Published online Feb 7, 2010. doi: 10.3748/wjg.v16.i5.636
Revised: December 4, 2009
Accepted: December 11, 2009
Published online: February 7, 2010
AIM: To explore the possibility and mechanism of inhibiting allogeneic T-cell responses by Kupffer cells (KC) pretreated with interferon-γ (IFN-γ) in vitro.
METHODS: The expressions of indoleamine 2,3-dioxygenase (IDO) mRNA and FasL mRNA in KC pretreated with IFN-γ were studied with real-time polymerase chain reaction (PCR). The catabolism of tryptophan by IDO from KC was analyzed by high performance liquid chromatography. Allogeneic T-cell response was used to confirm the inhibition of KC in vitro. The proliferation of lymphocytes was detected using [3H] thymidine incorporation. Cell cycle and lymphocyte apoptosis were evaluated by flow cytometric assay.
RESULTS: Real-time PCR revealed IDO mRNA and FasL mRNA expressions in KC pretreated with IFN-γ, and IDO catabolic effect was confirmed by a decrease in tryptophan and increase in kynurenine concentration. KC expressing IDO and FasL in BABL/c mice acquired the ability to suppress the proliferation of T-cells from C57BL/6, which could be blocked by addition of 1-methyl-tryptophan and anti-FasL antibody. KC expressing IDO could induce allogeneic T-cell apoptosis.
CONCLUSION: In addition to Fas/FasL pathway, IDO may be another mechanism for KC to induce immune tolerance.
- Citation: Yan ML, Wang YD, Tian YF, Lai ZD, Yan LN. Inhibition of allogeneic T-cell response by Kupffer cells expressing indoleamine 2,3-dioxygenase. World J Gastroenterol 2010; 16(5): 636-640
- URL: https://www.wjgnet.com/1007-9327/full/v16/i5/636.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i5.636
The acceptance of the fetus during pregnancy by the mother is a successful model of tolerance against allogeneic tissues. The exact mechanism of immunosuppression in pregnancy will help develop novel therapeutic strategies in clinical transplantation. Indoleamine 2,3-dioxygenase (IDO) plays an important role in maintenance of maternal T-cell tolerance to fetal alloantigen, and IDO-specific inhibitor, 1-methyl-tryptophan (1-MT), can selectively reject allogeneic fetuses[1,2], which was observed in liver allografts[3]. The mechanism of IDO immunosuppression may be the localized depletion of the essential amino acid tryptophan and formation of its metabolites[4-7].
Kupffer cells (KC), act as effective antigen-presenting cells (APC) in the liver, can directly interact with passenger leukocytes and may play an important role in intrahepatic immunoregulation. KC can regulate allogeneic T-cell response in vitro and in vivo by Fas/FasL pathway, but blocking anti-FasL antibody could not thoroughly suppress the effect of KC on T-cell proliferation[8], and some other mechanisms may contribute to KC-dependent suppression. Many experiments[9,10] showed that APC, such as dendritic cells and monocyte-derived macrophages, could induce IDO expression by certain treatment and acquire the capacity to suppress T-cell proliferation.
In this study, we hypothesized that IDO expressed by KC was another mechanism of KC immunoregulation. To confirm this, we analyzed whether KC pretreated with interferon-γ (IFN-γ) can be induced to express IDO and FasL, and the impact of KC expressing IDO and FasL on allogeneic T-cell response in vitro.
Male BABL/c and C57BL/6 mice were purchased from the West China Animal Center and those aged 10-12 wk were used for the experiment.
Spleens were harvested from male C57BL/6 mice and the lymphocyte (LC) was isolated as described previously[5]. KCs were isolated as described by Yan et al[11]. This technique of cell isolation yielded 10-20 million KCs per liver on average, with a 92%-95% viability, as determined by trypan blue exclusion. KCs were determined by phagocytosed carbon bead and stained positively for nonspecific esterase.
