Published online Feb 21, 2010. doi: 10.3748/wjg.v16.i7.897
Revised: December 16, 2009
Accepted: December 23, 2009
Published online: February 21, 2010
AIM: To test whether ethanol feeding could induce Toll-like receptor 4 (TLR4) responses, assess the hepatoprotective effect of betaine and its inhibitive effect on TLR4 in animal models of alcoholic liver injury.
METHODS: Forty-eight female Sprague-Dawley rats were randomly divided into four groups as control, model, low and high dose betaine groups. Except control group, all rats were fed with high fat-containing diet plus ethanol and fish oil gavages for 8 wk. Betaine was administered intragastrically after exposure of ethanol for 4 wk. The changes of liver histology were examined. The expression of TLR4 mRNA and protein was detected by RT-PCR and Western blotting, respectively. The serum aminotransferase activity [alanine transarninase (ALT), aspartate aminotransferase (AST)], serum endotoxin, and liver inflammatory factors [tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-18 (IL-18)] were also assayed.
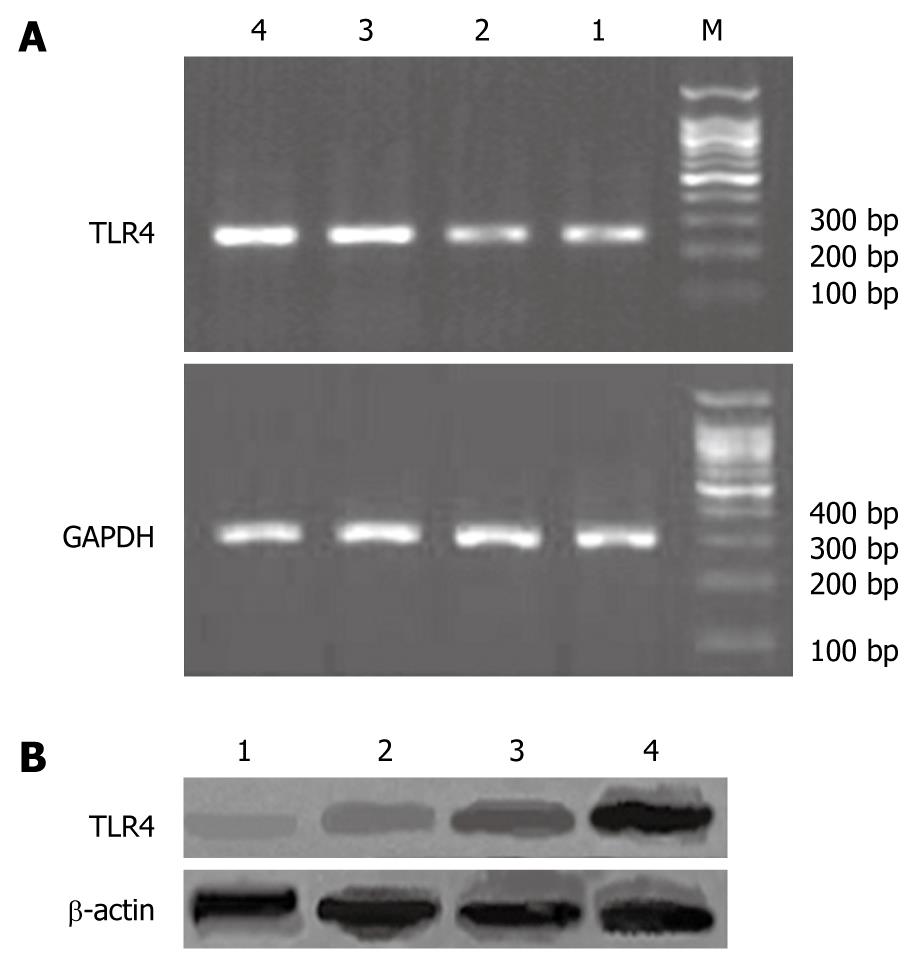
RESULTS: Compared with control group, rats of model group developed marked liver injury, accompanied by an increase of ALT (159.41 ± 7.74 U/L vs 59.47 ± 2.34 U/L, P < 0.0001), AST (248.25 ± 1.40 U/L vs 116.89 ± 3.48 U/L, P < 0.0001), endotoxin (135.37 ± 30.17 ng/L vs 44.15 ± 7.54 ng/L, P < 0.0001), TNF-α (20.81 ± 8.58 pg/mL vs 9.34 ± 2.57 pg/mL, P = 0.0003), IFN-γ (30.18 ± 7.60 pg/mL vs 16.86 ± 9.49 pg/mL, P = 0.0039) and IL-18 (40.99 ± 8.25 pg/mL vs 19.73 ± 9.31 pg/mL, P = 0.0001). At the same time, the expression of TLR4 mRNA and protein was markedly induced in the liver after chronic ethanol consumption (1.45 ± 0.07 vs 0.44 ± 0.04, P < 0.0001; 1.83 ± 0.13 vs 0.56 ± 0.08, P < 0.0001). Compared with model group, betaine feeding resulted in significant decreases of ALT (64.93 ± 6.06 U/L vs 159.41 ± 7.74 U/L, P < 0.0001), AST (188.73 ± 1.11 U/L vs 248.25 ± 1.40 U/L, P < 0.0001), endotoxin (61.80 ± 12.56 ng/L vs 135.37 ± 30.17 ng/L, P < 0.0001), TNF-α (9.79 ± 1.32 pg/mL vs 20.81 ± 8.58 pg/mL, P = 0.0003), IFN-γ (18.02 ± 5.96 pg/mL vs 30.18 ± 7.60 pg/mL, P = 0.0008) and IL-18 (18.23 ± 7.01 pg/mL vs 40.99 ± 8.25 pg/mL, P < 0.0001). Betaine also improved liver steatosis. The expression levels of TLR4 mRNA or protein in liver tissues were significantly lowered (0.62 ± 0.04 vs 1.45 ± 0.07, P < 0.0001; and 0.65 ± 0.06 vs 1.83 ± 0.13, P < 0.0001). There was a statistical difference of TLR4 mRNA and protein expression between high- and low-dose betaine groups (0.62 ± 0.04 vs 0.73 ± 0.05, P < 0.0001, and 0.65 ± 0.06 vs 0.81 ± 0.09, P < 0.0001).
