Published online Feb 7, 2010. doi: 10.3748/wjg.v16.i5.652
Revised: December 24, 2009
Accepted: December 31, 2009
Published online: February 7, 2010
Germ cell tumor (GCT) of the liver is extremely rare. Here, we describe a case of hepatic mixed GCT with significant sarcomatous components and elevated serum α-fetoprotein (AFP) in a 34-year-old man. Histopathologically, the tumor was composed of two GCTs components: yolk sac tumor and immature teratoma. The predominant components of immature teratoma consisted of several types of tissue that represented different germinal layers (endoderm, mesoderm and ectoderm) and showed varying degrees of differentiation with significant sarcomatous components. The yolk sac component showed positivity for AFP and cytokeratin (AE1/AE3). The immature teratoma components showed positivity for varying differentiation markers. Interphase cytogenetic analysis revealed that the yolk sac tumor and immature teratoma were positive for i(12p) and 12p over-representation. In particular, the rhabdomyoblastic components also showed typical i(12p) and 12p overrepresentation. This suggested that sarcomatous components may be associated with dedifferentiation or malignant transformation of certain mesenchymal components within teratoma.
- Citation: Xu AM, Gong SJ, Song WH, Li XW, Pan CH, Zhu JJ, Wu MC. Primary mixed germ cell tumor of the liver with sarcomatous components. World J Gastroenterol 2010; 16(5): 652-656
- URL: https://www.wjgnet.com/1007-9327/full/v16/i5/652.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i5.652
Germ cell tumors (GCTs) are a diverse family of neoplasms, and they are all presumed to arise from totipotent primordial germ cells[1]. This family of neoplasms includes tumors that arise from different anatomic sites, in different age groups, and that vary in clinical behavior. Most GCTs are gonadal in origin, others are extragonadal, which are located mainly along the midline of the body. GCT of the liver is extremely rare, and accounts for < 1% of all liver neoplasms[2]. Most of them are in children aged < 3 years old, and about half of these tumors are malignant. Approximately 20 cases of malignant GCTs have been reported in the English literature following presentation as teratoma[3-5], choriocarcinoma[6,7] or yolk sac tumor[8,9] in patients over 21 years of age. The presence of sarcomatous components in gonadal or extragonadal GCTs is also an infrequent phenomenon[10].
Here, we report the light microscopic and cytogenetic findings of a case of hepatic malignant mixed GCT with significant sarcomatous components in a 34-year-old Chinese man.
A 34-year-old man presented with right upper quadrant pain in 2006. Ultrasound, magnetic resonance imaging and computed tomography showed a 15 cm × 12 cm × 9 cm low density heterogeneous mass in the right liver lobe. Blood tests showed slightly elevated aspartate aminotransferase, γ-glutamyl transferase, alkaline phosphatase and a very high α-fetoprotein (AFP: 56 500 μg/L, normal level < 20 μg/L). Other biochemical parameters (renal and liver function) and tumor markers such as carbohydrate antigen 19-9, and carcinoembryonic antigen (CEA) were within normal limits. Results of serology testing showed that serum hepatitis B virus surface antigen and anti-hepatitis C virus antibody were negative. The patient had not received any treatment before operation. No other tumor site was detected by extensive preoperative staging. After the liver tumor was resected, the serum AFP level went down to 512 μg/L.
Macroscopically, the partial liver resection specimen from the right lobe contained a single, firm mass with partially necrotic areas. It measured 16 cm × 12 cm × 8 cm. The cut surface was reddish-purple, whitish, gray to tan, slightly lobular with fibrous septa, and showed cystic spaces and areas of hemorrhage.
