Published online Feb 14, 2010. doi: 10.3748/wjg.v16.i6.691
Revised: October 12, 2009
Accepted: October 19, 2009
Published online: February 14, 2010
It is essential in treating rectal cancer to have adequate preoperative imaging, as accurate staging can influence the management strategy, type of resection, and candidacy for neoadjuvant therapy. In the last twenty years, endorectal ultrasound (ERUS) has become the primary method for locoregional staging of rectal cancer. ERUS is the most accurate modality for assessing local depth of invasion of rectal carcinoma into the rectal wall layers (T stage). Lower accuracy for T2 tumors is commonly reported, which could lead to sonographic overstaging of T3 tumors following preoperative therapy. Unfortunately, ERUS is not as good for predicting nodal metastases as it is for tumor depth, which could be related to the unclear definition of nodal metastases. The use of multiple criteria might improve accuracy. Failure to evaluate nodal status could lead to inadequate surgical resection. ERUS can accurately distinguish early cancers from advanced ones, with a high detection rate of residual carcinoma in the rectal wall. ERUS is also useful for detection of local recurrence at the anastomosis site, which might require fine-needle aspiration of the tissue. Overstaging is more frequent than understaging, mostly due to inflammatory changes. Limitations of ERUS are operator and experience dependency, limited tolerance of patients, and limited range of depth of the transducer. The ERUS technique requires a learning curve for orientation and identification of images and planes. With sufficient time and effort, quality and accuracy of the ERUS procedure could be improved.
- Citation: Kav T, Bayraktar Y. How useful is rectal endosonography in the staging of rectal cancer? World J Gastroenterol 2010; 16(6): 691-697
- URL: https://www.wjgnet.com/1007-9327/full/v16/i6/691.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i6.691
Colorectal cancer is the most common gastrointestinal malignancy and the second most common cause of cancer-related deaths in Western countries[1]. Nearly 30% of these cancers arise in the rectum[2]. It is essential to determine prognostic factors in a patient before primary therapy is instituted. If examination has been delayed, it might be too late to influence the survival of a patient because of the lost opportunity to downstage the tumor before surgery. Primary surgery is no longer the only treatment due to recent advances in oncology and availability of therapeutic options. The potential advantages of preoperative treatment are to shrink the tumor size and thereby enhance the resectability rate and facilitate sphincter-saving surgery, to reduce local recurrences, and possibly to improve long-term survival[3]. The prognosis of rectal cancer is closely related to several factors, including depth of tumoral invasion, number of metastatic lymph nodes, and involvement of the circumferential margin. Assessment of the cancer invasion through the bowel wall (T stage) remains the primary and most important factor in treatment of patients with rectal cancer[1-4].
The TNM system for staging cancer of the colon and rectum to guide treatment and prognosis corresponds with the Dukes system: Stage I, Dukes A; stage II, Dukes B; and stage III, Dukes C. Stage IV corresponds to the presence of distant metastases[5]. Survival rates differ between T stages, and identifying poor prognostic groups within each stage has been the object of research. Early rectal cancers (T0) have a high, five-year survival rate of 95%. T3N0M0 and T4N0M0 lesions are stage II. Invasion of one or two lymph nodes but no distant metastasis (T1-4N1-2M0) with any T level represents stage III disease. Stage IV disease is the most severe, with distal metastasis (T1-4N1-2M1). The five-year survival rate for stage IV disease is poor (Table 1). There is a marked improvement in survival with early disease. A number of authors have shown a relationship between survival and the depth of extramural spread that is independent of other prognostic factors, including the circumferential margin status[6-8].
| Stage | T and N groups | Management | Five year survival |
| I | T 1-2, N0, M0 | Snare polypectomy, EMR-ESD, TAEX, LAR, APR | > 90% |
| II | T3 - 4, N0, M0 | 60%-85% | |
| III | T1-4, N1, M0 | LAR, RT followed by APR | 25%-60% |
| IV | T1-4, N0-2, M1 | RT-CT followed by APR | 5%-7% |
The presence of lymph node (LN) involvement is important for the clinical decision, as early and locally advanced disease are managed differently. Endorectal ultrasound (ERUS) is a safe diagnostic method that allows both tumor invasion and lymph node metastatic involvement to be staged, and it contributes significantly to the selection of an adequate surgical strategy in patients with rectal cancer[7-9].
Lesions confined to the wall may be resected by transanal excision or low anterior resection. Lesions involving, or in close proximity to, the anus might need abdominoperineal resection (APR). Patients with locoregionally-advanced lesions (extension onto the perirectal fat and/or perirectal or pelvic adenopathy) should be considered for neoadjuvant chemoradiotherapy. Neoadjuvant therapy has been shown to reduce local recurrence and permit an increased likelihood of a sphincter-sparing operation, with less toxicity compared with postoperative regimes. Thus, unlike more proximal colon cancers, the optimal method of management of rectal carcinoma is critically dependent on accurate preoperative staging of the disease[9,10].