CONCLUSION: Betaine can prevent the alcohol-induced liver injury effectively and improve the liver function. The expression of TLR4 increases significantly in ethanol-fed rats and betaine administration can inhibit TLR4 expression.
- Citation: Shi QZ, Wang LW, Zhang W, Gong ZJ. Betaine inhibits Toll-like receptor 4 expression in rats with ethanol-induced liver injury. World J Gastroenterol 2010; 16(7): 897-903
- URL: https://www.wjgnet.com/1007-9327/full/v16/i7/897.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i7.897
Previous studies have shown that the chronic ingestion of ethanol can induce functional and structural changes in liver. Bacterial lipopolysaccharide (LPS; endotoxin), an abundant and essential component of the outer membrane of gram negative bacteria, causes liver injury in many experimental models[1,2]. Chronic alcohol administration increases gut-derived endotoxin in the portal circulation, thereby activating Kupffer cells to produce several proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin (IL)-1[3,4].
Toll-like receptor 4 (TLR4), a transmembrane protein with a cytoplasmic domain that bears homology to the IL-1 receptor, is expressed in monocytes and macrophages, including Kupffer cells[5]. Recently, TLR4 has been shown to mediate LPS-induced signal transduction in peripheral blood monocytes[6]. Furthermore, it has been shown that Kupffer cell activation by LPS is dependent on the presence of a functional TLR4[7]. It has been confirmed that TLR4 is involved in the mechanism of early alcohol-induced liver injury[8-11]. Ethanol administration can lead to the synthesis of TLR4 protein and its gene expression in Kupffer cells, indicating that TLR4 may play a major role in the development of alcohol-induced liver injury.
Betaine, also known as trimethyl glycine, is the only methyl donor, which can replace folate or S-ademetionine in the human body[12,13]. Betaine is quaternary ammonium salt soluble alkaloids, which participates in the methionine recycling and phosphatidylcholine synthesis[14,15]. Many studies including our previous study have indicated that betaine can prevent the alcohol-induced liver injury effectively and improve the liver function[16,17]. The hepatoprotective mechanism of betaine is related to the inhibition of inflammatory factor, the decrease of lipid peroxidation, the promotion of endoplasmic reticulum stress and the prevention of apoptosis[18-21].
