Published online Feb 7, 2010. doi: 10.3748/wjg.v16.i5.625
Revised: November 30, 2009
Accepted: December 7, 2009
Published online: February 7, 2010
AIM: To investigate whether serum thymosin β4 can provide diagnostic or prognostic information in liver failure patients caused by chronic hepatitis B virus (HBV) infection.
METHODS: Serum thymosin β4 levels were measured in 30 patients with acute-on-chronic liver failure (ACLF), 31 patients with chronic liver failure (CLF), 30 patients with compensated liver cirrhosis (CR) and 32 patients with chronic hepatitis B and 30 healthy controls. Serum thymosin β4 levels were measured by enzyme-linked immunosorbent assay and Child-Pugh and model for end-stage liver disease (MELD) scores were calculated for each patient on admission.
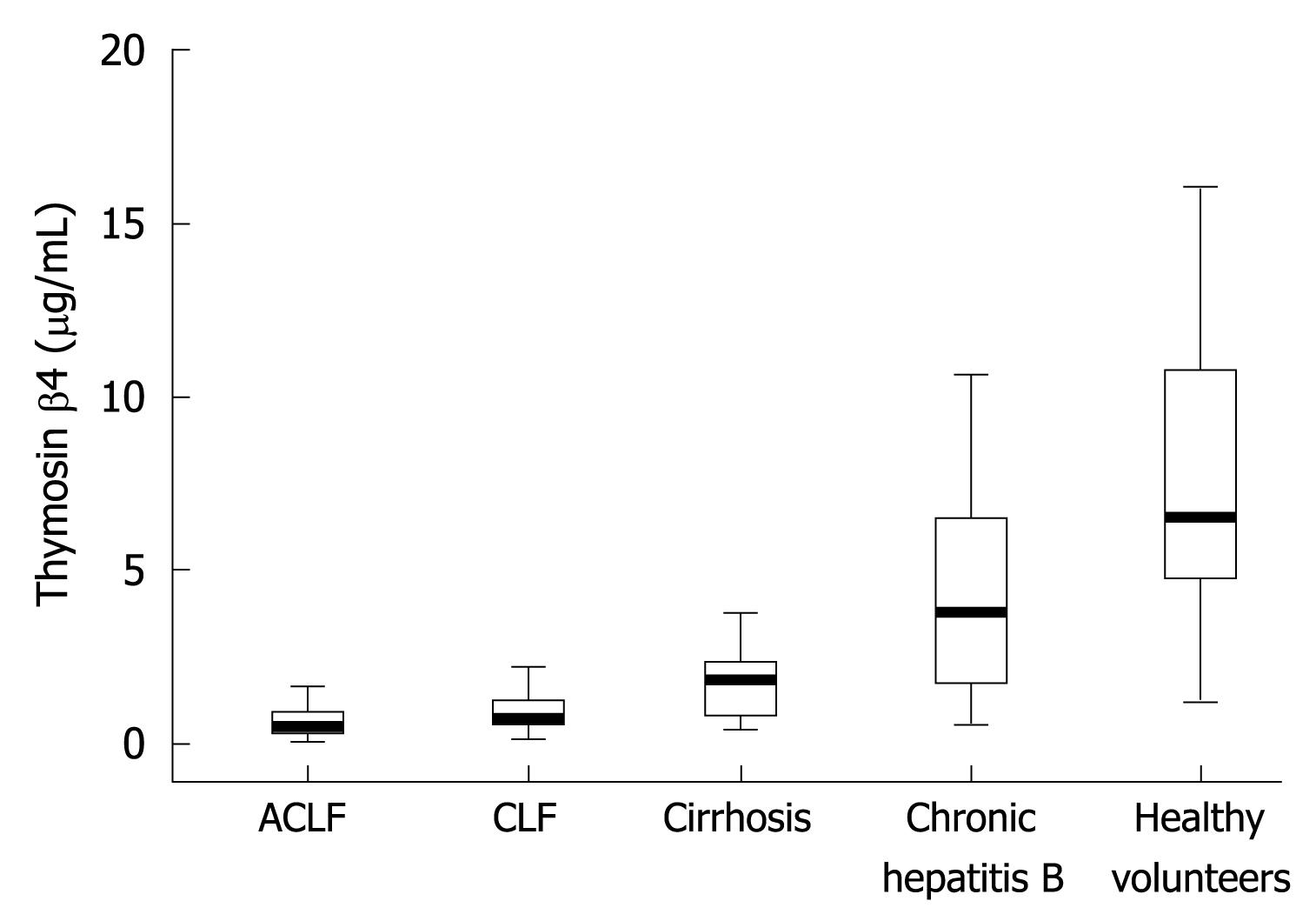
RESULTS: Compared with healthy controls, serum thymosin β4 levels in ACLF, CLF, CR and chronic hepatitis B patients were significantly lower, 6.5047 (4.7879-10.5314) μg/mL vs 0.4632 (0.2759-0.8768) μg/mL, 0.6981 (0.5209-1.2008) μg/mL, 1.8053 (0.8110-2.3397) μg/mL, 3.7803 (1.8570-6.4722) μg/mL, respectively (P < 0.001). The levels of thymosin β4 in liver failure (ACLF or CLF) patients were markedly lower than that in CR (P < 0.001), and a difference was also found between CLF and ACLF patients (P = 0.038). In patients with chronic liver disease, there was a positive relationship between thymosin β4 levels and albumin, choline esterase, and platelet (P < 0.001), and negative relationship with alanine aminotransferase (P = 0.020), aspartate aminotransferase, total bilirubin, international normalized ratio of prothrombin time, and Child-Pugh and MELD scores (P < 0.001). Of the 61 liver failure patients, the thymosin β4 levels of non-survivors were significantly lower than that of survivors (P = 0.007). Receiver operating characteristics analysis identified a thymosin β4 cutoff level of 0.5708 μg/mL for predicting poor prognosis in all liver failure patients. The serial thymosin β4 values were observed in 13 liver failure inpatients. Lower initial values were observed in the death. While greater improvement in thymosin β4 value was found in those who recovered from the disease.