These therapeutic strategies appear to reduce local recurrence rates, increase sphincter-preserving surgeries, and possibly improve overall survival. Surgeries, and possibly improve overall survival. Therefore, staging of rectal cancer is important for selecting patients for adequate management prior to disturbing the tumor bed and potentially disseminating the disease. In daily practice we have been using newly developed and improved technologies that enable us to assess the extent of rectal cancer, which in turn influences choice of therapy. At present, existing modalities for the preoperative staging of rectal cancer include computed tomography (CT); magnetic resonance imaging (MRI) with traditional body, endorectal, or phased-array coils; ERUS with rigid or flexible probes; and positron emission tomography (PET) with and without CT. The choice of modality is often influenced by local expertise and availability. This article reviews the current literature on the usefulness of ERUS in the staging of rectal cancer.
Endorectal sonography was introduced to clinical practice in 1983 and has been successfully used in clinical practice for the evaluation of both the prostate and the rectum. In 1985, Hildebrant and Feifel introduced endorectal ultrasound as a means of staging rectal carcinoma[11]. In the last decade ERUS has become a widely accepted tool for staging of gastrointestinal cancers. Availability of ERUS in developing countries is limited, and there is a variation in availability and use of ERUS across Europe; the United Kingdom being the country in which ERUS is most widely used[12]. When ERUS is available, oncologists usually prefer to use it for staging of rectal cancer, which is the second most common cause of consultation with endosonographic examination indicated by surveyed oncologists. Most oncologists (89.5%) thought ERUS made an important impact on the management of patients with rectal cancer[13].
Primary rectal adenocarcinoma is a common cause of a rectal mass on imaging. Other, less-common, lesions of the anorectum and perirectal tissues might resemble an adenocarcinoma. Transrectal sonography has proved to be a fast, safe, and accurate initial method for the staging of known rectal cancers or masses, although not for the screening of suspected rectum tumors, and is widely accepted as the diagnostic modality of first choice[14,15]. Imaging of the anorectum and perirectal tissues is technically challenging and can be difficult to interpret, as fecal material might be present, rectal lesions can be mobile or large, and general orientation is difficult[16]. The technique of transrectal sonography requires a learning curve for orientation and identification of ultrasound images and planes of rectal tumors. With sufficient effort, time, and meticulous technique, however, the rectum can be easily examined[15,16].
To perform ERUS it is preferable to have an empty rectum, because fecal material can distort the images obtained. Laxative enemas are usually sufficient for rectal lesions, but standard colonoscopy preparation, even for rectal end sigmoid lesions, could optimize imaging so that it is free of artifacts. For endosonographic examination of proximal colonic lesions, such a preparation is a prerequisite. Pre-examination sigmoidoscopy should be routinely performed to ensure the lumen is clear of debris. The procedure is well tolerated and can be performed without sedation. Intraluminal rectal ultrasound examination of rectal lesions can be done with a rigid probe or a flexible echoendoscope with a radial transducer. At our institution, we use a front-viewing upper echoendoscope, which can be advanced under direct vision to the level of the lesion. Linear echoendoscopes are normally used for fine-needle aspiration in case tissue sampling is needed, but could be used for routine ERUS[9]. For the purpose of this discussion, both techniques are considered as ERUS. Commonly, a dedicated blind rectal probe is used for ERUS. The probe is inserted and advanced into the rectum, where a water-filled balloon at the tip of the probe is inflated for evaluation of the rectum. High-frequency miniprobes are available and can be used with standard endoscopes to image the gastrointestinal wall and focal lesions under endoscopic vision. ERUS accurately visualizes the layers of the rectal wall and the precise localization of the layers of the rectal wall disrupted by the tumor, and the presence of perirectal lymph node metastases can be established[17].