In the present study, we employed the intragastric ethanol-fed rat model, which reproduces the pathological features of early alcohol-induced liver injury, to observe the changes of TLR4 expression and study the effect of betaine in alcohol-induced liver injury animal models.
Betaine hydrochlorides (99% of purity) were kindly presented by Juhua Group Co. (Zhejiang, China). Ferrous sulfate was obtained from Shanghai reagent chemicals Co. Ltd (Shanghai, China). Fish oil and 560 mL/L of alcohol were purchased from supermarkets. Limulus amoebocyte lysate assay kit for serum endotoxin assay was purchased from BioWhittaker Inc. (USA). Enzyme-linked immunosorbent (ELISA) kits for rat TNF-α, IFN-γ, and IL-18 detection were purchased from Shanghai Senxiong Biotech industry Co. Ltd. (Shanghai, China). TRIzol reagent was purchased from Invitrogen Co. (USA). DL1000 DNA ladder marker was purchased from TaKaRa Biotech Co. Ltd. (Japan). M-MLV reverse transcriptase, deoxyribonucleotide (dNTP, 10 mmol/L), oligo (dT)15 primer, Taq DNA polymerase, RNasin were purchased from Promega Biotech Co. Ltd. (USA). Polymerase chain reaction (PCR) primers for TLR4 and GAPDH were synthesized by Sai-Bai-Sheng Biocompany (Shanghai, China).
Forty-eight female specific pathogen free (SPF) Sprague-Dawley rats, weighing 150 ± 10 g, were purchased from the Experimental Animal Center of Wuhan University. After acclimation for 1 wk, animals were randomly divided into four groups as control, model, low dose and high dose betaine groups. Each group contains 12 rats. Except rats of control group fed with ordinary diet and administrated intragastrically with physiological saline, the rats of the other three groups were fed with fat-rich diet containing common animal feeds, lard and whole milk powder (80:10:10), and were administrated intragastrically with ethanol and 0.5 mL fish oil. The initial dose of ethanol was 6 g/kg per day (solutions maximally containing 560 mL/L alcohol). Within the first week, the dose was increased progressively to a maintenance dose of 8 g/kg per day that was continued for 8 more weeks. After exposure of ethanol for 4 wk, the rats of low dose and high dose groups were administrated intragastrically with betaine 200 and 400 mg/kg per day, respectively. Animals were weighted three times per week. At the end of the experiment, animals were anaesthetized with urethane (20%, 1.0 g/kg) and sacrificed by bleeding from femoral arteries. Blood samples were collected. Immediately after exsanguination, the livers were harvested. Small portions of the livers were kept frozen at -70°C for reverse transcriptase-polymerase chain reaction (RT-PCR), whereas other portions were separated and immersed in 10% buffered formalin solution for histological examination. All animals were given humane care in compliance with the institutional guidelines.
Blood samples were allowed to clot, and the sera were isolated by centrifugation at 1000 ×g for 10 min and kept at -20°C before determination. Serum alanine transaminase (ALT), aspartate transaminase (AST) and albumin (ALB) were determined by routine laboratory methods using a Hitachi Automatic Analyzer (Hitachi, Inc. Japan).
Serum levels of endotoxin, TNF-α, IFN-γ and IL-18 were measured using commercial kits according to the manufacturer’s protocol.