CONCLUSION: Serum thymosin β4 can be used as an important potential predictor for liver failure caused by chronic HBV infection.
- Citation: Han T, Liu Y, Liu H, Zhu ZY, Li Y, Xiao SX, Guo Z, Zhao ZG. Serum thymosin β4 levels in patients with hepatitis B virus-related liver failure. World J Gastroenterol 2010; 16(5): 625-630
- URL: https://www.wjgnet.com/1007-9327/full/v16/i5/625.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i5.625
Chronic hepatitis B virus (HBV) infection remains one of the most challenging public health problems in Asia. Acute-on-chronic liver failure (ACLF) and chronic liver failure (CLF) are common serious conditions with a high mortality among chronic HBV infected patients. The prognosis is clearly related to the stage of the disease and early diagnosis has resulted in a significant reduction in mortality[1-4]. Unfortunately, a differential diagnosis from non-liver failure patient is sometimes very difficult to make since the current biomarkers used in clinical diagnosis are still lack of sensitivity and reliability[5,6]. Thus, it is very important to discover new biomarkers for liver failure diagnosis.
Thymosin β4 is a 4.9-kDa polypeptide widely distributed in human tissues, which is considered as the major G-actin sequestering protein[7-9]. Thymosin β4 has been shown to have multiple biological activities involved in a variety of physiologic and pathologic processes. For example, thymosin β4 is known to promote wound healing, tumor metastasis and angiogenesis[10-12]. Recently thymosin β4 has been found to stimulate cardiac cell migration and survival related to cardiac repair. It was also considered to play an important role in healing hypoxic injury in the heart by preventing apoptotic cell death of cardiomyocytes and reducing scarring[13], meanwhile, hypoxia and oxidative stress also play a key role in the pathophysiology of liver failure. Recent findings suggested that thymosin β4 could be beneficial for the treatment of chronic liver disease (CLD)[14]. These data have attracted more consideration of whether thymosin β4 plays an important role in liver failure. However, the levels of thymosin β4 in liver failure patients are still unknown.
This present study aims to determine serum thymosin β4 levels in CLD patients with HBV infection, and to assess the potential usefulness of thymosin β4 in diagnosis and prognosis prediction of HBV-related liver failure, including ACLF and CLF.
The study included 61 patients with liver failure (44 males, 17 females), of whom 30 (23 males, 7 females) were diagnosed as having ACLF, the other 31 (21 males, 10 females) patients as having CLF. And 30 (20 males, 10 females) patients with Child-Pugh A cirrhosis, and 32 (26 males, 6 females) patients with chronic hepatitis B were also enrolled. All patients had chronic HBV infection. Those with concurrent hepatocellular carcinoma (HCC), splenectomy, fatty liver, and hepatitis C or alcohol-related liver diseases were excluded. ACLF was defined as acute deterioration of liver function within 4 wk in CLD, with severe jaundice [serum total bilirubin (TBIL) ≥ 171 μmol/L or an increase of TBIL ≥ 17.1 μmol/L per day] and coagulopathy [international normalized ratio of prothrombin time (INR) > 1.5 or PTA < 40%]. CLF was defined as chronic decompensation of an end-stage liver disease, complicated with refractory ascites and/or encephalopathy and hepatorenal syndrome[3]. The diagnosis of liver cirrhosis (CR) was based on the clinical manifestation, physical examination, biochemical, endoscopic and ultrasound findings and⁄or liver biopsy, with features of liver fibrosis, portal hypertension and hypersplenism. All patients infected with HBV were recruited from January to July 2009 at Tianjin Third Central Hospital. Thirty healthy volunteers served as controls, including 20 males and 10 females, aged 41 (31-49) years [median (inter-quartile range, IQR)].
The study was approved by Tianjin Third Central Hospital Ethics Committee. Informed consent was obtained from either the patients or their immediate family members.
INR, platelets (PLT), serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), choline esterase (CHE), albumin (ALB), TBIL and renal function were tested. Child-Pugh and model for end-stage liver disease (MELD) scores were calculated on admission.
All the samples were collected in the morning when the patients were admitted to our hospital. Blood collected without anticoagulant was spun at 2600 ×g for 15 min, and the serum obtained was immediately stored at -80°C until thymosin β4 measurement. Thymosin β4 was measured with a newly developed commercial enzyme-linked immunosorbent assay (ELISA) (Immunodiagnostik AG, Bensheim, Germany). The test principle was based on a competition between antigen in the sample or standards and the antigen coated on the wells of microplate. A peroxidase-conjugated antibody was used for detection and quantification, and tetramethylbenzidine as a peroxidase substrate. The enzymatic reaction was terminated by acidic stop solution. The results were obtained by measuring the absorbance at 450 nm in an ELISA reader.
