Published online Feb 7, 2010. doi: 10.3748/wjg.v16.i5.613
Revised: December 15, 2009
Accepted: December 22, 2009
Published online: February 7, 2010
AIM: To investigate the incidence of nocturnal dyspeptic symptoms in patients with functional dyspepsia (FD) and whether prokinetic drugs can alleviate them.
METHODS: Eighty-five consecutive Chinese patients with FD were included in this study. One week after single-blinded placebo run-in treatment, baseline nocturnal intragastric pH, bile reflux and nocturnal dyspeptic symptoms of eligible patients, including epigastric pain or discomfort, abdominal distention and belching, were investigated with questionnaires. Patients exhibiting nocturnal dyspeptic symptoms were randomly and double-blindly assigned to domperidone group or placebo group. Nocturnal intragastric pH and percentage of duodenogastric bile reflux time were determined after treatment.
RESULTS: Of the 85 FD patients, 2 females without nocturnal symptoms, who responded to placebo run-in treatment, were excluded from the study, 30 (36.1%) exhibited nocturnal dyspeptic symptoms with increased duodenogastric bile reflux time (intragastric bilirubin absorbance > 0.14) and mean gastric pH (confirming the existence of bile reflux) (P = 0.021, 0.023) at night were included in the study. Of these 30 patients, 21 (70%) had overt nocturnal duodenogastric bile reflux, which was significantly higher than that of those without nocturnal symptoms (P = 0.026). The 30 patients were allocated to domperidone group or placebo group (n = 15). The nocturnal duodenogastric bile reflux and gastric pH were significantly decreased after domperidone treatment (P = 0.015, 0.021). The severity score of nocturnal dyspeptic symptoms was also significantly decreased after domperidone treatment (P = 0.010, 0.015, 0.026), which was positively correlated with the reduced nocturnal bile reflux or gastric pH (r = 0.736, 0.784, 0.753 or r = 0.679, 0.715, 0.697, P = 0.039, 0.036, 0.037 or P = 0.043, 0.039, 0.040).
CONCLUSION: A subgroup of Chinese FD patients show overt nocturnal dyspeptic symptoms, which may be correlated with the excessive nocturnal duodenogastric bile reflux. Domperidone therapy can alleviate these symptoms.
- Citation: Chen SL, Ji JR, Xu P, Cao ZJ, Mo JZ, Fang JY, Xiao SD. Effect of domperidone therapy on nocturnal dyspeptic symptoms of functional dyspepsia patients. World J Gastroenterol 2010; 16(5): 613-617
- URL: https://www.wjgnet.com/1007-9327/full/v16/i5/613.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i5.613
Functional dyspepsia (FD) is a common chronic disorder with non-specific upper abdominal symptoms such as persistent or relapsing pain or discomfort[1]. Its underlying pathophysiological mechanism has not been fully elucidated. Besides the role of excessive chemical (both acidic and alkaline) stimuli in the gastric lumen and/or hypersensitivity to these stimuli, impaired gastrointestinal (GI) motility is intensively involved in FD pathogenesis[2-4]. Delayed gastric emptying or intestinal peristalsis may influence GI secretion function which may in turn further affect GI motility, resulting in alterations in luminal chemical environment, abnormal duodenogastric bile reflux and FD symptoms[5].
Although the optimal treatment of FD has not yet been established, empirical pharmacological interventions with prokinetic agents are effective in a subgroup of FD patients[6-8]. Domperidone, a peripheral dopamine (D2) receptor antagonist, acts as an antiemetic and prokinetic agent through its effects on the chemoreceptor trigger zone and motor function of the stomach and small intestine, thus promoting gastric emptying by augmenting gastric peristalsis and improving antroduodenal coordination[9,10]. Domperidone has been used in treatment of a variety of GI motility disorders, such as gastroparesis and gastroesophageal reflux disease, with an acceptable safety profile and a relatively good therapeutic efficacy for FD[7,10].