Total RNA was extracted from approximately 100 mg frozen liver tissue using TRIzol reagent according to the manufacturer’s protocol. The concentration of total RNA was assayed by ultraviolet spectrophotometric measurements at wavelength of 260 nm, and its purity was estimated by the ratio of A260/A280. The total RNA was reversely transcribed into single-stranded complementary DNA (cDNA) using the following methods: 2 μg RNA, 0.5 μg oligo(dT)15 primer and DEPC (diethylpyrocarbonate)-treated water were added to reach a total volume of 15 μL mixture at 70°C for 5 min, then rapidly chilled on ice. Finally, 5 μL 5 × reaction buffer, 1.25 μL dNTP (10 mmol/L, each), 25 units of RNasin, 200 units of M-MLV reverse transcriptase and DEPC-treated water were added to reach a total volume of 25 μL mixture and incubated at 42°C for 60 min, then terminated by placing it on ice after deactivation at 85°C for 5 min. The cDNA was amplified by PCR. The amplification primers for rat TLR 4 were 5'-ACTCGAGCCAGAATGAGGACT-3' and 5'-ACTGCCATGTCTGAGCAATCT-3', for rat GADPH were 5'-TCCCTCAAGATTGTCAGCAA-3' and 5'-AGATCCACAACGGATACATT-3'. The 50 μL PCR reaction mix contained 10 mmol/L dNTP, 2.5 mmol/L MgCl2, 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 25 pmol/L of sense and antisense primers, and 2U of Taq DNA polymerase. Amplification was performed with 35 cycles with initial incubation at 94°C for 3 min and final extension at 72°C for 7 min, each cycle consisted of denaturation for 45 s at 94°C, annealing for 45 s at 55°C, and extension for 1 min at 72°C. The PCR products were 237 bp and 309 bp for TLR 4 and GADPH, respectively. In all experiments, possible contamination with genomic DNA was excluded by PCR amplification in the absence of reverse transcriptase. The PCR products were electrophoresed on 2% agarose gel. Semiquantitative evaluation was performed using the Gel Doc 2000 System (BioRad Laboratories GmbH, München, Germany). GADPH was used as a positive internal control and was positive for each specimen. Its expression was used as a correction factor for TLR 4 mRNA, thus the results were calculated as the ratio of the intensity of bands of TLR 4 cDNA per GADPH cDNA on the gel.
Liver tissue samples of 100 mg were crushed in a liquid nitrogen-cooled grinding bowl and then were lysed in cold RIPA buffer (25 mmol/L Tris-HCl pH 7.6, 150 mmol/L NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) (Pierce Biotechnology, Inc., USA), supplemented with Halt™ Protease Inhibitor Cocktail (Pierce Biotechnology, Inc., USA). Whole cell lysates were obtained by subsequent centrifugation at 15 000 ×g for 10 min at 4°C. Protein concentrations were determined using Bradford Protein Assay Kit with bovine serum albumin (BSA) as standard (SinoBio Biotech Co., Ltd. Shanghai, China). Fifty μg of protein extracts were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a Protran® nitrocellulose membrane (Schleicher & Schuell BioScience GmbH, Whatman Group, Germany). The membrane was incubated with the rabbit anti-TLR4 polyclonal antibody (BioChain, USA) at 4°C overnight after being blocked with a 10% BSA solution. The membrane was washed with TBST buffer (20 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, 0.1% Tween-20) and incubated with a secondary goat anti-rabbit horseradish peroxidase (HRP)-conjugated antibody (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 2 h at room temperature, and finally detected by chemiluminescence using Enhanced NuGlo™ Chemiluminescent Substrate Kit (Alpha Diagnostic Intl. Inc., USA) followed by autoradiographic and densitometric analysis. β-actin was used as an internal control.
The liver specimens were fixed in 10% formaldehyde for 12-24 h, embedded in paraffin, sliced into sections of 5 μm thickness and stained with hematoxylin-eosin (HE). Histological assessment was performed by three pathologists independently. The severity of steatosis was scored as 0 (no hepatocytes), 1 (less than 25% of hepatocytes), 2 (26%-50%), 3 (51%-75%), and 4 (greater than 75% of hepatocytes). The severity of the inflammation was scored as 0 (none), 1 (minimal), 2 (mild), 3 (moderate) and 4 (severe) based on the degree of portal and lobular inflammation and the evidence of piecemeal and spotty necrosis. The degree of necrotic hepatocytes was also scored as 0 to 4 (none, minimal, mild, moderate, and severe, respectively) based on the evidence of piecemeal and spotty necrosis.
All data were presented as mean ± SE. Differences among groups were assessed using unpaired Student’s t test and one-way ANOVA. P value less than 0.05 was considered to be statistically significant. Calculations were performed with the SPSS11.0 statistical software package.
During the experiment, 4 rats in the model group died because fluids were poured mistakenly into trachea when they were administrated intragastrically. The other 44 rats survived.
The changes of the rat weight in models were significantly lower than that in controls (P < 0.01). Compared with the model group, there were no significant differences in betaine intervention groups (P > 0.05). Liver index was significantly higher in models than in controls (P < 0.01). Compared with the model group, liver index decreased significantly in the betaine intervention groups (P < 0.01), and there was no statistical difference between high dose betaine group and low dose betaine group (P > 0.05) (Table 1).