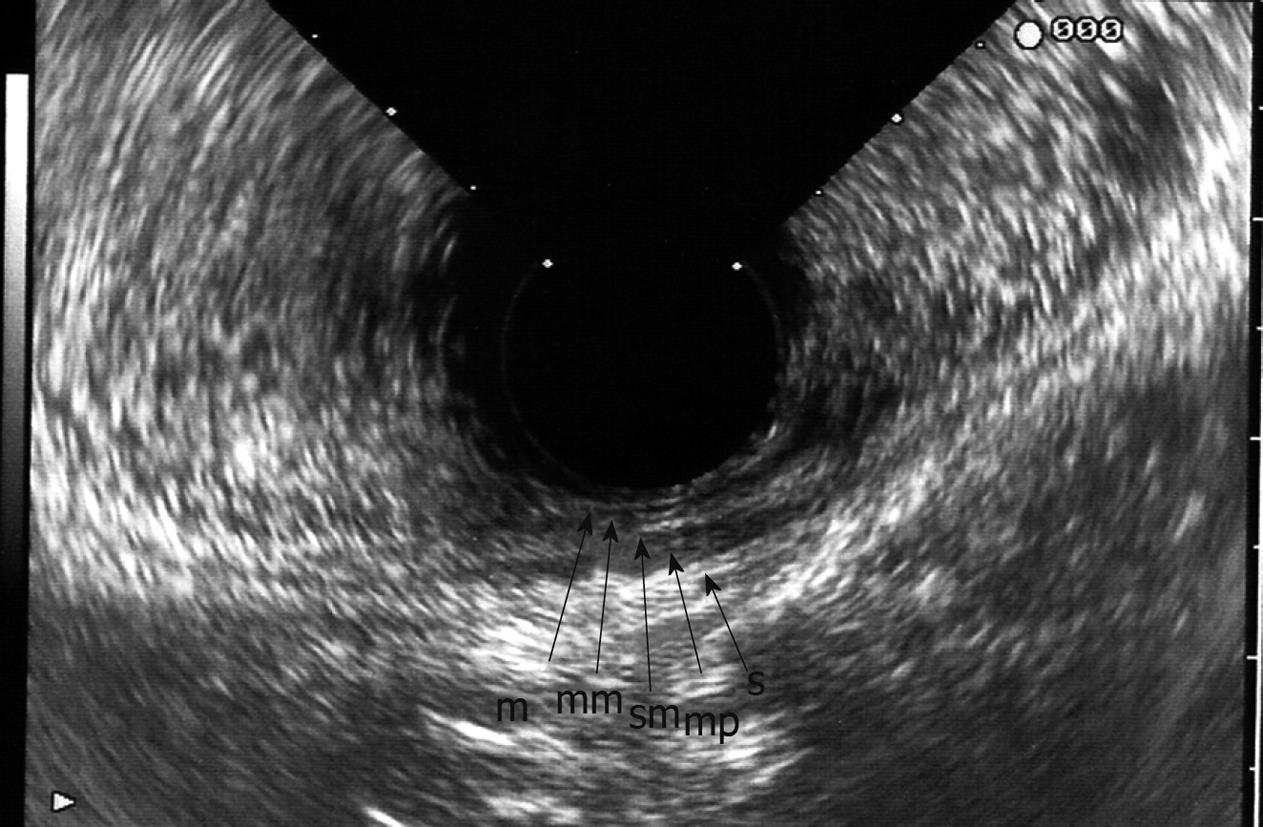
Endosonographically, the bowel wall is seen as five alternating hyper- and hypoechoic layers (Figure 1)[9,17-19], as a result of differences in acoustic impedance, corresponding to histological layers. The first (hyperechoic) layer is the interface between the superficial mucosa and water or a water-filled balloon; the second (hypoechoic) layer represents the mucosa and muscularis mucosae; the third (hyperechoic) layer denotes the submucosa and its interfaces; the fourth (hypoechoic) layer represents the muscularis propria; and the fifth (hyperechoic) layer is the interface between the serosa and perirectal fat.