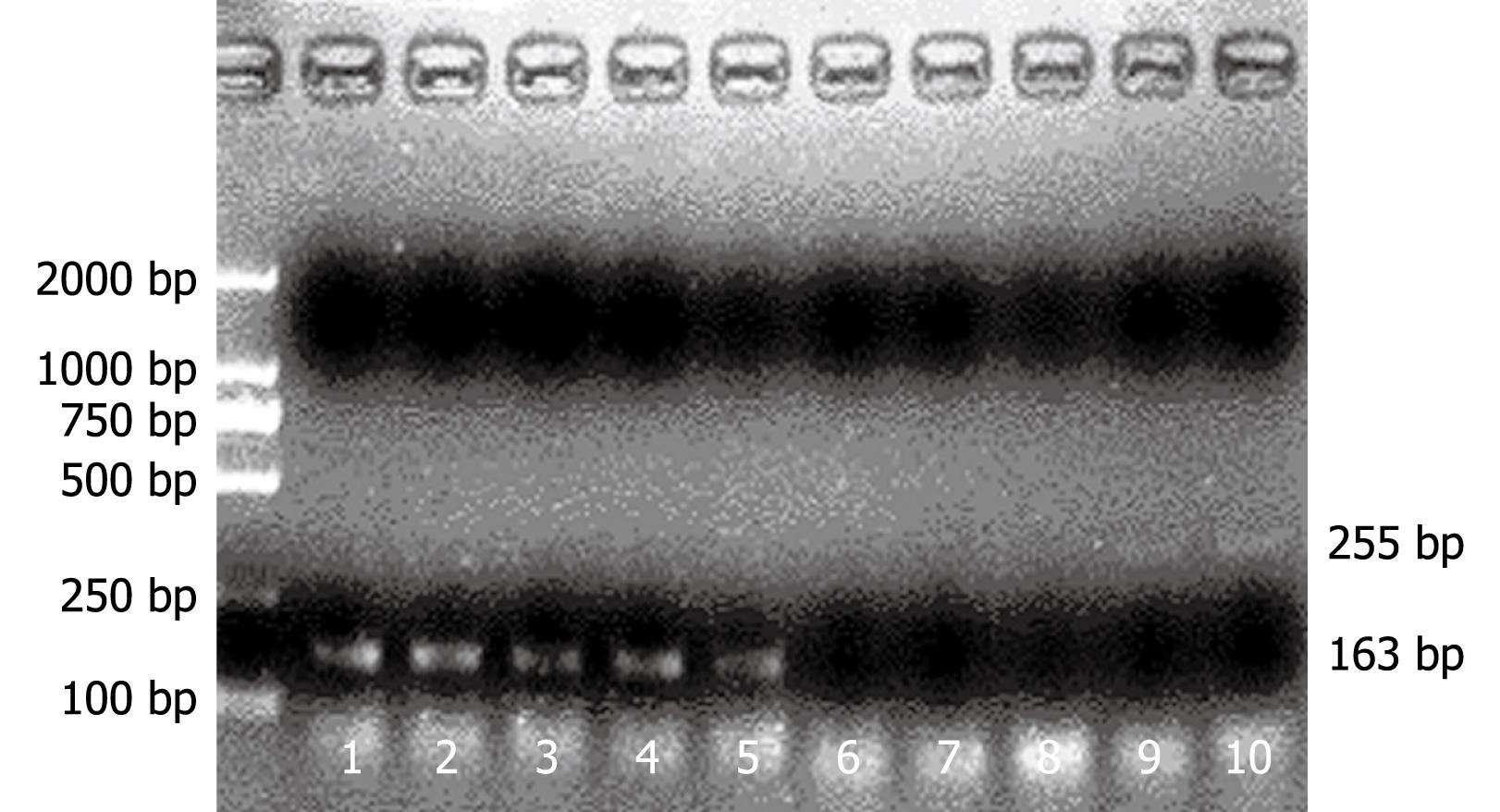
Total RNA of KC was extracted using TRIzol (Life Technologies, Rockville, USA) and subjected to reverse transcription polymerase reaction (RT-PCR) using the following primers: FasL 5'-GCACAGAAGGGAAGGAGTA-3' and 5'-CCAGGAGAATCGCAGTAGA-3'; IDO 5'-TATTGCTGTTCCCTACTG-3' and 5'-GGTCTTGACGCTCTACT-3'; GAPDH 5'-CCTCAAGATTGTCAGCAAT-3' and 5'-CCATCCACAGTCTTCTGAGT-3'. And they yielded a fragment size of 163, 255 and 141 bp, respectively. Amplification was performed on an iCycler (Bio-Rad, Hercules, USA). For quantitative PCR, amplification was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, USA). Fluorescence was detected at 490 nm using the iCycler IQ real-time PCR (Bio-Rad, Hercules, USA). The Ct of negative samples was defined as 45. A comparative Ct method was used to quantitate IDO and FasL cDNA levels[12]. Melting curve analysis consisted of a denaturation step at 95°C for 1 min, lowered to 55°C for 30 s, and followed by 40 cycles of incubation, in which the temperature was raised to 95°C at a rate of 1°C/30 s per cycle with continuous reading of fluorescence. The size of amplification products was verified by agarose gel electrophoresis.