In clinical practice, gastroenterological physicians frequently meet a subgroup of FD patients with obvious nocturnal dyspeptic symptoms such as epigastric pain or discomfort, abdominal distention or belching. Up to now, however, their prevalence and mechanism and whether prokinetic drugs can alleviate them are still unknown.
This study was aimed to evaluate the prevalence of nocturnal dyspeptic symptoms, the relation between such symptoms and nocturnal intragastric pH or duodenogastric bile reflux, and the effect of 2-wk oral administration of domperidone on these symptoms.
Eighty-five consecutively patients, including 38 males at the age of 22-56 years who were diagnosed as FD according to Rome II criteria, were enrolled in this study. All patients were admitted because of dyspeptic symptoms and underwent routine biochemistry, bilimetry, upper GI endoscopy and abdominal ultrasonography. Inclusion criteria included the presence of dyspeptic symptoms for at least 3 mo in the past year, the absence of organic, systemic or metabolic disease, 2 or more dyspeptic symptoms present at least in 3 d per week such as epigastric pain or discomfort, abdominal distention and belching. Exclusion criteria were the presence of esophagitis, severe atrophic gastritis, erosive or ulcerative gastroduodenal lesions on endoscopy, heartburn as a predominant symptom, a history of peptic ulcer, major abdominal surgery, or underlying psychiatric illness, and use of nonsteroidal anti-inflammatory drugs, steroids, or drugs affecting gastric motility and acid secretion during the last week.
This study was conducted at Department of Gastroenterology, Shanghai Institute of Digestive Diseases, Renji Hospital, Shanghai Jiaotong University School of Medicine, according to the ethical principles in Declaration of Helsinki and the requirements of local laws and regulations. The study protocol was approved by the Ethics Committee of Renji Hospital. Written informed consent was obtained from each participant prior to the study.
Patients with a severity score of at least two individual symptoms decreased by 50% were excluded. One week after single-blinded placebo run-in treatment, baseline nocturnal intragastric pH, bile reflux and nocturnal dyspeptic symptoms of eligible patients, including epigastric pain or discomfort, abdominal distention and belching, were investigated. Those exhibiting nocturnal symptoms were randomly and double-blindly assigned to either domperidone group or placebo group. Patients in domperidone group received domperidone (10 mg qid, before meal and at bedtime) for 2 wk and those in placebo group received placebo. Nocturnal dyspeptic symptoms, intragastric pH and percentage of bile reflux time during nighttime (22:00 PM to 6:00 AM) were determined after treatment.
Following 2-h fasting, an initial manometric localization (CTD-Synectics, Sweden) of the lower esophageal sphincter (LES) was carried out. Nocturnal intragastric pH was then recorded using a pH sensitive microelectrode (Synectics Digitrapper MKIII, Medtronic Synectics, Sweden) connected to a portable Synectics medical Digitrapper III (CTD-Synectics). The electrode was inserted transnasally and positioned at about 8-10 cm below the manometrically determined LES. A two-point calibration of the probe was made before each recording, using standard buffers of pH 1 and pH 7. The Digitrapper data were downloaded onto a personal computer to calculate the nocturnal mean intragastric pH.
A fiber-optic spectrophotometer, Bilitec 2000 (Medtronic Synectics), was used to quantify duodenogastric bile reflux. The system consists of a probe (1.5-mm in diameter) that carries light signals into the stomach and back via a plastic fiber-optic bundle. Before each study, the probe was calibrated in water and located at 8-10 cm below the LES. An episode of duodenogastric bile reflux was defined as a rise of bilirubin absorbance above the cut-off level (> 0.14 at 470 nm) lasting longer than 10 s[11]. The Digitrapper data were downloaded onto a personal computer to calculate the percentage of bile reflux time with the absorbance of bilirubin > 0.14.