Kruskal-Wallis and Mann-Whitney U tests were used. Correlations were analyzed by Spearman rank test. Results were expressed as median and IQR. Cutoff values for the identification of non-survivors with liver failure (ACLF and CLF) and survivors were determined using the receiver operating characteristic (ROC) analysis. A P value of < 0.05 was considered to be statistically significant. All analyses were performed with SPSS 13.0 software.
The clinical characteristics of CLD patients in the study are listed in Table 1.
| ACLF | CLF | CR | Chronic hepatitis B | Healthy subjects | |
| n | 30 | 31 | 30 | 32 | 30 |
| Age (yr) | 54 (48-61) | 50 (46-55) | 50 (37-55) | 39 (31-45) | 41 (31-49) |
| Gender (M:F) | 23:7 | 21:10 | 20:10 | 26:6 | 20:10 |
| ALB (g/L) | 30.9 (26.3-33.2) | 25.7 (22.6-28.9) | 42.2 (38.4-45.7) | 46.7 (42.0-49.1) | 39.3 (38.1-41.1) |
| ALT (IU/L) | 174.0 (104.3-653.0) | 38.0 (27.5-49.5) | 35.5 (24.0-52.8) | 57.5 (25.0-75.5) | 30.5 (25.1-34.7) |
| AST (IU/L) | 274.5 (74.0-521.0) | 34.0 (21.0-71.0) | 19.0 (9.3-32.3) | 15.0 (10.0-20.5) | 23.3 (19.2-31.3) |
| TBIL (μmol/L) | 409.7 (208.1-474.7) | 170.9 (98.2-215.2) | 14.3 (12.5-18.1) | 15.1 (12.7-20.5) | 9.7 (8.75-13.2) |
| CHE | 2699 (1760-3286) | 1586 (1129-2035) | 3526 (3287-4529) | 4938 (4168-5465) | 6784 (6553-7463) |
| INR | 2.29 (1.94-2.70) | 2.52 (2.27-2.85 | 1.08 (1.01-1.13) | 1.02 (1.01-1.04) | 1.01 (0.95-1.02) |
| PLT | 72.5 (44.5-104.0) | 44.0 (30.5-79.0) | 89.0 (78.0-99.8) | 197.5 (178.0-225.0) | 197.5 (187.0-222.3) |
| MELD | 23.75 (19.81-29.55) | 22.97 (19.16-27.98) | 3.52 (2.39-5.23) | 2.65 (1.72-4.44) | 2.65 (2.05-3.70) |
| Child-Pugh score | 11 (10-12) | 12 (11-12) | 6 (5-7) | 5 (5-6) | 5 (5-6) |
Thymosin β4 levels in the groups of ACLF, CLF, CR, chronic hepatitis B (CHB) and healthy controls are shown in Figure 1. Patients in ACLF, CLF, CR and CHB groups had significantly lower median (IQR) thymosin β4 levels than healthy controls, 0.4632 (0.2759-0.8768) μg/mL, 0.6981 (0.5209-1.2008) μg/mL, 1.8053 (0.8110-2.3397) μg/mL, 3.7803 (1.8570-6.4722) μg/mL vs 6.5047 (4.7879-10.5314) μg/mL, respectively (P < 0.001). The thymosin β4 in ACLF and CLF patients was markedly reduced as compared with that in CR patients (P < 0.001). A difference was also found between ACLF and CLF (P = 0.038).
Table 2 shows the relationship between thymosin β4 values and biochemical parameters in CLD patients (including ACLF, CLF, CR and CHB). There was a positive relationship between thymosin β4 levels and ALB, CHE, and PLT (P < 0.001). And in CLD patients, thymosin β4 was negatively correlated with ALT (r = -0.210, P = 0.020), AST (r = -0.553, P < 0.001), TBIL (r = -0.581, P < 0.001), INR (r = -0.605, P < 0.001), Child-Pugh (r = -0.629, P < 0.001) and MELD scores (r = -0.587, P < 0.001).