KC (2.0 × 104) from BABL/c was co-incubated with allogeneic spleen lymphocytes (4.0 × 105) from C57BL/6 in 0.2 mL RPMI1640 medium containing 10% fetal calf serum (FCS), 100 U/mL penicillin, and 100 mg/mL streptomycin. Positive controls consisted of KC pretreated with IFN-γ (Cytolab Ltd., USA) for 24 h and allogeneic lymphocytes with anti-FasL antibody (MFL4; BD PharMingen, San Diego, CA) or 1-MT (Aldrich Chemical, Milwaukee, WI), or KC pretreated with IFN-γ (200 U/mL) or without allogeneic lymphocytes,whereas negative controls included KC or lymphocytes only. These cells were cultured in a 37°C, 5% CO2 incubator in 96-well flat bottom plates (COSTAR, Corning, NY, USA). After 5 d, [3H] thymidine (3H-TdR) (Inotech Biosystems, USA) was added and kept for 12 h and the number of counts per minute (cpm) was determined in a β-counter.
To verify functional IDO expression, tryptophan and kynurenine levels were measured in culture media. IDO activity was expressed as the concentration of kynurenine in the sample. KC was treated with IFN-γ (200 U/mL) for 6 and 24 h in 200 μL RPMI1640 containing 24.5 μmol/L tryptophan. The supernatant was harvested and the quantity of kynurenine and tryptophan was determined by high performance liquid chromatography (HPLC) and UV detection (UV265 nm, Shimadzu, Japan). HPLC was performed according to Carlin et al[13] with minor modifications.
Protein-free supernatant of 100 μL was injected onto the column. Kynurenine and tryptophan concentrations in the supernatants were determined based on their peak heights relative to standard solutions obtained in the chromatograms. The standard solutions