Nocturnal dyspeptic symptoms were evaluated with a self-recorded questionnaire. Each patient was instructed on how to fill in the questionnaire. The symptom questionnaire consisted of 4 questions related to nocturnal upper gastrointestinal symptoms, including epigastric pain or discomfort, abdominal distention and belching. The intensity and frequency of each symptom were rated at 7 levels according to 3 grades with half steps between each rating: 0 = absent, 1 = mild, 2 = relevant and 3 = severe[12]. The severity of symptoms was scored as the product of intensity and frequency. The patients were asked to record the severity score of different nocturnal symptoms before and at end of the treatment.
All data were presented as mean ± SD. Student’s t-test and Wilcoxon rank sum test were used to compare the difference between means of before and after treatment. Incidence was compared using χ2 test and correlation was assessed by Pearson correlation analysis. P < 0.05 was considered statistically significant.
Of the 85 patients, 2 females without nocturnal symptoms with a severity score of at least 2 individual symptoms decreased by 50% after placebo run-in treatment were excluded from the study, 30 (36.1%) including 13 males at the age of 22-56 years who exhibited overt nocturnal dyspeptic symptoms were allocated to domperidone group or placebo group (n = 15). Demographic and baseline clinical characteristics did not differ between the two groups (Table 1).
| Characteristic | Placebo | Domperidone |
| Age (yr) | ||
| mean ± SD | 44.5 ± 8.6 | 43.9 ± 9.8 |
| Range | 25-56 | 22-50 |
| Gender | ||
| Male | 6 (40) | 7 (46.7) |
| Female | 9 (60) | 8 (53.3) |
| BMI (kg/m2) | ||
| mean ± SD | 21.3 ± 7.9 | 20.7 ± 8.1 |
| Range | 18.4-27.2 | 18.0-25.1 |
| Current smokers | 1 (4.2) | 3 (5.9) |
| Alcohol use | 5 (22.7) | 6 (11.8) |
| Positive for Helicobacter pylori | 0 (0) | 0 (0) |