| Characteristics | r value | P value |
| ALB | 0.536 | < 0.001 |
| ALT | -0.210 | 0.020 |
| AST | -0.553 | < 0.001 |
| TBIL | -0.581 | < 0.001 |
| CHE | 0.656 | < 0.001 |
| INR | -0.605 | < 0.001 |
| PLT | 0.541 | < 0.001 |
| Child-Pugh score | -0.629 | < 0.001 |
| MELD | -0.587 | < 0.001 |
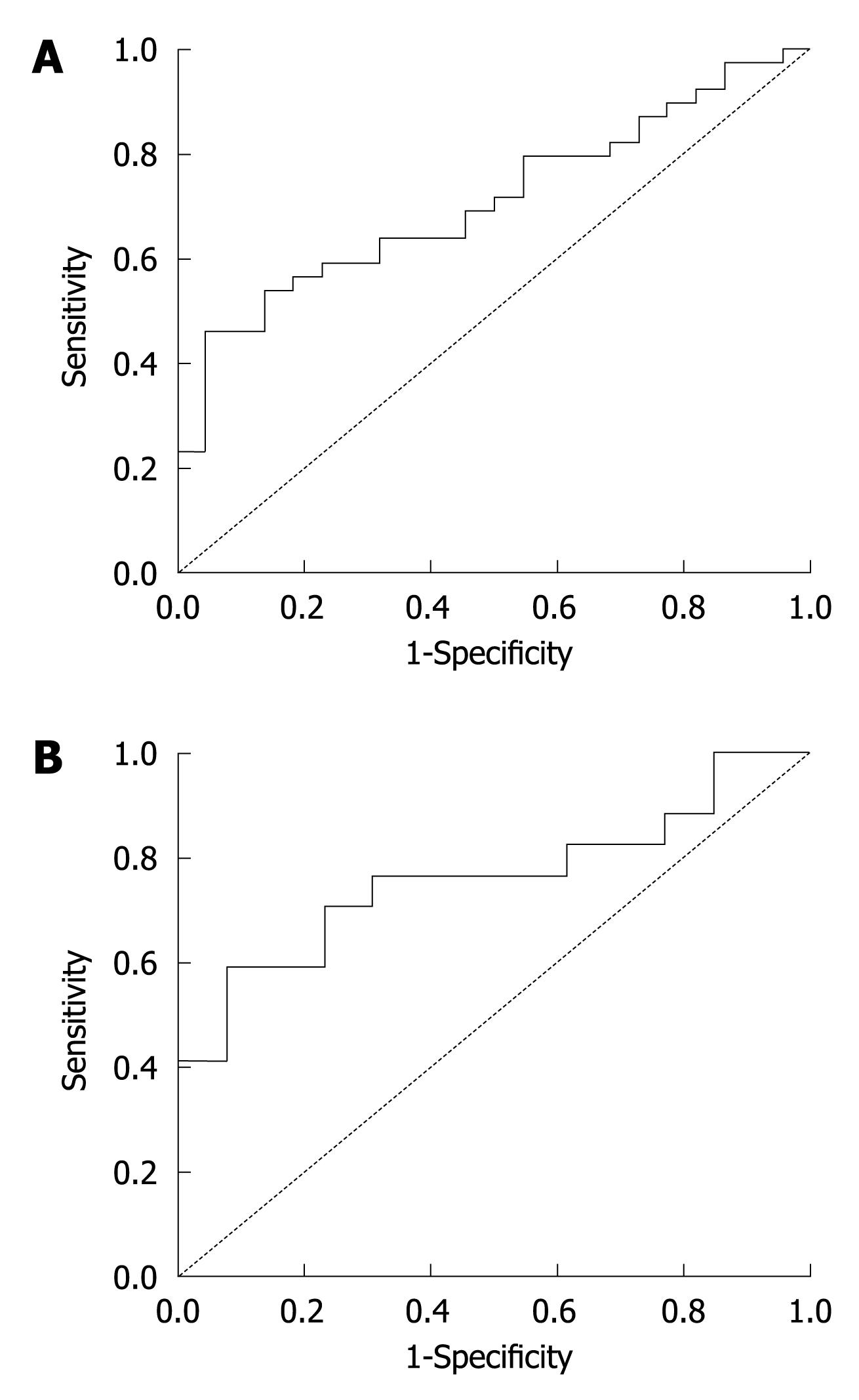
Among the 61 liver failure patients, the survival rate was 63.93% (39 patients), while the non-survival rate was 36.07% (22 patients). The thymosin β4 level of non-survivors was significantly lower than that of the survivors (P = 0.007) (Table 3). ROC analysis identified a thymosin β4 cutoff value of 0.5708 μg/mL [area under the ROC (AUROC) 0.710] with a sensitivity of 64.1% and a specificity of 68.2% for predicting poor prognosis in all liver failure patients (Figure 2A).
| Group | n | Survivors | Non-survivors | P value |