of kynurenine and tryptophan, purchased from Sigma, were prepared and kept fresh each day at concentrations ranging from 0 to 30 μmol/L and submitted to precipitation with 30% trichloroacetic acid and incubation at 50°C for 30 min. Chromatography of the standards and the samples was performed under the same conditions.
KCs (1 × 106) were seeded and after an overnight incubation, they were pretreated with IFN-γ (200 U/mL) for 24 h. T-cell alone was used as controls. KCs were cocultured with allogeneic T-cells (E/T = 1:1) for 24 h. T-cells were harvested and washed twice with 0.01 mol/L PBS and fixed with 70% ethanol for 2 h at 25°C. Finally, the cells were washed twice with PBS and stained with propidium iodide. The apoptosis of T-cells was analyzed by a flow cytometer (Beckman-Coulter, USA).
The Chinese law on the Protection of Animals was followed. The data were presented as mean ± SD. Parametric data was analyzed by Student’s t test. P < 0.05 was considered statistically significant.
We measured KC FasL mRNA and IDO mRNA using real-time RT-PCR. Treatment of mouse KC with IFN-γ resulted in the time-dependent induction of IDO and FasL. The results were expressed in terms of change in Ct values (ΔCt), ΔCt = average Ctspecific gene - average CtGAPDH. Because GAPDH is an abundant message, lower ΔCt values corresponded to a higher level of expression. Expression of FasL and IDO in KC pretreated with IFN-γ was significantly decreased in comparison with KC without IFN-γ pretreatment (Table 1). IDO and FasL expression was determined by RT-PCR in KCs before and after activation for 6 and 24 h with recombinant IFN-γ (Figure 1).