Of the 30 patients with nocturnal dyspeptic symptoms, 21 (70%) including 9 males had overt nocturnal duodenogastric bile reflux, which was higher than that of those without nocturnal symptoms (17.0%) including 2 males (P < 0.05). Moreover, the percentage of duodenogastric bile reflux time (intragastric bilirubin absorbance > 0.14) and mean gastric pH at night in the subgroup of patients with nocturnal symptoms was 5.9% ± 1.7% and 4.9% ± 1.9%, respectively, both were significantly higher than those in patients without nocturnal symptoms (bile reflux time = 2.4% ± 0.8%, mean gastric pH = 2.5 ± 1.4, P < 0.05).
In domperidone group, 11 patients (73.3%) including 5 males had overt nocturnal duodenogastric bile reflux at baseline and mean nocturnal gastric pH > 4, which were significantly decreased after domperidone treatment (P < 0.05). In placebo group, 10 patients (66.7%) including 4 males had obvious nocturnal bile reflux and mean gastric pH > 4 at baseline, which were not markedly changed after placebo treatment (Table 2).
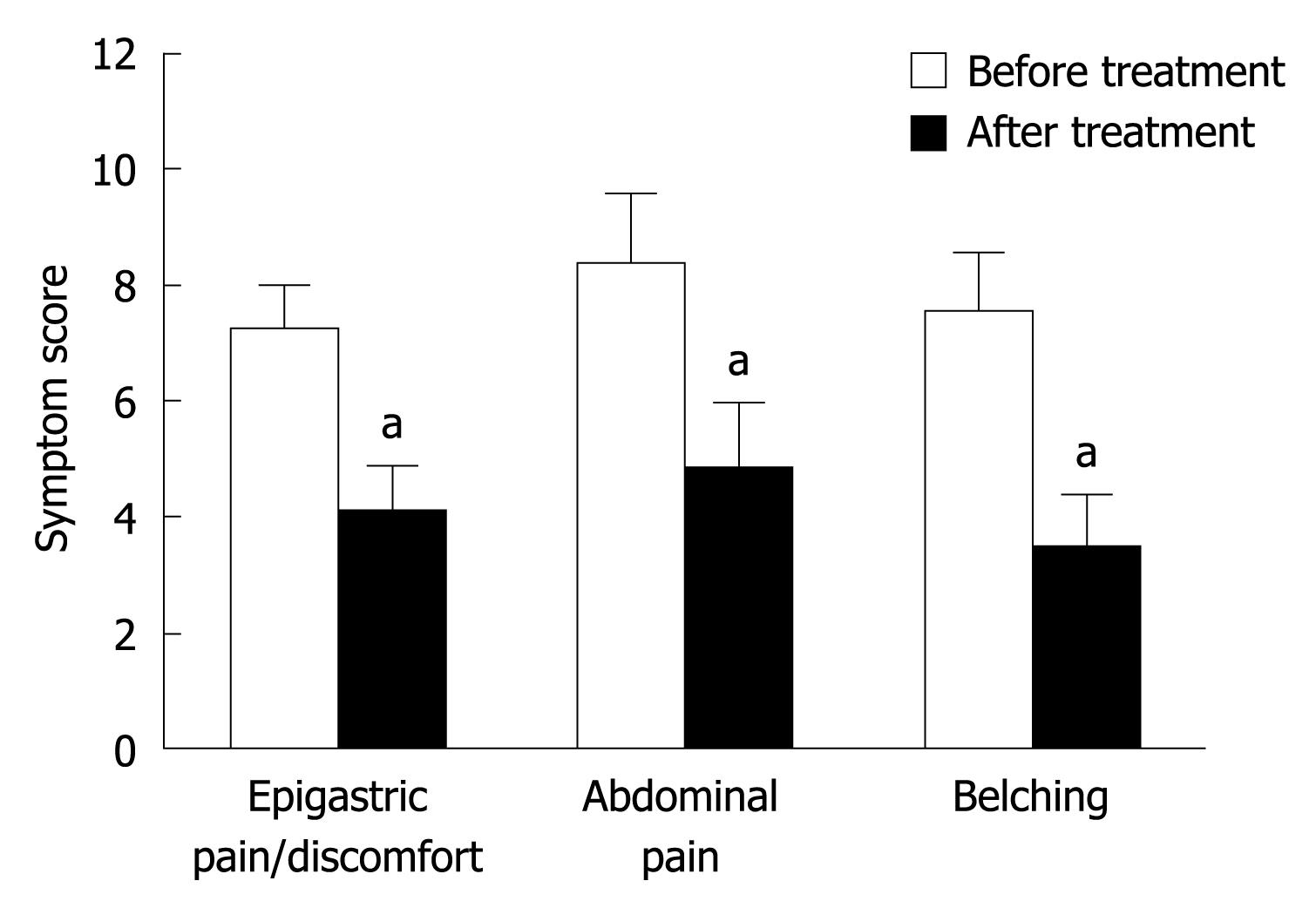
The severity score of nocturnal dyspeptic symptoms such as epigastric pain or discomfort, abdominal distention and belching was significantly lower in FD patients after domperidone treatment than before domperidone treatment (4.1 ± 0.8 vs 7.3 ± 0.7, 4.9 ± 1.1 vs 8.4 ± 1.2 and 3.5 ± 0.9 vs 7.6 ± 1.0, P < 0.05, Figure 1). Improved nocturnal symptoms after domperidone treatment were positively correlated with reduced nocturnal duodenogastric bile reflux or intragastric pH in patients with marked duodenogastric bile reflux and mean nocturnal gastric pH > 4 at baseline (r = 0.736-0.784 or r = 0.679-0.715, P < 0.05) (Table 3).
No patients stopped their medication or withdrew from the study due to side effects of domperidone during the study.
The pathophysiological mechanism underlying FD is complex and its treatment remains a clinical challenge. Impaired GI motility seems to play a pivotal role in pathogenesis of FD. Gastric emptying is delayed in about 30% of FD patients[4,13]. It has been shown that domperidone and other prokinetic agents can markedly improve dyspeptic symptoms of FD patients[6-8]. However, the prevalence of nocturnal symptoms and the effect of domperidone on these symptoms have not been extensively elucidated.