| LF | 61 | 0.8144 (0.3979-1.1510) | 0.4699 (0.2771-0.6658) | 0.007 |
| ACLF | 30 | 0.8144 (0.4042-1.0998) | 0.3656 (0.2291-0.4458) | 0.016 |
| CLF | 31 | 0.8329 (0.4306-1.4280) | 0.6581 (0.5603-0.7713) | 0.317 |
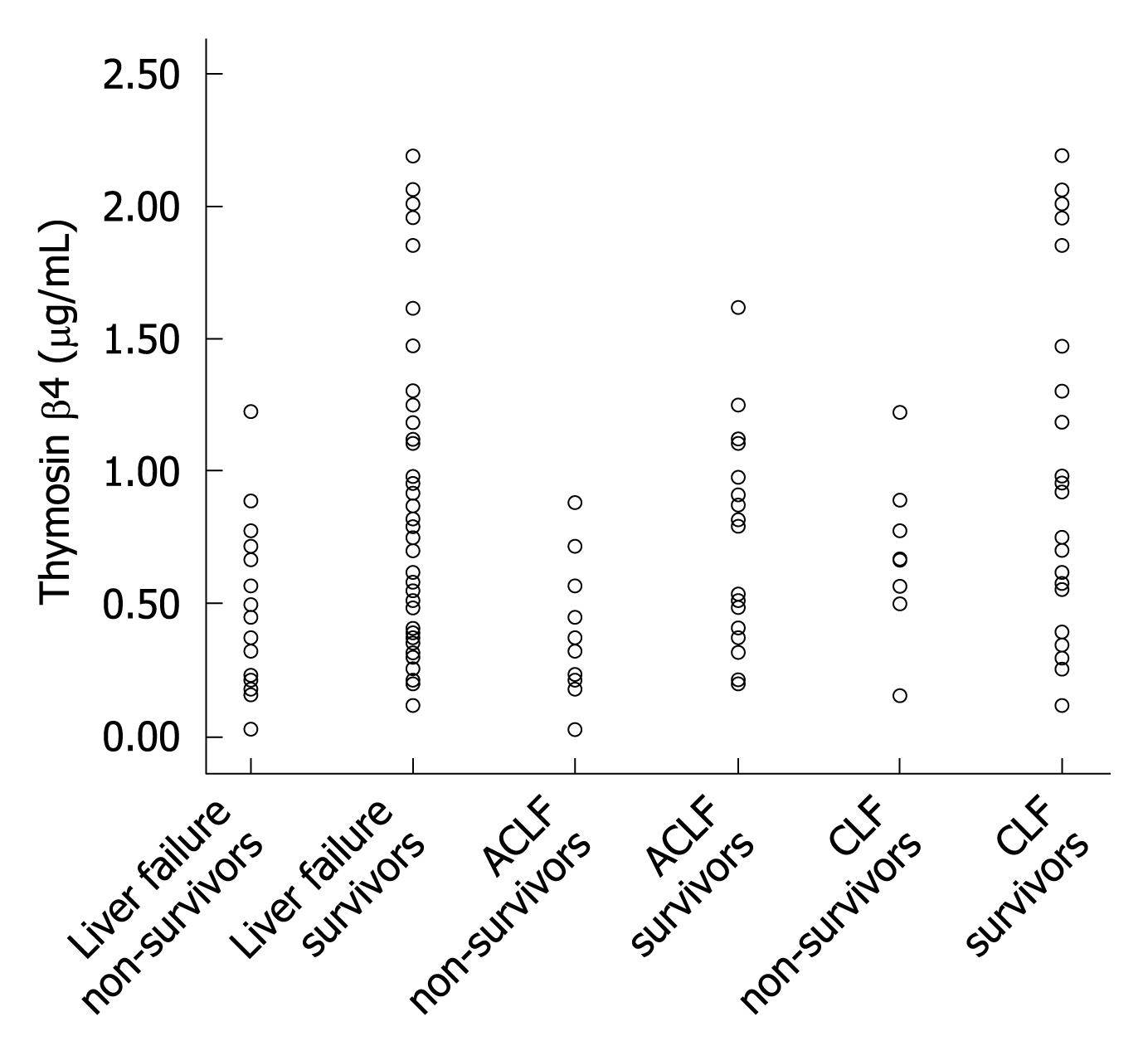
To further investigate the prediction value of thymosin β4 for different liver failure status, the 61 patients were divided into two groups: CLF and ACLF. Table 3 shows the median (IQR) thymosin β4 values of the two groups. In the ACLF group, ROC analysis identified a thymosin β4 cutoff value of 0.3873 μg/mL (AUROC 0.760) with a sensitivity of 76.5% and a specificity of 69.2 % for predicting poor prognosis (Figure 2B). In CLF group, lower thymosin β4 levels were observed in non-survivors, but there was no statistically significant difference between the survivors and non-survivors