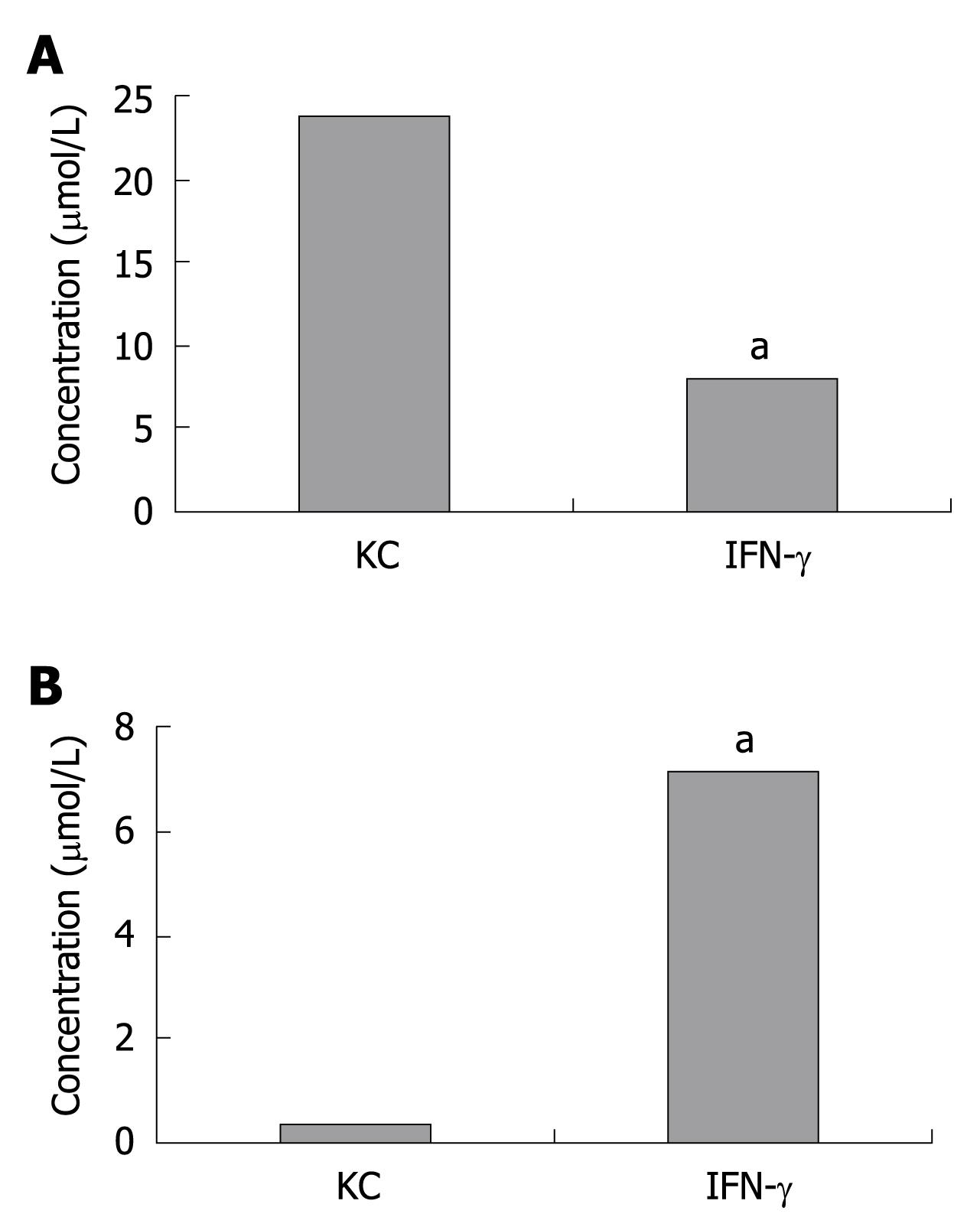
To verify whether IDO induced by KC exerted its catabolic effect, tryptophan as well as kynurenine were measured by HPLC in KCs pretreated with or without IFN-γ for 24 h. Tryptophan and kynurenine concentrations were determined using 1 mL of culture medium that was harvested from a 3.5 cm2 culture dish. As expected, the kynurenine concentration significantly increased while tryptophan decreased. Tryptophan concentration in KC group and IFN-γ group was 23.6 ± 0.9 μmol/L and 7.8 ± 0.6 μmol/L while kynurenine concentration was 0.3 ± 0.08 μmol/L and 7.1 ± 1.4 μmol/L, respectively (Figure 2).
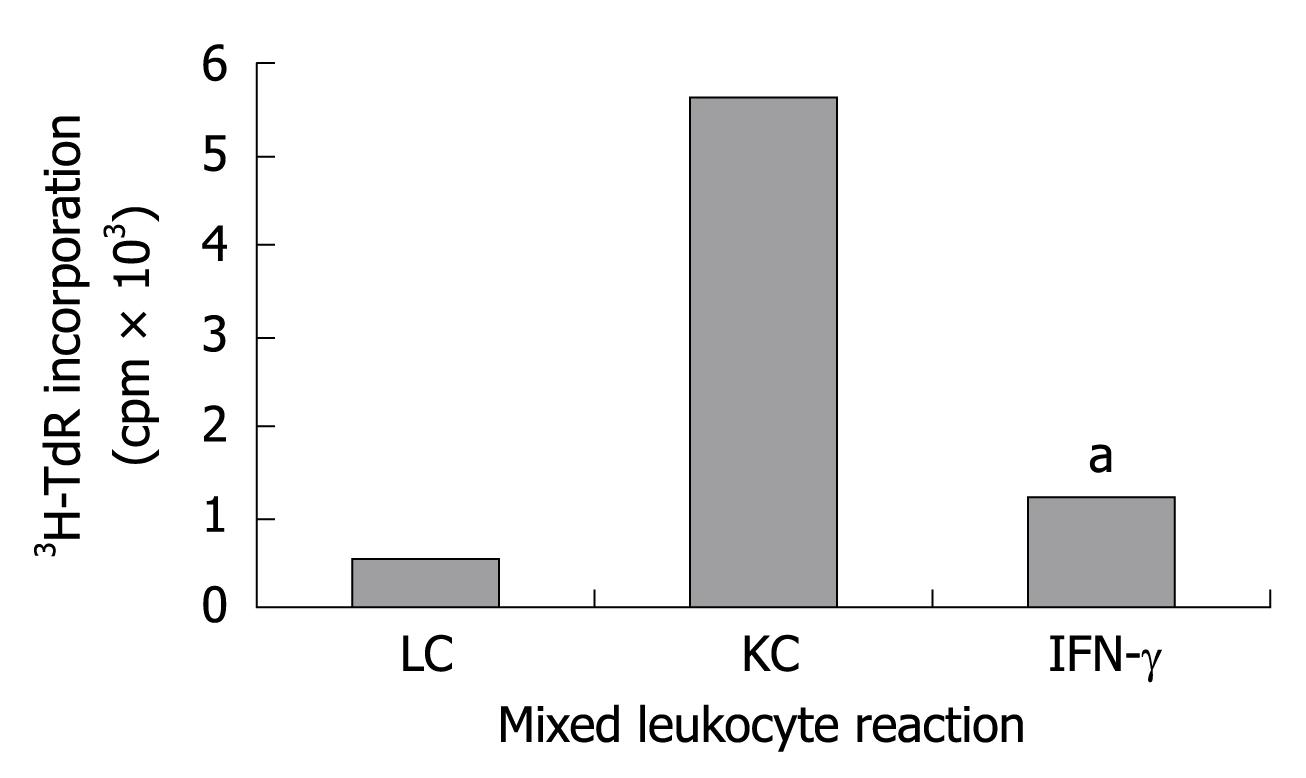
Whether KCs expressing IDO and FasL can inhibit the proliferation of allogeneic T-cells was confirmed in the experiment. KC pretreated with IFN-γ for 24 h and naive KC were coincubated with allogeneic T-cells, respectively, the proliferation was determined by 3H-TdR incorporation. KCs expressing IDO and FasL were significantly more effective than naive KC (Figure 3) in inhibiting the allogeneic T-cell proliferation (P < 0.05).
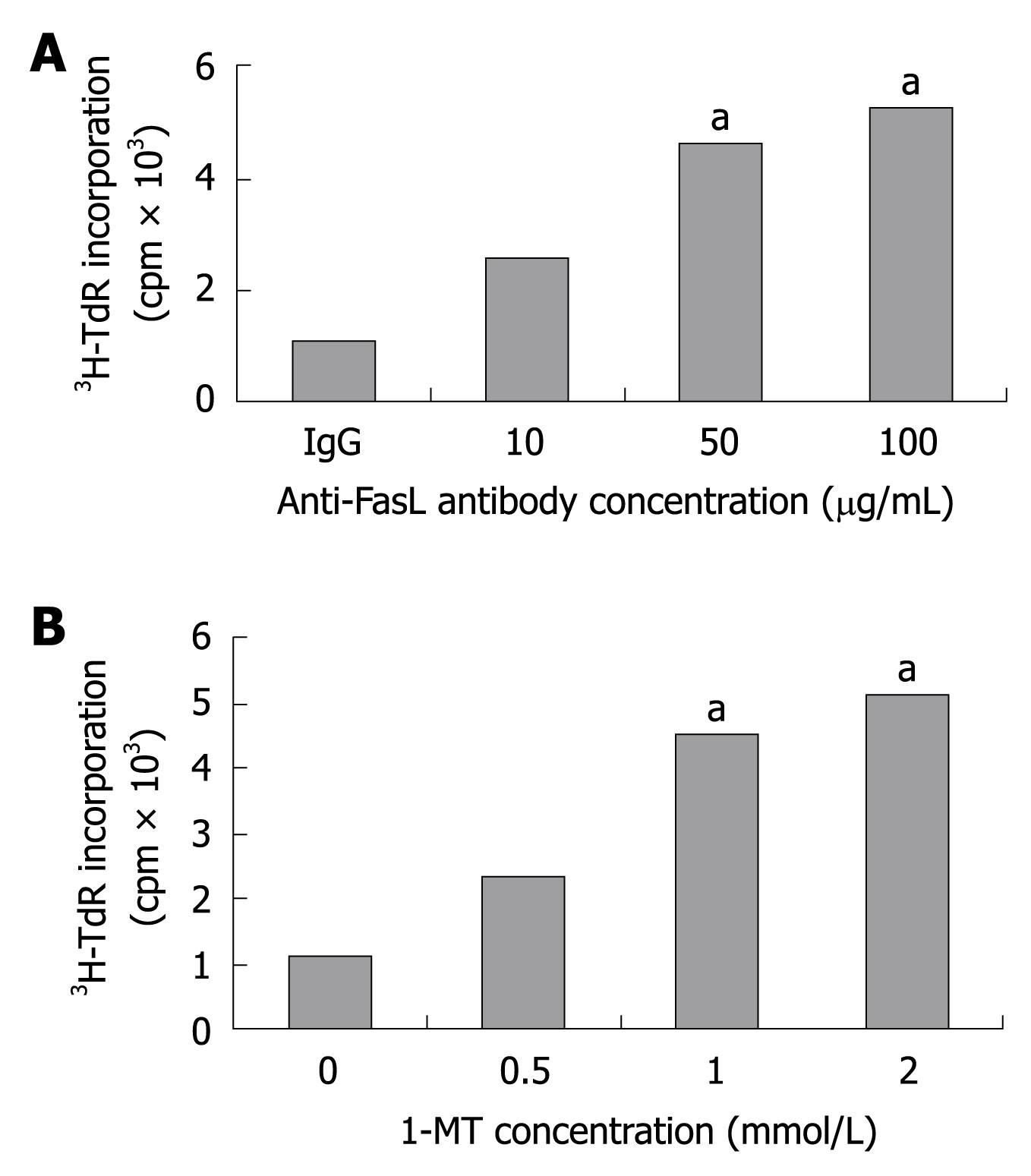
To confirm whether the inhibition of T-cell proliferation was due to the enzymatic activity of IDO, inhibitory tests were performed in the presence of 1-MT,anti-FasL antibody or IgG. The addition of 1-MT or anti-FasL antibody showed an augmented proliferative response in a dose-dependent manner as compared with the control group (Figure 4). These results indicate that the inhibitory effect of KC pretreated with IFN-γ was induced by the IDO activity and Fas/FasL interaction.