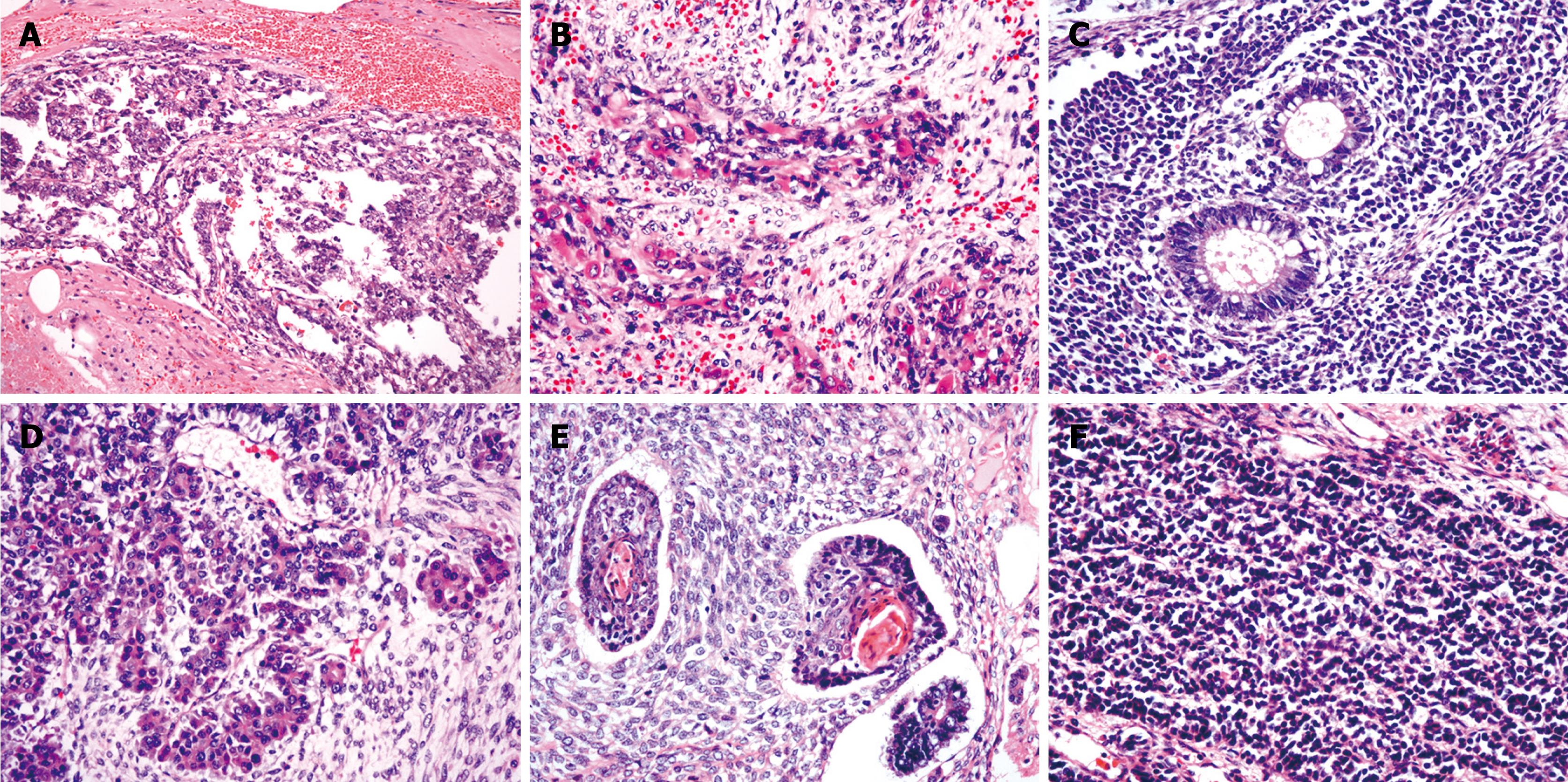
Histologically, the resected tumor was composed of two GCTs components: yolk sac tumor and immature teratoma. The morphological appearance of yolk sac tumor included endodermal sinus-like (with Schiller-Duval bodies), papillary and glandular components (Figure 1A). The predominant components of immature teratoma were composed of several types of tissue that represented different germinal layers (endoderm, mesoderm and ectoderm) and showed varying degrees of differentiation. The endoderm components were primitive mesenchyme and various mesenchyme-derived tissues, which were composed predominantly of primitive to spindle cells with rhabdomyoblastic (Figure 1B), chondroid and osteoblastic differentiation. The mesoderm components were composed of respiratory, intestinal-type epithelium (Figure 1C), and glandular, ductal or acinar structures that resembled pancreatic acinar tissue (Figure 1D). The ectoderm components were composed of keratinizing or non-keratinizing epithelium (Figure 1E), which resembled neuroblastoma tissue that exhibited rosettes formation (Figure 1F), ganglion differentiation or embryonic neural tube. Mitotic figures were found frequently in various tumorous components. Areas of necrosis were also present. Hepatocellular differentiation was not found in the tumor. Uninvolved liver demonstrated no obvious cirrhosis but slight steatosis. The surgical resection margins were clear.