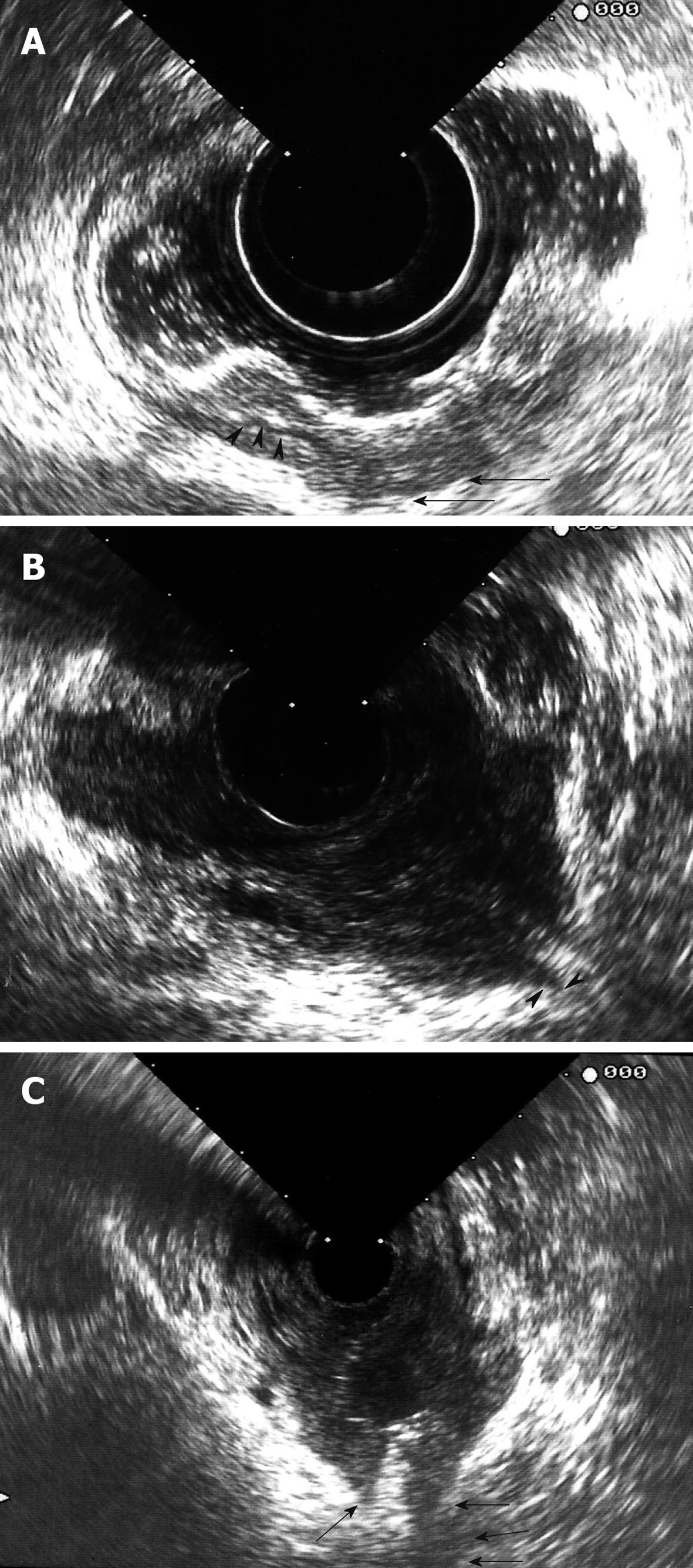
Carcinomas are hypoechoic, and the degree to which they disrupt and penetrate the rectal wall layers suggests the local stage[4]. Ultrasonographic staging of tumor depth is denoted by the prefix “u”. The ultrasonographic staging corresponds to the TNM classification[5]. A uT1 tumor does not penetrate the muscularis propria. A uT2 tumor penetrates the muscularis propria (Figure 2A). A uT3 tumor proceeds beyond the muscularis propria, infiltrating the perirectal fat to a variable degree (Figure 2B). A uT4 tumor infiltrates surrounding organs[10,18]. As the tumor stage is advanced, a marked decrease in survival is observed. ERUS, however, cannot reliably visualize the mesorectal fascia and thus cannot indicate whether the planned surgical circumferential resection margin will be successful[1-5].
The sonographic criteria for identifying involved lymph nodes consist of size greater than 5 mm, mixed signal intensity, irregular margins, and spherical rather than ovoid or flat shape. ERUS can distinguish the different anatomic layers of the bowel, and thus it appears to have advantages over both CT and MRI in assessing mural penetration, and is invaluable in assessing patients considered for local resection[10,20].
Indications for ERUS in rectal cancer are as follows[21]: (1) to choose endoscopic mucosal resection or transanal excision in case of a large polyp or small rectal cancer (lesion is T1 by ERUS); (2) to determine whether preoperative chemotherapy and radiation is needed; and (3) surveillance after surgery for rectal cancer.
As outlined above, appropriate staging guides the treatment. Many other modalities, including CT and MRI of the abdomen, have been utilized to correctly determine the TNM stage. In 80 consecutive patients with newly diagnosed rectal cancer who were prospectively evaluated, Harewood et al[22] reported T staging accuracy of 91%, compared to 71% for CT, and N staging accuracy of 82%, compared to 76% for CT.
The accuracy of ERUS for assessing local depth of invasion of rectal carcinoma (T stage) ranges from 80% to 95%, compared to 65%-75% for CT and 75%-85% for MRI[20]. ERUS has been demonstrated to be very accurate for staging superficial rectal tumors, with accuracy in evaluating tumor ingrowth into rectal wall layers ranging from 69% to 97%[23,24].
