Published online Feb 7, 2010. doi: 10.3748/wjg.v16.i5.563
Revised: December 5, 2009
Accepted: December 12, 2009
Published online: February 7, 2010
AIM: To evaluate effects of pre- and postnatal protein deprivation and postnatal recovery on the myenteric plexus of the rat esophagus.
METHODS: Three groups of young Wistar rats (aged 42 d) were studied: normal-fed (N42), protein-deprived (D42), and protein-recovered (R42). The myenteric neurons of their esophagi were evaluated by histochemical reactions for nicotinamide adenine dinucleotide (NADH), nitrergic neurons (NADPH)-diaphorase and acetylcholinesterase (AChE), immunohistochemical reaction for vasoactive intestinal polypeptide (VIP), and ultrastructural analysis by transmission electron microscopy.
RESULTS: The cytoplasms of large and medium neurons from the N42 and R42 groups were intensely reactive for NADH. Only a few large neurons from the D42 group exhibited this aspect. NADPH detected in the D42 group exhibited low reactivity. The AChE reactivity was diffuse in neurons from the D42 and R42 groups. The density of large and small varicosities detected by immunohistochemical staining of VIP was low in ganglia from the D42 group. In many neurons from the D42 group, the double membrane of the nuclear envelope and the perinuclear cisterna were not detectable. NADH and NADPH histochemistry revealed no group differences in the profile of nerve cell perikarya (ranging from 200 to 400 μm2).
CONCLUSION: Protein deprivation causes a delay in neuronal maturation but postnatal recovery can almost completely restore the normal morphology of myenteric neurons.
- Citation: Greggio FM, Fontes RB, Maifrino LB, Castelucci P, Souza RR, Liberti EA. Effects of perinatal protein deprivation and recovery on esophageal myenteric plexus. World J Gastroenterol 2010; 16(5): 563-570
- URL: https://www.wjgnet.com/1007-9327/full/v16/i5/563.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i5.563
Several studies have been published that deal with the effects of several forms of malnutrition on the myenteric plexus (MP) of the gastrointestinal tract. From the amassed data, it is obvious that different regions of the gut are differently affected in their morphology, both quantitatively and qualitatively[1-5]. However, information whether these changes are reversible following re-feeding are scarce.
The effects of re-feeding on both the peripheral and central nervous system (CNS) have been investigated. The optic nerve of rats undergoes a reduction in the number of myelinated fibers when subjected to malnourishment; that effect is reversed when adequate protein intake is restored early[6]. Similar results have been verified in the tibial and ulnar nerves of monkeys[7]. In the CNS, however, neurons lost in the hippocampal formation of rats or alteration of the dendritic orientation of pyramidal cells in the neocortex of rats are never recovered with a normal protein diet[8,9].
That behavior, however, should not be expected from all other neurons. Cell size and MP appearance in colonic neurons return to baseline after restoration of a normal protein-content diet, as shown in our previous work, in sharp contrast to CNS neurons[1]. The estimated number of myenteric neurons of the small intestine in protein-recovered animals of the same age does not differ from that of normally nourished rats[4]. The reaction to undernourishment would thus be more correctly described as a developmental delay from the morphological standpoint, but not fully recovered in functional terms.
Several ultrastructural changes have also been described after protein deprivation. Recently, we have demonstrated drastic alterations in the esophageal neurons of 21-d-old weanling rats submitted to pre- and postnatal protein deprivation, such as altered disposition of both nuclear chromatin and ribosomes in the granular reticulum, as well as a poorly developed granular component of the nucleolus. These data are further compatible with a delay in the development of the myenteric neurons[3]. However, information on the structural and ultrastructural characteristics of the MP of the esophagus specifically remains unknown as to whether these modifications are reversible after adequate protein rehabilitation in the early postnatal period. Therefore, in the present work, we evaluated some of the histochemical, immunohistochemical, and ultrastructural features of the myenteric neurons of the esophagi of protein-deprived and protein-recovered young rats (42 d old).
This study was conducted according to current legislation on animal experiments of the Instituto de Ciencias Biomedicas da Universidade de Sao Paulo (ICB-USP). Young adult male and female Wistar rats (200-240 g body weight) were mated during a period of 7-10 d. After conception, the females were placed in individual cages and maintained under standard conditions at 21°C with a 12-h light/dark cycle. During pregnancy, the nourished mothers (N) received an AIN-93G[10] normal protein diet and the malnourished (protein-deprived) mothers (D) received the AIN-93G low-protein diet (5% casein) (Rhoster, Sao Paulo, Brazil). Both groups were supplied with water ad libitum.
