Reasons for this difference might have included: (1) our specimens were taken by biopsy and immunohistochemical analysis was not performed for the entire lesion; (2) even in the original paper by Zen et al[1], not all cases showed the typical profile; and (3) MUC1-positivity implies that our case could have malignant potential and needs cautious observation in the future.
Surgical resection is often recommended because of the high malignancy rate, diffuse pattern of disease, and better survival after curative surgery[3-5]. Liver transplantation has been suggested as an alternative[9], while many patients with biliary papillomatosis, which is a disease of the elderly (mean age at time of diagnosis, 63 years)[10], would not be eligible for transplantation.
We consider that curative resection is not necessarily reasonable for every patient with IPNB for the following reasons. First, compared with the prognosis of usual cholangiocellular carcinoma, that of biliary papillomatosis including benign and malignant cases is much better[2]. This implies that the prognosis of benign biliary papillomatosis, in particular, may be good enough to be observed without radical resection, although precise information on the clinical course of patients with biliary papillomatosis is currently unclear. Second, the Whipple procedure and hemihepatectomy are the therapies of choice depending on the location and extension of the disease. In addition, malignant change is observed in 40%-50% of cases[11], which means that half of the cases remain benign. If radical resection were recommended for all patients, the therapy might be too invasive for the potentially large population of patients with benign disease.
A search of the English-language literature published in the past 5 years was performed using the MEDLINE database with keywords of ‘biliary papillomatosis’ and ‘intraductal papillary neoplasm of the bile duct’. Five patients with pathologically benign IPNB[10-13], including the present case, have been followed up using endoscopic procedures (Table 1). Two patients had been followed for > 10 years. Among these five patients, surgery was not considered a viable option because of the age of the patient in three cases, small range of the disease in one case, and for unknown reasons in the other.
| Author | Gender | Age (yr) | Follow-up after diagnosis of IPNB | Treatment | Reason surgery was not performed | Outcome |
| Bechmann et al[10] | Male | 65 | 10 years | Whipple, right hepatectomy, PDT | Patient’s age | Death without cholestasis |
| Park et al[11] | Female | 78 | 3 wk | EST, EPBD | Unknown | Well for a short time |
| Brauer et al[12] | Male | 86 | 1 mo | APC | Diffuse involvement of biliary system, patient’s age and comorbidities | Death due to hepatic encephalopathy |
| Jazrawi et al[13] | Male | 37 | 6 mo | Extrahepatic bile duct resection, APC | Patient’s refusal | Being evaluated for liver transplantation due to disease progression |
| Current case | Female | 86 | 11 years | Cholecystectomy, EST, ERBD | Patient’s age | Being well irrespective of slow disease progression |
The therapy offered to these patients was EST plus additional endoscopic papillary balloon dilation in two cases, argon plasma coagulation in two cases, and photodynamic therapy (PDT) in one case. Prognosis of these patients was unchanged with occasional cholangitis in two cases, exacerbation in one, death from another disease in one, and unknown in one. Based upon the summaries of these patients, cases in which malignant transformation cannot be ascertained pathologically could be cautiously followed using endoscopic procedures, and using radical resection as the gold standard is unnecessary. In addition, another patient similar to our own was followed up for 10 years, and treated using endoscopic procedures for recurrent biliary papillomatosis, because there was no surgical option left. As IPNB is considered as the biliary counterpart of IPNM-P[1,7], a follow-up period of over a decade for some patients with IPNB, as in our case, is not necessarily inappropriate.
To conclude, we encountered a patient with IPNB who was treated only with endoscopic procedures for 10 years, which suggests that some patients with benign IPNB could be followed conservatively without radical resection. Moreover, the present case might partly demonstrate the natural course of patients with the novel disease entity of IPNB.
Peer reviewer: Miguel Angel Mercado, MD, Surgical division, National Institute of Medical Sciences and Nutrition, Vasco de Quiroga 15, col. Seccion 16, Distrito Federal, 14000, Mexico
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH
| 1. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. |
| 2. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. |
| 3. | Yeh TS, Tseng JH, Chiu CT, Liu NJ, Chen TC, Jan YY, Chen MF. Cholangiographic spectrum of intraductal papillary mucinous neoplasm of the bile ducts. Ann Surg. 2006;244:248-253. |
| 4. | Paik KY, Heo JS, Choi SH, Choi DW. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97:508-512. |
| 5. | Yeung YP, AhChong K, Chung CK, Chun AY. Biliary papillomatosis: report of seven cases and review of English literature. J Hepatobiliary Pancreat Surg. 2003;10:390-395. |
| 6. | Adbores-Saavedra J, Scoazec JC, Wittekind C, Sripa B, Menck HR, Soehendra N, Sriram PVJ. Carcinoma of the gallbladder and extrahepatic bile ducts. World Health Organization Classification of Tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press 2000; 206. |
| 7. | Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327-338. |
| 8. | Osako M, Yonezawa S, Siddiki B, Huang J, Ho JJ, Kim YS, Sato E. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71:2191-2199. |
| 9. | Rambaud S, Nores JM, Meeus F, Paolaggi JA. Malignant papillomatosis of the bile ducts: a new indication for liver transplantation? Am J Gastroenterol. 1989;84:448-449. |
| 10. | Bechmann LP, Hilgard P, Frilling A, Schumacher B, Baba HA, Gerken G, Zoepf T. Successful photodynamic therapy for biliary papillomatosis: a case report. World J Gastroenterol. 2008;14:4234-4237. |
| 11. | Park JH, Park do H, Park SH, Lee SH, Kim SJ, Cho HD. Non-mucin-producing biliary papillomatosis diagnosed by transpapillary endoscopic curettage (with video). Gastrointest Endosc. 2007;65:519-520, discussion 520. |
| 12. | Brauer BC, Fukami N, Chen YK. Direct cholangioscopy with narrow-band imaging, chromoendoscopy, and argon plasma coagulation of intraductal papillary mucinous neoplasm of the bile duct (with videos). Gastrointest Endosc. 2008;67:574-576. |
| 13. | Jazrawi SF, Nguyen D, Barnett C, Tang SJ. Novel application of intraductal argon plasma coagulation in biliary papillomatosis (with video). Gastrointest Endosc. 2009;69:372-374. |