A recent meta-analysis evaluating all ERUS studies from 1980 to 2008 showed that accuracy was high (88%-95%). The sensitivity and specificity of ERUS to diagnose stage T1 cancer were 87.8% and 98.3%, respectively. For stage T2, ERUS had a sensitivity and specificity of 80.5% and 95.6%, respectively. For stage T3, ERUS had a sensitivity and specificity of 96.4% and 90.6%, respectively. In diagnosing stage T4 cancer, ERUS had a sensitivity of 95.4% and specificity of 98.3%[25]. One common finding is a lower accuracy for T2 tumors. Several reasons have been suggested, including the difficulty in distinguishing those tumors that have deep invasion into the muscularis propria from those with microscopic invasion into the perirectal fat. This could raise problems with sonographic T3 cancers that have been overstaged, because there is an increased tendency to give preoperative radiotherapy to T3 cancers.
Zorcolo et al[26] evaluated the accuracy of ERUS for the distinction of early vs advanced rectal lesions before transanal endoscopic microsurgery and they found ERUS differentiated early and advanced rectal lesions with 96% sensitivity, 85% specificity, and 94% accuracy. Similarly, another retrospective series reached 89.2% accuracy for staging of early rectal carcinomas[27].
ERUS is also helpful in determining the presence of residual cancer in the rectal wall. A retrospective series with 63 patients showed the presence of residual cancer in patients who underwent surgery (n = 30) with 54% accuracy. Authors stated that ERUS was more useful than morphological or histological criteria for determining residual cancer[28].
Transanal endoscopic microsurgery (TEM) and endoscopic submucosal dissections have been becoming more popular because of they offer function-preserving resections. An important problem that has arisen in this setting is the assessment of the tumor breach to the submucosa, which changes the mode of surgery. Other imaging modalities are known to be poor at staging in early cancers. According to a prospective study involving 156 patients, of whom 62 underwent TEM, no understaging was observed with an accuracy of 95%, and only 5% were overstaged. ERUS is accurate at predicting early disease[29].
ERUS was useful in detecting cancer recurrence at the anastomosis site. This often requires serial examination to differentiate postoperative scars from local recurrences. In sonographically equivocal cases, tissue characterization and sampling via FNA make ERUS very accurate; although the surveillance period was not assessed, a recommendation was made of every 3-6 mo during the first two years after low anterior resection[20].
Assessment for nodal metastases is less accurate than that for tumor depth. According to a recent meta-analysis of 35 studies by Puli et al[30], which involved more than 2700 patients, the sensitivity of ERUS in diagnosing nodal involvement in rectal cancer was 73.2% and it had a specificity of 75.8%.
Discrepancies in accuracies could be partly due to the variable criteria used for defining nodal metastases. For rectal cancer in particular, over half of the metastatic nodes secondary to rectal cancer are ≤ 5 mm and are located within 3 cm of the primary tumor[31]. In a large trial, lymph node metastatic disease was shown to predict local recurrence. There is a wide variation in accuracy for metastatic nodal detection with ERUS (62%-87%), CT (22%-73%), and MRI (39%-95%)[32]. ERUS criteria are a lack of ovoid morphology and central echogenic nidus, but its limited field of view is a major limitation[4]. Data from pooled analyses, as well as from recent smaller studies, reveal that the sensitivity of ERUS in detecting LN metastasis ranges from 50% to 83%, which is comparable with that of MRI (sensitivity 45% to 79%)[9,10]. Assessment of nodal metastases is difficult because most small lymph nodes are not easily observed with ERUS, and 18% of lymph nodes less than 5 mm harbor metastases[17]. More recent studies suggest that multiple criteria should be used to improve accuracy. Gleeson et al[20] conducted a study with ERUS guided FNA to identify nodal echo characteristics and size for prediction of malign infiltration, and to determine if any combination of standard nodal criteria had sufficient predictive value to preclude FNA. Nodal hypoechogenicity and short axis ≥ 5 mm were independent factors for malignancy. If all four malignant nodal echo features of node were present, it distinguished the malignant from the benign node. These US features were node size, echogenicity, shape, and the border. A long axis length greater than 9 mm was 95% specific for the presence of malignancy.
Accuracies of ERUS might vary with different tumor stages. Overstaging is more frequent than understaging. As with MRI, overstaged T2 lesions are the most common causes of inaccuracy. ERUS cannot reliably or precisely differentiate an irregular outer rectal wall due to peritumoral inflammation or real transmural tumor extension[7-9,33]. Staging of the stenotic lesions might also be difficult; they are probably suboptimally staged because of the inability of the probe to traverse the lesion. This problem is greater with rigid probes. Flexible probes have the ability to evaluate the iliac region for adenopathy, which is clinically important because these nodes are retained in standard resection with total mesorectal excision. In one study, up to 28% of lymph node-positive distal tumors showed iliac adenopathy, with 6% of patients having only iliac adenopathy. Thus, failure to evaluate this region could lead to inadequate surgical margins in up to 6% of patients with low rectal lesions. Lymph nodes > 5 mm in size have a 50% to 70% chance of being malignant compared with only 20% of nodes < 4 mm. ERUS-guided FNA allows confirmation of malignancy in suspicious nodes during the same examination, as long as the primary tumor does not lie in the path of the needle[33].