The changes of the ALT and AST in models were significantly higher than in controls (P < 0.01). Compared with the model group, the ALT and AST levels were significantly lowered in betaine intervention groups, indicating that the betaine can greatly improve the alcohol-induced liver injury, and there was no statistical difference between high dose betaine group and low dose betaine group (P > 0.05). There were no significant differences in ALB and A/G between model group and betaine intervention groups (P > 0.05) (Table 2).
The levels of serum endotoxin, TNF-α, IFN-γ and IL-18 were significantly higher in model group than in control group (P < 0.01). Compared with model group, serum endotoxin, TNF-α, IFN-γ and IL-18 significantly decreased in betaine intervention groups (P < 0.01). There was a statistical difference between high dose betaine group and low dose betaine group (P < 0.01) (Table 3).
| Groups | n | Endotoxin (ng/L) | TNF-α (pg/mL) | INF-γ (pg/mL) | IL-18 (pg/mL) |
| Control | 12 | 44.15 ± 7.54b | 9.34 ± 2.57b | 16.86 ± 9.49b | 19.73 ± 9.31b |
| Model | 8 | 135.37 ± 30.17 | 20.81 ± 8.58 | 30.18 ± 7.60 | 40.99 ± 8.25 |
| Low dose betaine | 12 | 87.36 ± 15.93b | 12.61 ± 1.70b | 22.63 ± 4.90b | 26.51 ± 5.59b |
| High dose betaine | 12 | 61.80 ± 12.56bd | 9.79 ± 1.32bd | 18.02 ± 5.96bd | 18.23 ± 7.01bd |
The software Quantiscan was used to analyze the absorbance of the products of TLR4 mRNA or protein quantitatively. The expression of TLR4 mRNA and protein was both significantly higher in model group than in normal (P < 0.01). Compared with the model group, the expression of TLR4 mRNA and protein was significantly reduced in betaine intervention groups (P < 0.01). There was a statistical difference of TLR4 expression between high dose betaine group and low dose betaine group (P < 0.01) (Table 4 and Figure 1).
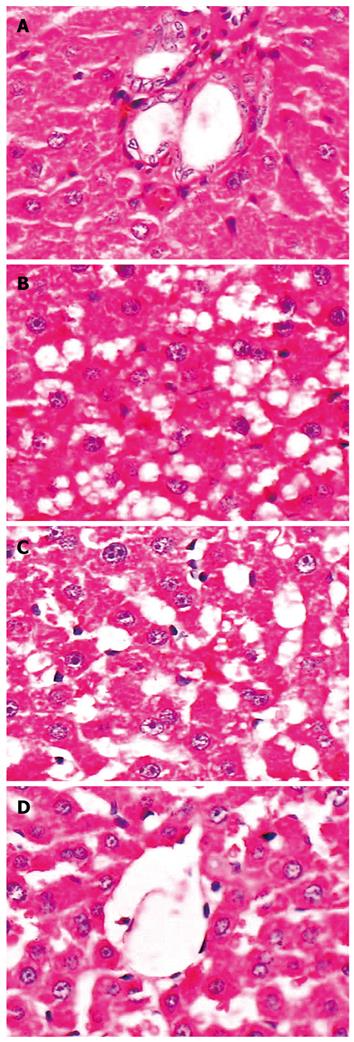
The liver structure in control group was normal, and no obvious inflammation and hepatic steatosis were observed (Figure 2A). In model group, the structure of hepatic cord was deranged, and various degrees of diffuse hepatic steatosis and intralobular inflammation could be found obviously (Figure 2B). Compared with model group, the degree of hepatic steatosis and inflammation was greatly reduced in betaine intervention groups. The improvement of liver histopathology in the high dose betaine group was most significant (Figure 2C and D).
In this study, by establishing the intragastric fat-rich and ethanol diet mouse model, we found that the rats in model group had lower weight and higher liver index, obvious liver injury and hepatic steatosis, higher serum endotoxin, TNF-α, IFN-γ and IL-18 levels compared with the rats in control group. Endotoxemia and oxidative stress are two key factors for the progression of alcoholic liver diseases[22]. There are solid data supporting the hypothesis that endotoxin is indeed involved in alcoholic liver injury. First, it has been shown that excessive alcohol intake increases gut permeability of normally nonabsorbed substances[23,24]. Second, intestinal gram-negative bacteria, as well as blood endotoxin levels, are increased both in alcoholic patients and in the Tsukamoto-French enteral alcohol feeding model[25,26]. Third, intestinal sterilization with antibiotics and displacement of gram-negative bacteria with lactobacillus treatment prevents alcohol-induced liver injury[27,28]. Alcohol can increase the levels of circulating endotoxin in the portal blood. Once bound to LPS-binding protein (LBP), this complex is formed with the endotoxin receptor and CD14, activates Kupffer cells via TLR4 [29]. Kupffer cell activation leads to the up-regulation of key cytokines, including TNF-α. Besides direct toxic effects on hepatocytes, TNF-α can indirectly damage the liver by increasing expression of intercellular adhesion molecule-1 (ICAM-1) on endothelial cells, as well as increasing the production of chemoattractant molecules from inflammatory cells[30].