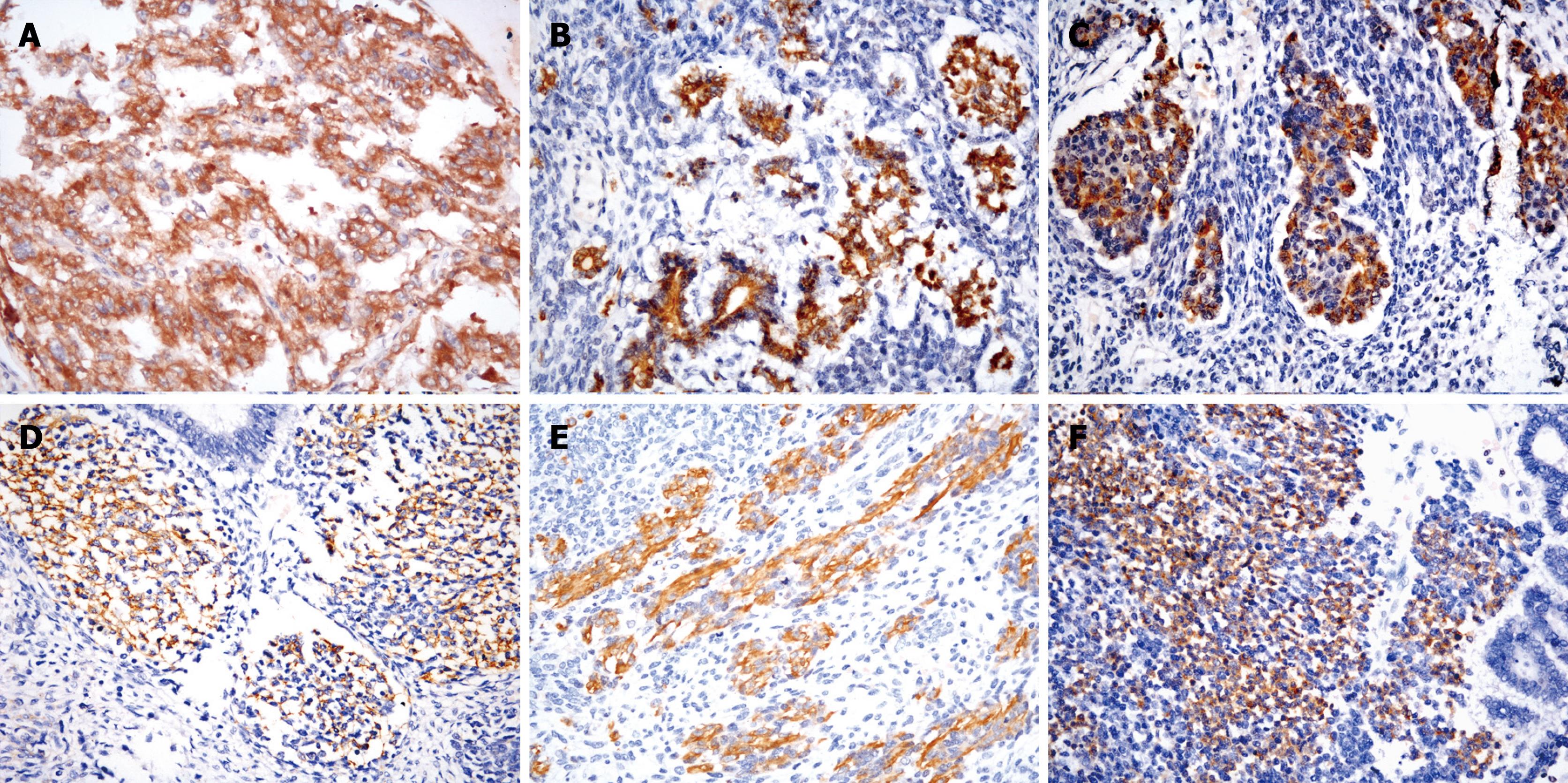
Immunohistochemically, the yolk sac tumor stained positively for AFP (Figure 2A) and cytokeratin (AE1/AE3). The cells of immature glandular or ductal structures were positive for pCEA, mCEA, AE1/AE3, CK7, CK18 and CK19. Focal hepatocyte marker (HepPar1) protein reaction was seen in the glandular epithelium. The acinar structures that resembled pancreatic acinar cells were positive for AFP (Figure 2B), α-1-antitrypsin, α-chymotrypsin (Figure 2C) and AE1/AE3. The primitive mesenchymal spindle cells were stained prominently with vimentin, and focally stained with smooth muscle actin, CD56 (Figure 2D), CD117 and CD34. Desmin and myoglobin were positive in the immature skeletal muscle tissue (Figure 2E). The cells of immature chondroid and osteoblastic components were positive for both vimentin and S-100 protein. The neuroblastoma-like areas and ganglion cells were positive for vimentin, synaptophysin (Figure 2F), neuron-specific enolase (NSE), glial fibrillary acid protein (GFAP), and focally stained with HMB45, CD99 and CD117. The immature glandular structures and neuroblastoma-like areas showed a high proliferative activity of Ki-67 positivity, whereas proliferative activity in the spindle cell areas was lower. P53 showed strong nuclear expression in all epithelial and