Nocturnal intragastric pH and bilirubin level are hardly affected by food taking and other diurnal activities, thus relatively more stable and better reflecting the chemical environment in the gastric cavity. Increased gastric pH is thought to be an indicator of gastric bile reflux, which is related to impaired antroduodenal motility[14-16]. Therefore, the profile of gastric pH and bile reflux at night were selected as the main endpoints in the present study.
In this study, over 35% of FD patients exhibited nocturnal dyspeptic symptoms, and about 70% of the patients in this subgroup had a marked nocturnal duodenogastric bile reflux at baseline, manifested as prolonged time of absorbance of gastric bilirubin > 0.14 confirmed by alkalinization of the acidic intragastric environment (mean pH > 4), suggesting that nocturnal dyspeptic symptoms of FD patients may be associated with abnormal nocturnal duodenogastric bile reflux. There is evidence that decreased antroduodenal motility is the main factor for increased duodenogastric reflux[15,16]. Our findings also indicate that nocturnal dyspeptic symptoms are related with impaired antroduodenal motility.
In our study, the severity score of nocturnal epigastric pain or discomfort, abdominal distention or belching, as well as nocturnal gastric pH and bile reflux in FD patients, were significantly lower after domperidone treatment than before domperidone treatment. Furthermore, alleviation of bile reflux was well correlated with the improved nocturnal symptoms, suggesting that the efficacy of domperidone therapy may be associated with the inhibition of nocturnal duodenogastric bile reflux resulting from improved gastric peristalsis and antroduodenal coordination.
Since the placebo effect on FD can be high[17], 1-wk single-blind placebo run-in treatment was used in patients before they received domperidone or placebo treatment in this study.
In conclusion, a subgroup of FD patients exhibit overt nocturnal dyspeptic symptoms, which may be related to an increased nocturnal duodenogastric bile reflux. Domperidone therapy can alleviate such symptoms by attenuating nocturnal duodenogastric bile reflux resulting from improved GI motility.
The pathophysiological mechanism of functional dyspepsia (FD) is complex and its treatment remains a clinical challenge. There exists a subgroup of FD patients with nocturnal dyspeptic symptoms, such as epigastric pain or discomfort, abdominal distention or belching. Up to now, the prevalence and mechanism of FD and its effective drug therapy have not been established.
Impaired gastrointestinal motility plays a pivotal role in pathogenesis of FD, and empirical pharmacological interventions with prokinetic agents have been shown to be effective on FD. However, whether abnormal antroduodenal motility, which can lead to increased nocturnal duodenogastric bile reflux, contributes to nocturnal dyspepsia symptoms of FD patients and whether prokinetic drugs can alleviate these symptoms are still unknown.
This is the first study to report that a subgroup of FD patients exhibit overt nocturnal dyspeptic symptoms, which may be related to excessive nocturnal duodenogastric bile reflux. Prokinetic therapy can alleviates these syndromes by attenuating nocturnal bile reflux, thus representing a promising treatment modality for nocturnal dyspeptic symptoms of FD patients.
The incidence of nocturnal dyspeptic symptoms and the effect of prokinetic drugs on FD were studied in this study, which may provide valuable data for the treatment of nocturnal dyspeptic symptoms of FD patients.
A RCT in dyspeptic patients with nocturnal symptoms was described. The results are clear cut in the sense that many FD patients in China complain of nocturnal symptoms and that domperidone is a promising agent in treatment of these patients. The study also suggests that duodenogastric reflux during night is of a pathophysiological relevance.