Preoperative chemoradiation is a main reason for lower staging accuracy rate. Napoleon et al[34] found a variation in the accuracy of T staging from 86% (in patients referred directly to surgery) to 46% (in patients after neoadjuvant radiation therapy).
Overstaging is mainly caused by inflammatory and associated reactive changes in the rectum wall after preoperative radiotherapy. They are presented as hypoechoic lesions and can be confused with carcinoma. However, radiotherapy affects the wall thickness but does not change the five-layered image. In one particular study, comparison of postradiation ERUS correlated with histopathology findings revealed that ultrasound was actually assessing the fibrosis that had replaced the tumor; therefore, after radiotherapy, what is staged by ERUS is no longer the tumor but the extent of fibrosis in the rectal wall. A histopathological examination showed that the residual tumor, when present, was always within the fibrosis, never outside or separate from it[33,35].
In general, ERUS is better at detecting lymph nodes in the distal and middle thirds of the rectum[21,33]. The overstaging of lymph node status is primarily caused by the presence of reactive swollen lymph nodes that could be considered as malignant. The small blood vessels, urethra, and seminal vesicle are known to be mistaken for metastatic lymph nodes. Blood vessels can simulate malignant nodes, but they can be differentiated by moving the transducer to outline the linear or branching course of the vessel and by power Doppler. The main reasons for nodal status understaging are difficulty in detecting very small involved nodes (less than 2 mm) and nodes outside the perirectal tissue[21,33].
The three-dimensional reconstruction is also thought to improve visualization of subtle protrusions of tumors infiltrating into adjacent tissues and organs, allowing for improved T and N staging. A study of 25 patients undergoing three-dimensional ERUS, two-dimensional ERUS, and MRI showed no significant difference in T- or N-stage accuracy, but it was thought that MRI and three-dimensional ERUS improved understanding of the spatial relationship of the tumor due to their ability to obtain multiplanar images[36].
The limitations of ERUS are that it is heavily operator dependent; it has poor patient acceptability; it has limited depth of penetration; it cannot be performed in stenotic tumors[16,21]; and it is unable to visualize tumors located in the upper rectum with a rigid probe, detect lymph nodes outside the range of the transducer, or visualize mesorectal fascia because of its limited field of view. In addition, accuracy is affected by postbiopsy peritumoral inflammation, hemorrhage, and villous or pedunculated tumors[22,31]. Overstaging of tumor depth frequently occurs as a result of paraneoplastic inflammation, as ultrasound cannot clearly differentiate between inflammatory and neoplastic tissue[22,31].
Several authors suggest that obstructive tumors interfere with accurate staging. In such tumors, inadequate probe contact perpendicular to the tumor makes it more likely to be mis-staged. Some authors reported better accuracy rates for high compared to low rectal tumors, while others found the opposite[32].
The tumor margin cannot be assessed accurately, which in turn causes mis-staging, because of inadequate bowel preparation and bulky tumors that lie outside the focal length of the transducer.
There is a learning curve with operator variability[33]. Badger et al[37] found that experience does not affect the T and N staging accuracy, suggesting that there was no learning curve. Others supported the effect of experience and appropriate training for accurate staging[38]. Inexperience has been cited as contributing to many of the poor accuracies in tumor depth infiltration[17].
Orrom et al[39] reported an increase in diagnostic accuracy of ultrasound from 59.3% to 95% over a period of three years when ERUS was performed by several operators and a single skilled operator performed the later exams. More time and meticulous training are required for improvements in the accuracy of ERUS, a statement supported by different studies showing a progress from 50% to over 90%[39-42]. It is has also been suggested that centralization of ERUS could provide more caseloads to experienced operators and result in a high-quality service[41].
Studies suggest a learning curve of up to 50 cases for tumor depth and more than 75 cases for accurate node status assessments[41]. Interpretation is often more difficult after a partial excision or neoadjuvant chemoradiation, which can result in a hematoma or local inflammation with obliteration of sonographic layers of the rectal wall. Presence of inflammatory changes, desmoplastic changes, and hypervascularity could lead to overstaging because the echogenicity of tumors is similar to that of muscularis propria and inflammatory infiltrate[42].