rhabdomyoblastic cells. All tumor cells stained negatively for CD30, human chorionic gonadotropin or placental alkaline phosphatase (PLAP). The immunohistochemical profile of tumor in the differential diagnosis of GCT of the liver is shown in Table 1.
| Markers | Yolk sac tumor component | Immature glandular structure | Primitive mesenchymal component | Rhabdomyoblastic component | Chondroid and osteoblastic components | Neuroblastoma component |
| Antigen | ||||||
| AFP | +++ | +1 | - | - | + | - |
| AE1/AE3 | +++ | +++ | - | - | - | - |
| Hep par 1 | - | + | - | - | - | - |
| CK7 | - | + | - | - | - | - |
| CK18 | + | +++ | - | - | - | - |
| CK19 | +++ | +++ | - | - | - | - |
| CK20 | - | + | - | - | - | - |
| EMA | - | + | - | - | - | - |
| α-1-antitrypsin | + | + | - | - | - | - |
| α-1-chymotrypsin | - | + | - | - | - | - |
| pCEA | - | ++ | - | - | - | - |
| mCEA | - | ++ | - | - | - | - |
| Vimentin | - | - | +++ | +++ | ++ | +++ |
| Desmin | - | - | - | +++ | - | - |
| Myoglobin | - | - | - | +++ | - | - |
| SMA | - | - | +1 | - | - | - |
| CD30 | - | - | - | - | - | - |
| HCG | - | - | - | - | - | - |
| GFAP | + | - | - | - | - | ++ |
| NSE | - | - | ++ | - | - | +++ |
| S-100 | - | - | - | - | ++ | ++ |
| Synaptophysin | - | - | - | - | - | ++ |
| PLAP | - | - | - | - | - | - |
| CD56 | - | - | + | - | - | ++ |