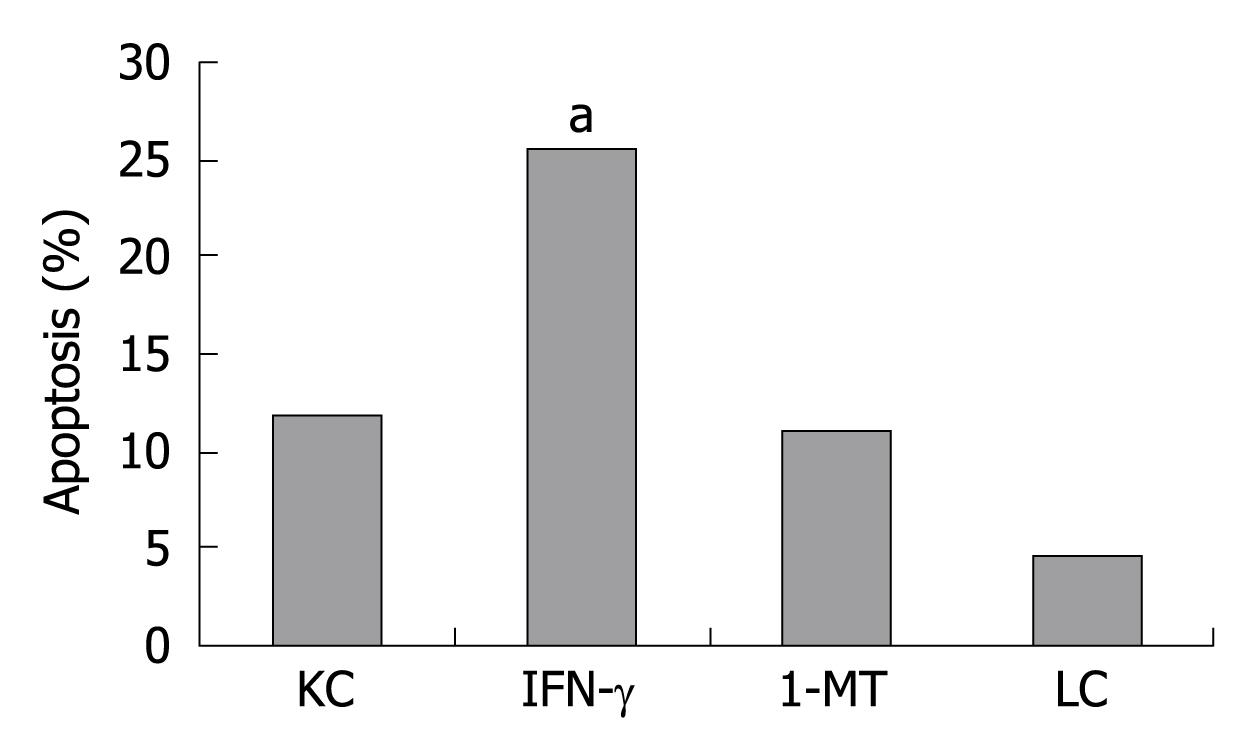
In order to clarify the underlying mechanism by which KC inhibits the proliferation of allogeneic T-cells, we confirmed that KC has the ability to induce apoptosis of allogeneic T-cells. The apoptosis rate of allogeneic T cells was 11.7% ± 1.2% in KC group, 25.53% ± 1.31% in IFN-γ group, 10.9% ± 0.25% in 1-MT group and 4.53% ± 0.65% in LC group. Figure 5 shows that KC pretreated with IFN-γ was more effective to induce T-cell apoptosis than naive KC, but the effect was abrogated by addition of 1-MT (1 mmol/L).