In this study, we also found that the rats in model group fed with ethanol had a significantly higher expression of TLR4 mRNA and protein than normal rats. Many studies have confirmed that TLR4 is critical for early alcoholic liver injury[8-11]. It was shown that a functional mutation in TLR4 prevents early alcohol-induced liver injury in mice. Specifically, no differences in alcohol levels or plasma endotoxin were observed between the groups fed with ethanol. Moreover, a functional mutation in the TLR4 receptor prevents all downstream events, including increased TNF-α expression, inflammation, and liver injury[11]. These results support the hypothesis that endotoxin and TLR4 play a major role in the development of early alcohol-induced liver injury. CD14, a glycosylphosphatidylinositol-anchored receptor for LPS, is important in mediating the effects of LPS/LPB complexes on peripheral blood monocytes[31], and it is known that ethanol increases expression of CD14 on Kupffer cells[32]. However, CD14 lacks the ability to transduce LPS-induced cytoplasmic signals across a cell membrane, because it is not a transmembrane protein[33]. It had been suggested that LPS-induced inflammatory cell activation via CD14 also requires TLR4, which associates with CD14 on the cell surface, mediating LPS-induced signal transduction[34]. The finding that alcoholic liver injury is blocked in both CD14 and TLR4-deficient mice suggests that both of these receptors are necessary to initiate liver damage caused by alcohol[35]. Therefore, pharmacologic manipulation and targeting of the endotoxin-CD14/TLR4 signaling pathways could prove to be useful in alcoholic liver disease.
Previous studies have shown that betaine can prevent the alcohol-induced liver injury effectively and improve the liver function, which is related to the inhibition of inflammatory factor, the decrease of lipid peroxidation, the rivalry of endoplasmic reticulum stress and the prevention of apoptosis[16-21]. Our study indicates that in rats with alcohol-induced liver injury, betaine feeding can decrease the levels of serum ALT, AST, endotoxin, TNF-α, IFN-γ and IL-18, and reduced the expressions of TLR4, and improved the degree of hepatic steatosis and inflammation in liver tissues. It is suggested that betaine can prevent the alcohol-induced liver injury effectively and improve the liver function. The hepatoprotective mechanism of betaine is probably related to the inhibition of endotoxin/TLR4 signaling pathways.
In summary, the results of this study show that the expression of TLR4 increased significantly in ethanol-fed rats. Betaine administration can inhibit TLR4 expression, which may be one of mechanisms of alcoholic liver injury protected by betaine.
Chronic ethanol ingestion increases gut-derived endotoxin (LPS) in the portal circulation, thereby activating Kupffer cells to produce proinflammatory cytokines and induce liver injury. Toll-like receptor 4 (TLR4), via mediating LPS-induced signal transduction, plays a major role in the development of alcohol-induced liver injury. Blocking TLR4 signaling pathways is a therapeutic target of alcoholic liver disease. Many studies reported that betaine can prevent the alcohol-induced liver injury effectively and improve the liver function, but there are few reports about the effects of betaine on TLR4 and endotoxin in alcohol-induced liver injury.
Betaine is the only methyl donor, which can replace folate or S-ademetionine to participate in methionine recycling and phosphatidylcholine synthesis in the human body. The hepatoprotective effect and mechanism of betaine is a research hotspot in the area of prevention and cure of alcoholic liver disease. Current studies show that the hepatoprotective mechanism of betaine is related to the inhibition of inflammatory factor, the decrease of lipid peroxidation, the promotion of endoplasmic reticulum stress and the prevention of apoptosis. However, whether the inhibition of TLR4 expression and reduction of endotoxin are involved in hepatoprotective effect of betaine in the alcoholic liver injury remains unclear.