| HMB45 | - | - | - | - | - | +1 |
| CD117 | - | - | ++ | - | - | - |
| CD34 | - | - | +1 | - | - | - |
| Ki67 | +++ | +++ | + | ++ | - | +++ |
| P53 | +++ | +++ | - | ++ | - | ++ |
| CD45 | - | - | - | - | - | - |
| FISH | + | + | - | + | - | - |
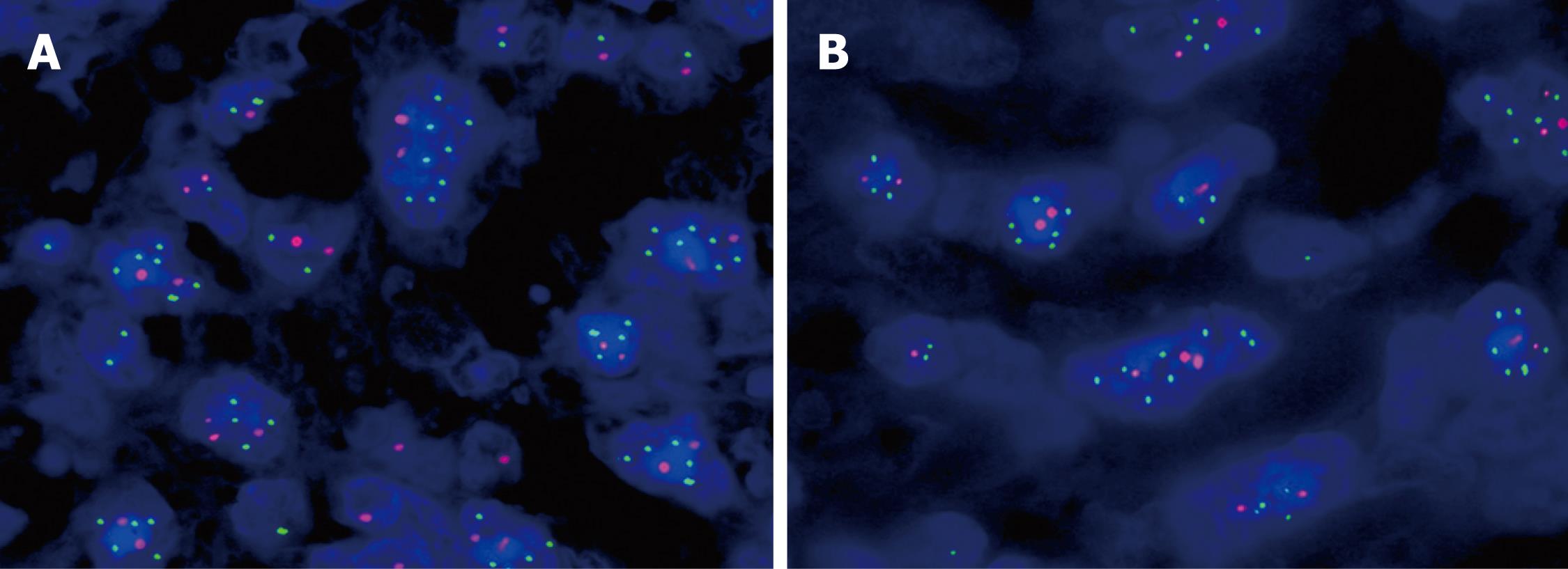
Interphase cytogenetic analysis using fluorescence in situ hybridization (FISH) revealed that the yolk sac tumor and immature teratoma components were positive for i(12p) and 12p overrepresentation (Figure 3A). In particular, the rhabdomyoblastic cell components also showed typical i(12p) and 12p overrepresentation (Figure 3B). The FISH ratios ranged from 1.21 to 3.30 in various tumor components (mean: 2.1 ± 0.8).
Based on the clinical features, histological findings, immunohistochemical stains and cytogenetic studies, the pathological diagnosis of hepatic mixed tumor with significant sarcomatous components was made. Gonads and retroperitoneal lymph nodes were examined, and did not reveal any abnormality. This patient was cured with a standard protocol of cisplatin, etoposide and bleomycin. Five months after surgery, the patient died of hepatic failure with tumor recurrence and thrombosis of the intrahepatic veins, which indicates that this tumor had the low chemosensitivity. Permission for an autopsy was not granted.