KC pretreated with IFN-γ can be induced to express IDO mRNA and FasL mRNA as shown in this study. KC expressing FasL and IDO can inhibit the proliferation of allogeneic T-cells and induce their apoptosis by increasing kynurenine and reducing tryptophan in vitro, which could be blocked by addition of 1-MT and anti-FasL antibody. These results are consistent with our hypothesis that IDO is another mechanism of KC immunoregulation.
The interaction between T-cell and KC has an important impact on antigen-specific immune response in the liver. The expansion of specifically activating T-cell requires the presence of KC, which holds a key position in regulation of hepatic immune response[14,15]. Apoptosis induced by Fas/FasL interactions have been proposed as a mechanism of immune privilege and peripheral tolerance[16]. Sun et al[8] identified that KC induced T-cell apoptosis and may be involved in the development of hepatic immune tolerance through Fas/FasL pathway. But the addition of anti-FasL antibody could not thoroughly block the inhibitory effect of KC on allogeneic T-cell proliferation. We propose that KC-induced T-cell apoptosis is dependent on the Fas/FasL pathway and IDO. IDO as well as FasLare known to be expressed in immune-privileged sites and believed to induce peripheral tolerance[1,2,17]. Our data showed that KC pretreated with IFN-γ induced the expression of both FasL and IDO. The blockade of IDO activity by 1-MT would negate the induction of apoptosis in vitro by IFN-γ-treated KC. This suggests that IDO induction is another mechanism by which IFN-γ acts on KC to mediate apoptosis of T-cells.
Munn et al[7] confirmed that macrophages expressing IDO caused the lack of tryptophan in a cell-culture system. In the current series of experiments, the amount of tryptophan reduced by KC expressing IDO was not so dramatic as by macrophages. The phenomena were due to different activities of the IDO gene. In the presence of tryptophan, IDO-expressing KCs also suppressed the proliferation of allogeneic T-cells. So the concentration of tryptophan in culture may not be the only reason contributing to the effect. Some reports[4,6,18,19] have shown that tryptophan-derived catabolites, such as kynurenine, 3-hydroxykynurenine, 3-hydroxyanthranilic acid and picolinic acid, also suppress the allogeneic T-cell responses in vitro and in vivo. The concentration of kynurenine in our study also increased after KCs were pretreated with IFN-γ. Tryptophan metabolites may be another reason that IDO-expressing KCs inhibit the proliferation of allogeneic T-cells.
KCs expressing IDO and FasL may drive the differentiation of Th2/Th3. Th2 cells expressed higher levels of FAP-1 that inhibited Fas/FasL pathway and were not sensitive to FasL-induced apoptosis, but Fas/FasL pathway promoted the rapid death of Th1 cells and selective survival of Th2 cells[20]. It may be a mechanism for differential regulation of Th cells by KC. Furthermore, tryptophan metabolites, such as 3-hydroxykynurenine and quinolinic acid, can selectively induce apoptosis of Th1 cells, but not Th2 cells[19]. It was associated with the activation of caspase 28 and cytochrome C released by mitochondrion without the participation of Fas/FasL interaction. KC pretreated with IFN-γ may control the differentiation of Th cells by the two mechanisms.
In conclusion, our results showed that IDO expressed by KC is another mechanism associated with induction of allogeneic T-cell apoptosis in addition to Fas/FasL interaction.
The acceptance of the fetus during pregnancy by the mother is a successful model of tolerance against allogeneic tissue. Indoleamine 2,3-dioxygenase (IDO) plays an important role in maintenance of maternal T-cell tolerance to fetal alloantigens. The mechanism of IDO immunosuppression may be the localized depletion of tryptophan and formation of its metabolites. Kupffer cells (KC) plays an important role in intrahepatic immunoregulation and induction of tolerance by Fas/FasL pathway. But blocking the anti-FasL antibody could not thoroughly suppress the effect of KC on T-cell proliferation. IDO expressed by KC may be another mechanism of KC immunoregulation.
How to induce specific immune tolerance is a hot topic of research. Killing the transplantation antigen-activated T cells is an important way to induce specific immune tolerance. IDO plays an important role in inducing the apoptosis of antigen-activated T cells. The activation and apoptosis of T cell is the central link in the formation of immune tolerance, but the apoptosis requires the activation of antigen presenting cells in the liver mainly by KC. KC can regulate allogeneic T-cell response in vitro and in vivo by Fas/FasL pathway.
The study found that KC pretreated with interferon-γ (IFN-γ) could express IDO and FasL. KCs expressing IDO and FasL from BABL/c mice acquire the ability to suppress the proliferation of T-cells from C57BL/6, which could be blocked by 1-methyl-tryptophan and anti-FasL antibody. In addition to Fas/FasL pathway, IDO may be another mechanism in KC to induce immune tolerance.