of CLF group. Figure 3 shows the distribution of thymosin β4 levels in the survivors and non-survivors with liver failure in the groups of ACLF and CLF.
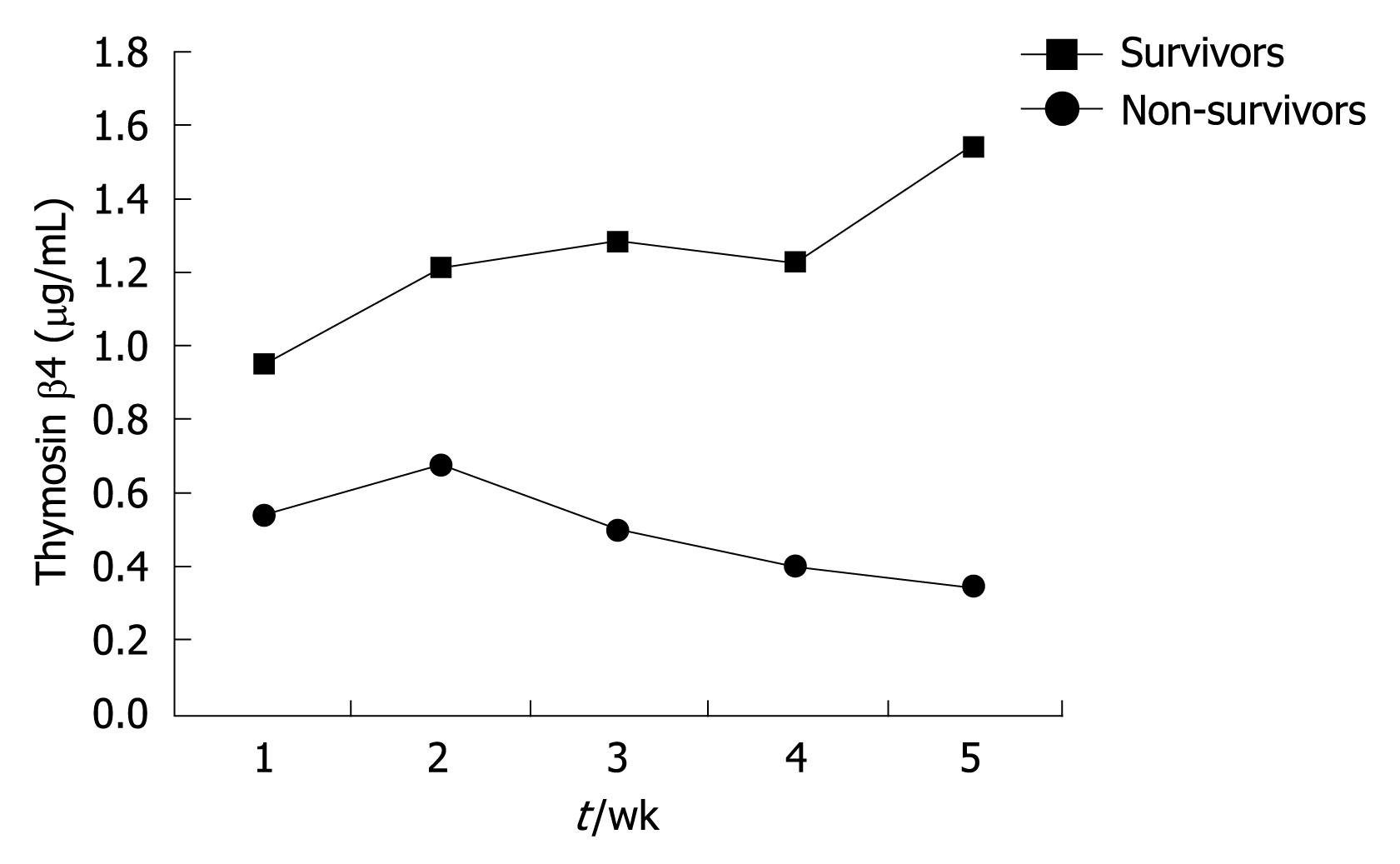
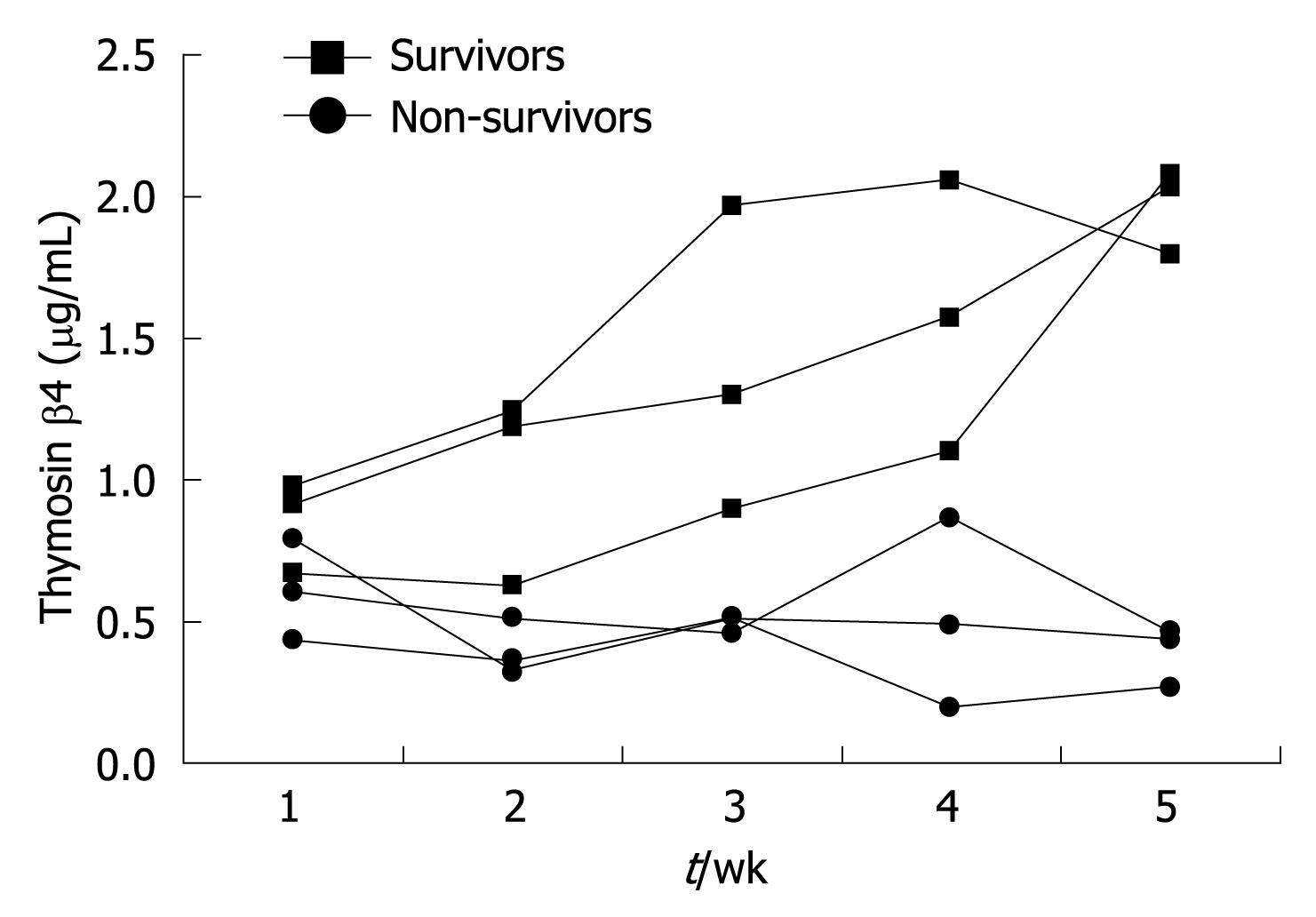
In this study, 13 liver failure patients were selected to observe the dynamic changes of their serum thymosin β4 values. Among them, 6 patients survived and the other 7 patients died. Blood samples were taken from these patients every week during their hospitalization. The median thymosin β4 concentrations of non-survivors were significantly lower than those of survivors (P < 0.001, Table 4). And greater improvement in thymosin β4 values was observed in those who recovered than in the non-survivors (Figure 4). Figure 5 shows the serial thymosin β4 changes in 6 typical liver failure patients of the 13 patients.