Transrectal sonography is useful to differentiate extramural lesions, extrinsic compression, vascular lesions, and solid tumors. Other types of malignancies resembling rectal adenocarcinoma include neuroendocrine tumors, which usually manifest as small, mobile, submucosal nodules or focal areas of submucosal thickening, and primary squamous cell carcinomas, which seem to be frequently locally invasive and involve regional lymphatic vessels. Lymphomas are rare, and can be a primary lesion or a secondary infiltration of the large intestine, which characteristically involves the deeper layers of the intestinal wall. Anorectal melanoma is another rare rectal tumor. Gastrointestinal stromal tumor (GIST) is the most common mesenchymal tumor that originates in the alimentary tract, but it rarely involves the anorectal region. The tissue of origin is the muscle layer of the bowel wall, and the size can be variable. On sonography, a GIST appears as a hypoechoic mass[15]. ERUS is also helpful for determining local invasion of the rectum by other pelvic malignancies (Figure 2C).
ERUS is a safe and accurate technique for the local staging of rectal carcinoma with reported high accuracy rates for T and N staging. Although availability is limited, it has been implemented into clinical practice in clinical decision making regarding treatment modality. A growing body of expertise has confirmed the clinical impact. ERUS is also helpful in assessing recurrence of rectal cancer and evaluation of subepithelial masses. Technological improvements in ultrasound might improve accuracy and reduce the overstaging problem.
Peer reviewer: Dr. Herwig R Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, A-8036 Graz, Austria
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH
| 1. | Rovera F, Dionigi G, Boni L, Cutaia S, Diurni M, Dionigi R. The role of EUS and MRI in rectal cancer staging. Surg Oncol. 2007;16 Suppl 1:S51-S52. |
| 2. | Meredith KL, Hoffe SE, Shibata D. The multidisciplinary management of rectal cancer. Surg Clin North Am. 2009;89:177-215, ix-x. |
| 3. | Wolpin BM, Meyerhardt JA, Mamon HJ, Mayer RJ. Adjuvant treatment of colorectal cancer. CA Cancer J Clin. 2007;57:168-185. |
| 4. | Karantanas AH, Yarmenitis S, Papanikolaou N, Gourtsoyiannis N. Preoperative imaging staging of rectal cancer. Dig Dis. 2007;25:20-32. |
| 5. | Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20-31. |
| 6. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR. Can Endoscopic Ultrasound Predict Early Rectal Cancers That Can Be Resected Endoscopically? A Meta-Analysis and Systematic Review. Dig Dis Sci. 2009;Epub ahead of print. |
| 7. | Siddiqui AA, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol. 2006;3:36. |
| 8. | Assenat E, Thezenas S, Samalin E, Bibeau F, Portales F, Azria D, Quenet F, Rouanet P, Saint Aubert B, Senesse P. The value of endoscopic rectal ultrasound in predicting the lateral clearance and outcome in patients with lower-third rectal adenocarcinoma. Endoscopy. 2007;39:309-313. |
| 9. | Bhutani MS. Colorectal endoscopic ultrasound. Endoscopic ultrasonography. 2nd ed. West Sussex: Wiley-Blackwell 2009; 160-171. |
| 10. | Giovannini M, Ardizzone S. Anorectal ultrasound for neoplastic and inflammatory lesions. Best Pract Res Clin Gastroenterol. 2006;20:113-135. |
| 11. | Hildebrandt U, Feifel G. Preoperative staging of rectal cancer by intrarectal ultrasound. Dis Colon Rectum. 1985;28:42-46. |
| 12. | Kalaitzakis E, Panos M, Sadik R, Aabakken L, Koumi A, Meenan J. Clinicians’ attitudes towards endoscopic ultrasound: a survey of four European countries. Scand J Gastroenterol. 2009;44:100-107. |
| 13. | Reddy NK, Markowitz AB, Abbruzzese JL, Bhutani MS. Knowledge of indications and utilization of EUS: a survey of oncologists in the United States. J Clin Gastroenterol. 2008;42:892-896. |
| 14. | Berton F, Gola G, Wilson SR. Perspective on the role of transrectal and transvaginal sonography of tumors of the rectum and anal canal. AJR Am J Roentgenol. 2008;190:1495-1504. |
| 15. | Rouse HC, Godoy MC, Lee WK, Phang PT, Brown CJ, Brown JA. Imaging findings of unusual anorectal and perirectal pathology: a multi-modality approach. Clin Radiol. 2008;63:1350-1360. |
| 16. | Saranovic D, Barisic G, Krivokapic Z, Masulovic D, Djuric-Stefanovic A. Endoanal ultrasound evaluation of anorectal diseases and disorders: technique, indications, results and limitations. Eur J Radiol. 2007;61:480-489. |