In the present study, the authors employed the intragastric ethanol-fed rat model, which reproduces the pathological features of early alcohol-induced liver injury, to observe the changes of TLR4 and endotoxin, and to study the protective effect of betaine on alcohol-induced liver injury. The authors found that the ethanol-fed rats had obvious liver injury and hepatic steatosis, higher serum endotoxin and inflammatory factor (TNF-α, IFN-γ and IL-18) levels, and significantly higher TLR4 expression, whereas betaine feeding can improve the liver function, reduce the expressions of TLR4 and endotoxin levels, and improve the degree of hepatic steatosis and inflammation in liver tissues.
The study results suggest that betaine can prevent the alcohol-induced liver injury effectively, and one of the hepatoprotective mechanisms of betaine is probably related to the inhibition of endotoxin/TLR4 signaling pathways.
Betaine, also known as trimethylglycine, is a chemical compound similar to folic acid and S-ademetionine. These compounds function as “methyl donors” that carry methyl molecules throughout the body, thus helping in the completion of several vital chemical processes. Toll-like receptor 4 (TLR4): TLR4 is a member of the Toll-like receptors (TLRs) family which can recognize pathogen-associated molecular patterns (PAMPs) that are expressed on infectious agents, and mediate the production of cytokines necessary for the development of effective immunity, and thereby plays a fundamental role in pathogen recognition and activation of innate immunity.
This is a well conducted and well written study. The experiments are described in detail, the results are shown nicely and the figures are impressive. This study for the first time shows that Betaine reduces the expression of TLR4 in rats with ethanol-induced liver injury, and proposes that the hepatoprotective mechanism of betaine is secondary to inhibition of endotoxin/TLR4 signaling pathways.
Peer reviewers: Dr. BS Anand, Professor, Digestive Diseases Section (111D), VA Medical Center, 2002 Holcombe Blvd., Houston, TX 77030, United States; Dr. Radha Krishna Yellapu, MD, DM, Department of Hepatology, Mount Sinai, 121 E 97 Street, NY 10029, United States
S- Editor Wang JL L- Editor Ma JY E- Editor Ma WH
| 1. | Fukui H. Relation of endotoxin, endotoxin binding proteins and macrophages to severe alcoholic liver injury and multiple organ failure. Alcohol Clin Exp Res. 2005;29:172S-179S. |
| 2. | Fang ZH, Cui JW, Hu YY. [Endotoxin injury in alcoholic liver disease]. Zhonghua Ganzangbing Zazhi. 2005;13:636-638. |
| 3. | Xu FL, You HB, Li XH, Chen XF, Liu ZJ, Gong JP. Glycine attenuates endotoxin-induced liver injury by downregulating TLR4 signaling in Kupffer cells. Am J Surg. 2008;196:139-148. |
| 4. | Enomoto N, Takei Y, Yamashima S, Ikejima K, Kitamura T, Sato N. Protective effect of pioglitazone against endotoxin-induced liver injury through prevention of Kupffer cell sensitization. Alcohol Clin Exp Res. 2005;29:216S-219S. |
| 5. | Dai Q, Pruett SB. Ethanol suppresses LPS-induced Toll-like receptor 4 clustering, reorganization of the actin cytoskeleton, and associated TNF-alpha production. Alcohol Clin Exp Res. 2006;30:1436-1444. |
| 6. | Tavener SA, Kubes P. Is there a role for cardiomyocyte toll-like receptor 4 in endotoxemia? Trends Cardiovasc Med. 2005;15:153-157. |
| 7. | Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven AJ, Van der Meer JW, Netea MG. Functional consequences of toll-like receptor 4 polymorphisms. Mol Med. 2008;14:346-352. |
| 8. | Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76-85. |
| 9. | Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Acute exposure to ethanol affects Toll-like receptor signaling and subsequent responses: an overview of recent studies. Alcohol. 2004;33:235-239. |
| 10. | Zuo G, Gong J, Liu C, Wu C, Li S, Dai L. Synthesis of Toll-like receptor 4 in Kupffer cells and its role in alcohol-induced liver disease. Chin Med J (Engl). 2003;116:297-300. |