| Group | 1 wk | 2 wk | 3 wk | 4 wk | 5 wk |
| Survivors | 0.9402 | 1.2146 | 1.2762 | 1.2226 | 1.5486 |
| Non-survivors | 0.5458 | 0.6765 | 0.5056 | 0.3986 | 0.3488 |
In this study, we found that serum thymosin β4 levels were significantly lower in patients with chronic hepatitis B infection. The magnitude of reduction of thymosin β4 was closely related to the severity of the hepatic injury. Serum thymosin β4 concentrations were most significantly decreased in ACLF and CLF. Dynamic changes of serum thymosin β4 values could reflect the recovery or death in some liver failure patients.
Liver failure occurs in the hepatocytes with extensive injury, which represents either a failure to regenerate after accelerated destruction of hepatocytes from necrosis or apoptosis[15]. As a result, surviving hepatocytes cannot maintain adequate metabolic functions. ALB and CHE are synthesized by hepatocyte, and their content can directly reflect the state of liver function. In this study, the levels of thymosin β4 in CLD patients had a significantly positive correlation with ALB and CHE. In addition, a negative correlation between serum levels of thymosin β4 and other parameters (INR, ALT, AST and TBIL) was found, which reflected the severity of acute hepatocellular injury in patients with liver failure. There was a strong correlation between composite scores of CLF (Child-Pugh and MELD scores) and thymosin β4. Thymosin β4 significantly decreased in ACLF and CLF patients. These results suggested that serum thymosin β4 can be used as an important potential predictor of liver failure caused by chronic HBV infection.
Liver failure occurs when the extent of hepatocyte death exceeds the liver’s regenerating capacity. Liver regeneration is considered to be suppressed in liver failure. Hepatocyte growth factor (HGF) is a promising therapeutic agent for the treatment of liver failure. HGF not only stimulates liver regeneration, but also acts as an antiapoptotic factor in experimental liver failure models[16]. Recent findings showed that thymosin β4 upregulates the expression of HGF and downregulates the expression of PDGF-β receptor in human hepatic stellate cells[14]. HGF could induce apoptosis of hepatic stellate cells and hepatocyte regeneration[17,18]. So it is conceived that thymosin β4 protects the liver from injury. The study demonstrated that thymosin β4 level was significantly lowered in liver failure patients, suggesting that thymosin β4 might become a new therapeutic agent for liver failure caused by chronic HBV infection. Of course, more investigations will be needed to answer this question.
In addition, thymosin β4 promotes wound healing and modulates inflammatory mediators in different tissue injury[19-21]. Thymosin β4 reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock[22]. Liver failure is a systemic inflammatory reaction, which is characterized by a predominantly proinflammatory cytokine profile, causing the transition from stable clinical condition to severe deterioration in liver function. So it is considered that the more severe the liver inflammatory reaction is, the lower level of thymosin β4. In this study, thymosin β4 significantly decreased in liver failure patients.
In this study, we also found that serum thymosin β4 was an attractive parameter in the assessment of prognosis for liver failure patients. Thymosin β4 levels were markedly lower in the non-survivors than the survivors in liver failure cohorts. ACLF group had the same result with the liver failure group. A trend toward higher thymosin β4 levels was seen in survivors as compared with non-survivors of CLF patients. A possible explanation for the absence of a statistically significant difference could relate to heterogeneity in complications of CLF. In addition, the relatively small number of patients may not permit small differences to be detected. Dynamic changes of thymosin β4 concentration may further help determine the prognosis of liver failure patients.
In conclusion, our study has demonstrated a clear relationship between reductions in serum thymosin β4 level and severity of liver failure. Serum thymosin β4 level could be used as an important potential marker for predicting the prognosis of HBV-related liver failure (ACLF and CLF) patients. Further investigations including comparison with Child-Pugh or MELD scores are needed to assess the thymosin β4 value and explore the mechanism of lower thymosin β4 levels in liver failure caused by chronic HBV infection. More studies in different etiologies and a larger number of subjects of liver failure should also be considered.
Chronic hepatitis B virus (HBV) infection remains one of the most challenging public health problems in Asian region. Acute-on-chronic liver failure (ACLF) and chronic liver failure (CLF) are common serious conditions with a high mortality among chronic HBV infected patients. The prognosis is clearly related to the stage of the disease and early diagnosis has resulted in a significant reduction in mortality. This study aims to discover a new biomarker for diagnosing liver failure caused by chronic HBV infection.
Thymosin β4 is a 4.9-kDa polypeptide widely distributed in human tissues, which is considered as the major G-actin sequestering protein. Thymosin β4 has been shown to have multiple biological activities involved in a variety of physiologic and pathologic processes. Recent findings suggested that thymosin β4 could be beneficial for the treatment of chronic liver diseases (CLD). However, the levels of thymosin β4 in liver failure patients are still unknown.