GCTs are a diverse family of neoplasms, and they are gonadal and extragonadal in origin[1]. Mixed GCTs include mature/immature teratoma, and one or more malignant germ cell components. Sarcomatous differentiation has been observed previously in primary and metastatic GCTs[10,11]. We describe a hepatic mixed GCT with significant sarcomatous components and elevated serum AFP in an adult man. The tumor was composed of two GCT components: yolk sac tumor and immature teratoma. The components of immature teratoma included epithelial (keratinizing or non-keratinizing, glandular, ductal or acinar), mesenchymal (rhabdomyoblastic, chondroid and osteoblastic differentiation) and neuroectodermal (neuroblastoma exhibiting rosettes formation, and ganglia) differentiation structures. GCTs in the liver may be primary or metastatic, therefore, it is important to exclude a gonadal tumor before making a diagnosis of malignant GCT. The present case was without any history of pretreatment, because no other tumor site was detected by extensive preoperative staging. This suggested that the tumor was primary to the liver. Therefore, malignant mixed GCT with significant sarcomatous components arising in the liver was diagnosed, based on the clinical features, histological findings, immunohistochemical stains and cytogenetic studies. To the best of our knowledge, this is the first report of hepatic malignant mixed GCT with significant sarcomatous components in adults.
Sarcomatous components are more common in mediastinal GCTs than those of other locations[10,11]. Although the pathogenesis of the development of sarcomatous components in GCTs has not been fully elucidated, it has been proposed to include a dedifferentiation phenomenon; malignant transformation of certain mesenchymal components within teratomas; origin from primitive germ cells; and transformation of the blastematous stroma in yolk sac tumor[10,11]. In the present case, the rhabdomyoblastic cell components also showed typical i(12p) and 12p overrepresentation, which supports the origin of the sarcomatous components from malignant transformation of mesenchymal components within the teratoma, and GCTs have the potential to develop almost any type of sarcoma[10,11].
Another interesting finding is the presence of focal pancreatic acinar differentiation, with positive staining for AFP, α-1-antitrypsin, and chymotrypsin. AFP production is normally a feature of yolk sac, embryonal liver, and embryonal gastrointestinal tract, and it is recapitulated in neoplasms of these structures. Such a phenomenon has been described under the term hepatoid carcinoma in tumors at numerous sites[12]. The production of AFP by pancreatic acinar neoplasms is well recognized, and Cingolani et al[12] have reported a case of such a tumor arising in a mediastinal teratoma, similar to our case but lacking the sarcomatoid components. Therefore, our case supports the hypothesis that AFP production in pancreatic neoplasms is related to acinar cell differentiation.
In the differential diagnosis, the tumor has to be compared with several entities, including carcinosarcoma, hepatocellular carcinoma or cholangiocarcinoma with sarcomatoid dedifferentiation, yolk sac tumor, mixed hepatoblastoma with teratoid features, lymphoma, and melanoma. The latter two are excluded by negativity of the tumor markers[13,14]. Carcinosarcoma of the liver is a malignant tumor that contains an intimate mixture of carcinomatous (either hepatocellular or cholangiocellular) and differentiated sarcomatous elements (such as osteosarcoma, angiosarcoma, rhabdomyosarcoma or malignant schwannoma). Unlike GCT, true carcinosarcoma consists of only a single malignant epithelial component and a single malignant mesenchymal component. They must be distinguished from sarcomatoid carcinoma or spindle-cell carcinoma, morphological variants of hepatocellular carcinoma and/or cholangiocarcinoma. The sarcomatoid part that consists of spindle cells is epithelial marker positive. HepPar1 and pCEA are useful markers for hepatocytes[15]. A true immature teratoma does not contain fetal and embryonal hepatoblastoma areas.
The role of chemotherapy remains speculative. Although the prognosis of GCTs depends on the site and clinical stage, the emergence of sarcomatous components in a GCT portends a worse prognosis, and appears to be highly resistant to the standard combination chemotherapy commonly employed for the treatment of GCT. However, numerous authors have administered chemotherapy to GCTs with sarcomatous differentiation, and some have achieved complete remission[10,11]. Our case was also treated with adjuvant chemotherapy after complete surgical excision according to sarcoma protocols, but the effectiveness did not appear to be obvious. Therefore, the role of chemotherapy needs to be clarified in this extremely rare tumor. Addition of sarcoma specific treatment modalities should be explored in such patients to increase the chances for survival.