| 11. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. |
| 12. | Kim YC, Jung YS, Kim SK. Effect of betaine supplementation on changes in hepatic metabolism of sulfur-containing amino acids and experimental cholestasis induced by alpha-naphthylisothiocyanate. Food Chem Toxicol. 2005;43:663-670. |
| 13. | Kharbanda KK, Rogers DD 2nd, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. A comparison of the effects of betaine and S-adenosylmethionine on ethanol-induced changes in methionine metabolism and steatosis in rat hepatocytes. J Nutr. 2005;135:519-524. |
| 14. | Krueger KJ, McClain CJ, McClave SA, Dryden GW. Nutritional supplements and alternative medicine. Curr Opin Gastroenterol. 2004;20:130-138. |
| 15. | Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539-549. |
| 16. | Zhang P, Gong ZJ. Effects of betaine on expression of caspase-12 in ethanol-induced liver injury in rats. Shijie Huaren Xiaohua Zazhi. 2005;13:2437-2440. |
| 17. | Zhang P, Gong ZJ, Wang LW, Sun XM, Zhou XR. Effects of Betaine on hyperhomocysteinemia and lipid peroxidation in rats with ethanol-induced liver injury. Zhongxiyi Jiehe Ganbing Zazhi. 2006;16:30-32. |
| 18. | Ji C, Shinohara M, Vance D, Than TA, Ookhtens M, Chan C, Kaplowitz N. Effect of transgenic extrahepatic expression of betaine-homocysteine methyltransferase on alcohol or homocysteine-induced fatty liver. Alcohol Clin Exp Res. 2008;32:1049-1058. |
| 19. | Samara K, Liu C, Soldevila-Pico C, Nelson DR, Abdelmalek MF. Betaine resolves severe alcohol-induced hepatitis and steatosis following liver transplantation. Dig Dis Sci. 2006;51:1226-1229. |
| 20. | Kharbanda KK, Mailliard ME, Baldwin CR, Sorrell MF, Tuma DJ. Accumulation of proteins bearing atypical isoaspartyl residues in livers of alcohol-fed rats is prevented by betaine administration: effects on protein-L-isoaspartyl methyltransferase activity. J Hepatol. 2007;46:1119-1125. |
| 21. | Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488-1499. |
| 23. | Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220-1230. |
| 25. | Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742-747. |
| 26. | Zuo GQ, Gong JP, Liu CA, Li SW, Wu XC, Yang K, Li Y. Expression of lipopolysaccharide binding protein and its receptor CD14 in experimental alcoholic liver disease. World J Gastroenterol. 2001;7:836-840. |
| 27. | Zeng MD, Li YM, Chen CW, Lu LG, Fan JG, Wang BY, Mao YM. Guidelines for the diagnosis and treatment of alcoholic liver disease. J Dig Dis. 2008;9:113-116. |
| 28. | Barve A, Khan R, Marsano L, Ravindra KV, McClain C. Treatment of alcoholic liver disease. Ann Hepatol. 2008;7:5-15. |
| 29. | Su GL, Klein RD, Aminlari A, Zhang HY, Steinstraesser L, Alarcon WH, Remick DG, Wang SC. Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology. 2000;31:932-936. |
| 30. | Kono H, Uesugi T, Froh M, Rusyn I, Bradford BU, Thurman RG. ICAM-1 is involved in the mechanism of alcohol-induced liver injury: studies with knockout mice. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1289-G1295. |
| 31. | Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301-304. |
| 32. | Dai LL, Gong JP, Zuo GQ, Wu CX, Shi YJ, Li XH, Peng Y, Deng W, Li SW, Liu CA. Synthesis of endotoxin receptor CD14 protein in Kupffer cells and its role in alcohol-induced liver disease. World J Gastroenterol. 2003;9:622-626. |
| 33. | Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903-914. |
| 34. | Muta T, Takeshige K. Essential roles of CD14 and lipopolysaccharide-binding protein for activation of toll-like receptor (TLR)2 as well as TLR4 Reconstitution of TLR2- and TLR4-activation by distinguishable ligands in LPS preparations. Eur J Biochem. 2001;268:4580-4589. |
| 35. | Miyaso H, Morimoto Y, Ozaki M, Haga S, Shinoura S, Choda Y, Murata H, Katsuno G, Huda K, Takahashi H. Protective effects of nafamostat mesilate on liver injury induced by lipopolysaccharide in rats: possible involvement of CD14 and TLR-4 downregulation on Kupffer cells. Dig Dis Sci. 2006;51:2007-2012. |