In this study, the authors examined the serum thymosin β4 level in the HBV-related CLD patients and investigated the relationship between thymosin β4 values and biochemical parameters and observed the dynamic changes of serum thymosin β4 in liver failure patients. The results suggested that a lower serum thymosin β4 level could be a potential marker for predicting HBV-related liver failure (ACLF and CLF) prognosis.
Serum thymosin β4 level could become an important potential marker for liver failure diagnosis and is helpful in predicting the prognosis of patients with liver failure caused by chronic HBV infection.
ACLF was defined as acute deterioration of liver function within 4 wk in CLD, with severe jaundice and coagulopathy. CLF was defined as chronic decompensation of an end-stage liver disease, complicated with refractory ascites and/or encephalopathy, and hepatorenal syndrome.
This study is an interesting piece of work investigating whether serum thymosin β4 can provide diagnostic or prognostic information in liver failure patients caused by chronic HBV infection.
Peer reviewers: Dr. Richard Parker, MB, ChB, MRCP (London), Department of Gastroenterology, University Hospital of North Staffordshire, Newcastle Road, Stoke on Trent, North Staffordshire, ST4 6QG, United Kingdom; Christopher O’Brien, MD, Professor of Clinical Medicine, Chief of Clinical Hepatology, Center for Liver Diseases, Divisions of Liver and GI Transplantation, University of Miami School of Medicine, 1500 Northwest 12th Ave., Suite #1101, Miami, FL 33136, United States
S- Editor Wang YR L- Editor Ma JY E- Editor Zheng XM
| 1. | Sarin SK, Kumar A, Almeida JA, Chawla YK, Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int. 2009;3:269-282. |
| 2. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;1118-1129. |
| 3. | Sen S, Williams R, Jalan R. The pathophysiological basis of acute-on-chronic liver failure. Liver. 2002;22 Suppl 2:5-13. |
| 4. | Rolando N, Wade J, Davalos M, Wendon J, Philpott-Howard J, Williams R. The systemic inflammatory response syndrome in acute liver failure. Hepatology. 2000;32:734-739. |
| 5. | Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002;20:252-261. |
| 6. | Renner EL. How to decide when to list a patient with acute liver failure for liver transplantation? Clichy or King's College criteria, or something else? J Hepatol. 2007;46:554-557. |
| 7. | Hannappel E, Xu GJ, Morgan J, Hempstead J, Horecker BL. Thymosin beta 4: a ubiquitous peptide in rat and mouse tissues. Proc Natl Acad Sci USA. 1982;79:2172-2175. |
| 8. | Low TL, Goldstein AL. Chemical characterization of thymosin beta 4. J Biol Chem. 1982;257:1000-1006. |
| 9. | Marx J. Biomedicine. Thymosins: clinical promise after a decades-long search. Science. 2007;316:682-683. |
| 10. | Cha HJ, Jeong MJ, Kleinman HK. Role of thymosin beta4 in tumor metastasis and angiogenesis. J Natl Cancer Inst. 2003;95:1674-1680. |
| 11. | Philp D, Huff T, Gho YS, Hannappel E, Kleinman HK. The actin binding site on thymosin beta4 promotes angiogenesis. FASEB J. 2003;17:2103-2105. |
| 12. | Li X, Zheng L, Peng F, Qi C, Zhang X, Zhou A, Liu Z, Wu S. Recombinant thymosin beta 4 can promote full-thickness cutaneous wound healing. Protein Expr Purif. 2007;56:229-236. |
| 13. | Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177-182. |
| 14. | Barnaeva E, Nadezhda A, Hannappel E, Sjogren MH, Rojkind M. Thymosin beta4 upregulates the expression of hepatocyte growth factor and downregulates the expression of PDGF-beta receptor in human hepatic stellate cells. Ann N Y Acad Sci. 2007;1112:154-160. |
| 15. | Rutherford A, Chung RT. Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis. 2008;28:167-174. |
| 16. | Tomiya T, Omata M, Imamura H, Fujiwara K. Impaired liver regeneration in acute liver failure: the significance of cross-communication of growth associated factors in liver regeneration. Hepatol Res. 2008;38:S29-S33. |
| 17. | Nishino M, Iimuro Y, Ueki T, Hirano T, Fujimoto J. Hepatocyte growth factor improves survival after partial hepatectomy in cirrhotic rats suppressing apoptosis of hepatocytes. Surgery. 2008;144:374-384. |
| 18. | Ido A, Tsubouchi H. Translational research to identify clinical applications of hepatocyte growth factor. Hepatol Res. 2009;39:739-747. |
| 19. | Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Müller S, Willhauck M, Spitzweg C, Gildehaus FJ, Münzing W. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232-2240. |
| 20. | Bock-Marquette I, Shrivastava S, Pipes GC, Thatcher JE, Blystone A, Shelton JM, Galindo CL, Melegh B, Srivastava D, Olson EN. Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol. 2009;46:728-738. |
| 21. | Ho JH, Tseng KC, Ma WH, Chen KH, Lee OK, Su Y. Thymosin beta-4 upregulates anti-oxidative enzymes and protects human cornea epithelial cells against oxidative damage. Br J Ophthalmol. 2008;92:992-997. |
| 22. | Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin beta(4) reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol. 2003;3:1225-1233. |