Peer reviewer: Joachim Labenz, Associate Professor, Jung-Stilling Hospital, Wichernstr 40, Siegen 57074, Germany
S- Editor Wang YR L- Editor Wang XL E- Editor Zheng XM
| 1. | Geeraerts B, Tack J. Functional dyspepsia: past, present, and future. J Gastroenterol. 2008;43:251-255. |
| 2. | Mizuta Y, Shikuwa S, Isomoto H, Mishima R, Akazawa Y, Masuda J, Omagari K, Takeshima F, Kohno S. Recent insights into digestive motility in functional dyspepsia. J Gastroenterol. 2006;41:1025-1040. |
| 3. | Sha W, Pasricha PJ, Chen JD. Correlations among electrogastrogram, gastric dysmotility, and duodenal dysmotility in patients with functional dyspepsia. J Clin Gastroenterol. 2009;43:716-722. |
| 4. | Haruma K, Kusunoki H, Manabe N, Kamada T, Sato M, Ishii M, Shiotani A, Hata J. Real-time assessment of gastroduodenal motility by ultrasonography. Digestion. 2008;77 Suppl 1:48-51. |
| 5. | Mearin F, De Ribot X, Balboa A, Antolín M, Varas MJ, Malagelada JR. Duodenogastric bile reflux and gastrointestinal motility in pathogenesis of functional dyspepsia. Role of cholecystectomy. Dig Dis Sci. 1995;40:1703-1709. |
| 6. | Hiyama T, Yoshihara M, Matsuo K, Kusunoki H, Kamada T, Ito M, Tanaka S, Nishi N, Chayama K, Haruma K. Meta-analysis of the effects of prokinetic agents in patients with functional dyspepsia. J Gastroenterol Hepatol. 2007;22:304-310. |
| 7. | Ang TL, Fock KM, Teo EK, Chan YH, Ng TM, Chua TS, Tan JY. Helicobacter pylori eradication versus prokinetics in the treatment of functional dyspepsia: a randomized, double-blind study. J Gastroenterol. 2006;41:647-653. |
| 8. | Passos Mdo C, Duro D, Fregni F. CNS or classic drugs for the treatment of pain in functional dyspepsia? A systematic review and meta-analysis of the literature. Pain Physician. 2008;11:597-609. |
| 9. | Brogden RN, Carmine AA, Heel RC, Speight TM, Avery GS. Domperidone. A review of its pharmacological activity, pharmacokinetics and therapeutic efficacy in the symptomatic treatment of chronic dyspepsia and as an antiemetic. Drugs. 1982;24:360-400. |
| 10. | Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol. 2007;102:2036-2045. |
| 11. | Koek GH, Vos R, Sifrim D, Cuomo R, Janssens J, Tack J. Mechanisms underlying duodeno-gastric reflux in man. Neurogastroenterol Motil. 2005;17:191-199. |
| 12. | Miwa H, Osada T, Nagahara A, Ohkusa T, Hojo M, Tomita T, Hori K, Matsumoto T, Sato N. Effect of a gastro-protective agent, rebamipide, on symptom improvement in patients with functional dyspepsia: a double-blind placebo-controlled study in Japan. J Gastroenterol Hepatol. 2006;21:1826-1831. |
| 13. | Talley NJ, Locke GR 3rd, Lahr BD, Zinsmeister AR, Tougas G, Ligozio G, Rojavin MA, Tack J. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933-939. |
| 14. | Fuchs KH, Fein M, Maroske J, Heimbucher J, Freys SM. The role of 24-hr gastric pH-monitoring in the interpretation of 24-hr gastric bile monitoring for duodenogastric reflux. Hepatogastroenterology. 1999;46:60-65. |
| 15. | Defilippi C, Mamani N, Gomez E. Relationship between antropyloric and intestinal motility and duodenogastric reflux in fasting dogs. Dig Dis Sci. 1987;32:171-176. |
| 16. | Cucchiara S, Bortolotti M, Colombo C, Boccieri A, De Stefano M, Vitiello G, Pagano A, Ronchi A, Auricchio S. Abnormalities of gastrointestinal motility in children with nonulcer dyspepsia and in children with gastroesophageal reflux disease. Dig Dis Sci. 1991;36:1066-1073. |
| 17. | Talley NJ, Locke GR, Lahr BD, Zinsmeister AR, Cohard-Radice M, D'Elia TV, Tack J, Earnest DL. Predictors of the placebo response in functional dyspepsia. Aliment Pharmacol Ther. 2006;23:923-936. |