In summary, primary mixed malignant GCT with sarcomatous components in the liver is extremely rare. The presence of sarcomatous components in GCT is a factor that portends a more aggressive behavior, and appears to be highly resistant to standard chemotherapy. As a result of the extreme rarity of this disease, it appears very important to add additional cases of this pathology to the literature. Further studies are needed to identify prognostic factors, and the role of chemotherapy in the management of GCT.
Peer reviewer: Miguel Angel Mercado, MD, Surgical Division, National Institute of Medical Sciences and Nutrition, Vasco de Quiroga 15, col. Section 16, Distrito Federal 14000, Mexico
S- Editor Wang JL L- Editor Kerr C E- Editor Ma WH
| 1. | Palmer RD, Barbosa-Morais NL, Gooding EL, Muralidhar B, Thornton CM, Pett MR, Roberts I, Schneider DT, Thorne N, Tavaré S. Pediatric malignant germ cell tumors show characteristic transcriptome profiles. Cancer Res. 2008;68:4239-4247. |
| 2. | Theegarten D, Reinacher A, Graeven U, Philippou S. Mixed malignant germ cell tumour of the liver. Virchows Arch. 1998;433:93-96. |
| 3. | Cöl C. Immature teratoma in both mediastinum and liver of a 21-Year-old female patient. Acta Med Austriaca. 2003;30:26-28. |
| 4. | Nirmala V, Chopra P, Machado NO. An unusual adult hepatic teratoma. Histopathology. 2003;43:306-308. |
| 5. | Martin LC, Papadatos D, Michaud C, Thomas J. Best cases from the AFIP: liver teratoma. Radiographics. 2004;24:1467-1471. |
| 6. | Lee CH, Hsieh SY. Case report: Clostridium septicum infection presenting as liver abscess in a case of choriocarcinoma with liver metastasis. J Gastroenterol Hepatol. 1999;14:1227-1229. |
| 7. | Arai M, Oka K, Nihei T, Hirota K, Kawano H, Kawasaki T, Hakozaki H. Primary hepatic choriocarcinoma--a case report. Hepatogastroenterology. 2001;48:424-426. |
| 8. | Lenci I, Tariciotti L, Baiocchi L, Manzia TM, Toti L, Craboledda P, Callea F, Angelico M, Tisone G. Primary yolk sac tumor of the liver: incidental finding in a patient transplanted for hepatocellular carcinoma. Transpl Int. 2008;21:598-601. |
| 9. | Toumi N, Chaumette-Plankaert MT, Cherqui D, de Revel T, Duvillard P, Theodore C. Germ cell tumors. Case 3. Primary yolk sac tumor of the liver. J Clin Oncol. 2004;22:1756-1758. |
| 10. | Malagón HD, Valdez AM, Moran CA, Suster S. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol. 2007;31:1356-1362. |
| 11. | Guo CC, Punar M, Contreras AL, Tu SM, Pisters L, Tamboli P, Czerniak B. Testicular germ cell tumors with sarcomatous components: an analysis of 33 cases. Am J Surg Pathol. 2009;33:1173-1178. |
| 12. | Cingolani N, Shaco-Levy R, Farruggio A, Klimstra DS, Rosai J. Alpha-fetoprotein production by pancreatic tumors exhibiting acinar cell differentiation: study of five cases, one arising in a mediastinal teratoma. Hum Pathol. 2000;31:938-944. |
| 13. | Okada T, Mibayashi H, Hasatani K, Hayashi Y, Tsuji S, Kaneko Y, Yoshimitsu M, Tani T, Zen Y, Yamagishi M. Pseudolymphoma of the liver associated with primary biliary cirrhosis: a case report and review of literature. World J Gastroenterol. 2009;15:4587-4592. |
| 14. | Wu XN, Zhao XY, Jia JD. Nodular liver lesions involving multiple myeloma: a case report and literature review. World J Gastroenterol. 2009;15:1014-1017. |
| 15. | Sumiyoshi S, Kikuyama M, Matsubayashi Y, Kageyama F, Ide Y, Kobayashi Y, Nakamura H. Carcinosarcoma of the liver with mesenchymal differentiation. World J Gastroenterol. 2007;13:809-812. |