IDO may be another mechanism in KC to induce immune tolerance, indicating that KC may be the potential target for immune tolerance in liver transplantation.
IDO is a monomeric protein heme iron, which was found in rabbit intestinal tissues in 1967. IDO has been found widely expressed in the immune system, including the thymus, placenta, gastrointestinal mucosa, anterior chamber, epididymis and other immune tolerance or immunologically privileged sites, particularly in a number of inhibitory antigen-presenting cells. IDO is involved in the regulation of T-cell proliferation and apoptosis.
This is an interesting paper devoted to the role of IDO in KC in inhibiting allogenic T-cell responses.In this study, the authors investigated whether KC pretreated with IFN-γ can be induced to express IDO and FasL, and analyzed the impact of KC expressing IDO and FasL on allogeneic T-cell response in vitro. The study shows IDO is another mechanism of KC immune regulation. Overall, the study is interesting and significant, and the data are presented in a clear and logical manner.
Peer reviewer: Natalia A Osna, MD, PhD, Liver Study Unit, Research Service (151), VA Medical Center, 4101 Woolworth Avenue, Omaha, NE 68105, United States
S- Editor Wang YR L- Editor Ma JY E- Editor Lin YP
| 1. | Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191-1193. |
| 2. | Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64-68. |
| 3. | Laurence JM, Wang C, Park ET, Buchanan A, Clouston A, Allen RD, Mccaughan GW, Bishop GA, Sharland AF. Blocking indoleamine dioxygenase activity early after rat liver transplantation prevents long-term survival but does not cause acute rejection. Transplantation. 2008;85:1357-1361. |
| 4. | Belladonna ML, Puccetti P, Orabona C, Fallarino F, Vacca C, Volpi C, Gizzi S, Pallotta MT, Fioretti MC, Grohmann U. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation. 2007;84:S17-S20. |
| 5. | Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl Int. 2005;18:95-100. |
| 6. | Funeshima N, Fujino M, Kitazawa Y, Hara Y, Hara Y, Hayakawa K, Okuyama T, Kimura H, Li XK. Inhibition of allogeneic T-cell responses by dendritic cells expressing transduced indoleamine 2,3-dioxygenase. J Gene Med. 2005;7:565-575. |
| 7. | Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363-1372. |
| 8. | Sun Z, Wada T, Maemura K, Uchikura K, Hoshino S, Diehl AM, Klein AS. Hepatic allograft-derived Kupffer cells regulate T cell response in rats. Liver Transpl. 2003;9:489-497. |
| 9. | Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396-5404. |
| 10. | Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124:89-101. |
| 11. | Yan ML, Wang YD, Tian YF, Lai ZD, Zhou SQ, Qiu FN. The isolation of Kupffer cells in BALB/c mouse. Fujian Yike Daxue Xuebao. 2008;42:526-528. |
| 12. | Liu FS, Chen JT, Dong JT, Hsieh YT, Lin AJ, Ho ES, Hung MJ, Lu CH. KAI1 metastasis suppressor gene is frequently down-regulated in cervical carcinoma. Am J Pathol. 2001;159:1629-1634. |
| 13. | Carlin JM, Borden EC, Sondel PM, Byrne GI. Biologic-response-modifier-induced indoleamine 2,3-dioxygenase activity in human peripheral blood mononuclear cell cultures. J Immunol. 1987;139:2414-2418. |
| 14. | Chen Y, Liu Z, Liang S, Luan X, Long F, Chen J, Peng Y, Yan L, Gong J. Role of Kupffer cells in the induction of tolerance of orthotopic liver transplantation in rats. Liver Transpl. 2008;14:823-836. |
| 15. | You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978-990. |
| 16. | Miyagawa-Hayashino A, Tsuruyama T, Egawa H, Haga H, Sakashita H, Okuno T, Toyokuni S, Tamaki K, Yamabe H, Manabe T. FasL expression in hepatic antigen-presenting cells and phagocytosis of apoptotic T cells by FasL+ Kupffer cells are indicators of rejection activity in human liver allografts. Am J Pathol. 2007;171:1499-1508. |
| 17. | van der Marel AP, Samsom JN, Greuter M, van Berkel LA, O'Toole T, Kraal G, Mebius RE. Blockade of IDO inhibits nasal tolerance induction. J Immunol. 2007;179:894-900. |
| 18. | Wee JL, Christiansen D, Li YQ, Boyle W, Sandrin MS. Suppression of cytotoxic and proliferative xenogeneic T-cell responses by transgenic expression of indoleamine 2,3-dioxygenase. Immunol Cell Biol. 2008;86:460-465. |
| 19. | Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC, Puccetti P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069-1077. |
| 20. | Xu H, Zhang GX, Ciric B, Rostami A. IDO: a double-edged sword for T(H)1/T(H)2 regulation. Immunol Lett. 2008;121:1-6. |