| 17. | Skandarajah AR, Tjandra JJ. Preoperative loco-regional imaging in rectal cancer. ANZ J Surg. 2006;76:497-504. |
| 18. | Halefoglu AM, Yildirim S, Avlanmis O, Sakiz D, Baykan A. Endorectal ultrasonography versus phased-array magnetic resonance imaging for preoperative staging of rectal cancer. World J Gastroenterol. 2008;14:3504-3510. |
| 19. | Bartram C, Brown G. Endorectal ultrasound and magnetic resonance imaging in rectal cancer staging. Gastroenterol Clin North Am. 2002;31:827-839. |
| 20. | Gleeson FC, Clain JE, Papachristou GI, Rajan E, Topazian MD, Wang KK, Levy MJ. Prospective assessment of EUS criteria for lymphadenopathy associated with rectal cancer. Gastrointest Endosc. 2009;69:896-903. |
| 21. | Bhutani MS. Recent developments in the role of endoscopic ultrasonography in diseases of the colon and rectum. Curr Opin Gastroenterol. 2007;23:67-73. |
| 22. | Harewood GC, Wiersema MJ, Nelson H, Maccarty RL, Olson JE, Clain JE, Ahlquist DA, Jondal ML. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123:24-32. |
| 23. | Garcia-Aguilar J, Pollack J, Lee SH, Hernandez de Anda E, Mellgren A, Wong WD, Finne CO, Rothenberger DA, Madoff RD. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum. 2002;45:10-15. |
| 24. | Gualdi GF, Casciani E, Guadalaxara A, d'Orta C, Polettini E, Pappalardo G. Local staging of rectal cancer with transrectal ultrasound and endorectal magnetic resonance imaging: comparison with histologic findings. Dis Colon Rectum. 2000;43:338-345. |
| 25. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254-265. |
| 26. | Zorcolo L, Fantola G, Cabras F, Marongiu L, D'Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23:1384-1389. |
| 27. | Kulig J, Richter P, Gurda-Duda A, Gach T, Klek S. The role and value of endorectal ultrasonography in diagnosing T1 rectal tumors. Ultrasound Med Biol. 2006;32:469-472. |
| 28. | García-Aguilar J, Hernández de Anda E, Rothenberger DA, Finne CO, Madoff RD. Endorectal ultrasound in the management of patients with malignant rectal polyps. Dis Colon Rectum. 2005;48:910-916; discussion 916-917. |
| 29. | Glancy DG, Pullyblank AM, Thomas MG. The role of colonoscopic endoanal ultrasound scanning (EUS) in selecting patients suitable for resection by transanal endoscopic microsurgery (TEM). Colorectal Dis. 2005;7:148-150. |
| 30. | Puli SR, Reddy JB, Bechtold ML, Choudhary A, Antillon MR, Brugge WR. Accuracy of endoscopic ultrasound to diagnose nodal invasion by rectal cancers: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:1255-1265. |
| 31. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. |
| 32. | Maor Y, Nadler M, Barshack I, Zmora O, Koller M, Kundel Y, Fidder H, Bar-Meir S, Avidan B. Endoscopic ultrasound staging of rectal cancer: diagnostic value before and following chemoradiation. J Gastroenterol Hepatol. 2006;21:454-458. |
| 33. | Muthusamy VR, Chang KJ. Optimal methods for staging rectal cancer. Clin Cancer Res. 2007;13:6877s-6884s. |
| 34. | Napoleon B, Pujol B, Berger F, Valette PJ, Gerard JP, Souquet JC. Accuracy of endosonography in the staging of rectal cancer treated by radiotherapy. Br J Surg. 1991;78:785-788. |
| 35. | Gavioli M, Bagni A, Piccagli I, Fundaro S, Natalini G. Usefulness of endorectal ultrasound after preoperative radiotherapy in rectal cancer: comparison between sonographic and histopathologic changes. Dis Colon Rectum. 2000;43:1075-1083. |
| 36. | Hünerbein M, Pegios W, Rau B, Vogl TJ, Felix R, Schlag PM. Prospective comparison of endorectal ultrasound, three-dimensional endorectal ultrasound, and endorectal MRI in the preoperative evaluation of rectal tumors. Preliminary results. Surg Endosc. 2000;14:1005-1009. |
| 37. | Badger SA, Devlin PB, Neilly PJ, Gilliland R. Preoperative staging of rectal carcinoma by endorectal ultrasound: is there a learning curve? Int J Colorectal Dis. 2007;22:1261-1268. |
| 38. | Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43:440-446. |
| 39. | Orrom WJ, Wong WD, Rothenberger DA, Jensen LL, Goldberg SM. Endorectal ultrasound in the preoperative staging of rectal tumors. A learning experience. Dis Colon Rectum. 1990;33:654-659. |
| 40. | Carmody BJ, Otchy DP. Learning curve of transrectal ultrasound. Dis Colon Rectum. 2000;43:193-197. |