Following birth, the dams and pups of both groups continued to receive their respective assigned diets. At the end of the weanling period (21 d) N and D male pups were separated from the dams and maintained on their respective diets until day 42. They were studied as N42 and D42 groups, respectively. Exactly half of the malnourished pups from the first phase (up to 21 d) received the AIN-93G normal protein diet from day 22 to 42 and formed the protein-recovery group, R42.
The animals from all groups (N42, D42 and R42) were weighed, euthanized with hypnol (Fontoveter, Sao Paulo, Brazil) and had their esophagi entirely removed. After measurement of the surface area using a planimeter[11] (OTT, Germany), the esophagi were processed using the following techniques.
NADH-diaphorase histochemistry was employed as our “pan-neuronal” marker for the MP; the controversy about the ideal neuronal marker has not been satisfactorily settled and shall not be addressed here[11]. Five animals from each group were used for NADH-diaphorase reaction histochemistry[12,13]. After being washed in Krebs solution (120 mmol/L NaCl, 5 mmol/L KCl, 25 mmol/L NaHCO3, 1.2 mmol/L NaH2PO4, 1.2 mmol/L MgSO4, 2.5 mmol/L CaCl2, and 11.5 mmol/L glucose), the oral portion of each esophagus was ligated with cotton threads and filled with Krebs solution using a syringe needle introduced in the lumen. When the walls of the viscera were slightly distended, the needle was withdrawn and a ligature was performed in the aboral section. After incubation in Krebs solution at room temperature for 15-30 min, the specimens were transferred to a permeabilizing agent (0.3% Triton-X in Krebs solution) for 90 s and then washed in three 10-min cycles with Krebs solution.
The esophagi were incubated for 60 min at 20°C in 20 mL of a solution that contained 0.5 mg/mL nitro blue tetrazolium (Sigma, St Louis, MO, USA) in 5 mL distilled water, 5 mL 0.1 mol/L sodium phosphate buffer (pH 7.3), 10 mL distilled water and 0.5 mg/mL β-nicotinamide adenine dinucleotide (reduced form). The reaction was stopped by fixation in 4% buffered formaldehyde (24 h). Subsequently, the esophagi from all groups (N42, D42 and R42) were opened longitudinally and three circular fragments, 2 mm2 each, were obtained from the oral (Or), medium (Me) and aboral (Ab) parts using a puncture device. The mucosa and submucosa from the fragments were then removed, washed in distilled water, arranged as whole-mount preparations in glycerol on a microscope slide, and sealed with Entellan (Merck KGaA, Darmstadt, Germany).
The histochemical NADPH-diaphorase reaction[14,15] was performed in five esophagi from each animal group (N42, D42 and R42). The specimens were washed in Krebs solution and filled with 4% paraformaldehyde in phosphate buffer pH 7.4. After ligation, the oral and aboral parts from each esophagus were immersed in the same fixative solution for 2 h at 4°C, washed in phosphate-buffered 0.9% NaCl for 2.5 h, and incubated in medium that contained 1 mg/mL β-NADPH (reduced form; Sigma) in phosphate buffer for 35 min. Thereafter the Or, Me and Ab circular fragments were obtained from each esophagus and prepared as whole-mount preparations as mentioned above.
In an attempt to characterize further the cytoarchitecture of the MP, indirect detection of cholinergic neurons was attempted by demonstration of AChE activity[3]. Three esophagi from each group (N42, D42 and R42) were subjected to the direct coloring method to demonstrate AChE[16-18]. The specimens were filled with 4% paraformaldehyde in phosphate buffer (pH 7.4), ligated at their oral and aboral limits, and immersed in the same fixative solution for 2 h at 4°C. After this period, the esophagi were opened and the Or, Me and Ab circular fragments incubated overnight at 4°C in solution A - hyaluronidase (Hialozime; Apsen, Brazil), Krebs solution and tetraisopropylpyrophosphoramide (Sigma). The fragments were then washed in Krebs solution and further incubated overnight at 4°C in a solution that contained 50% of solution A plus 0.17 mol/L acetylthiocholine iodide, 0.1 mol/L phosphate buffer (pH 7.1), 100 mmol/L sodium citrate, 30 mmol/L cupric sulfate, 5 mmol/L potassium ferricyanide and 0.3% Triton X-100. The mucosal and submucosal layers were removed and the fragments were dehydrated in an increasing alcohol series (70%-100%), immersed in benzene for 20 min, and mounted on microscope slides with Entellan.
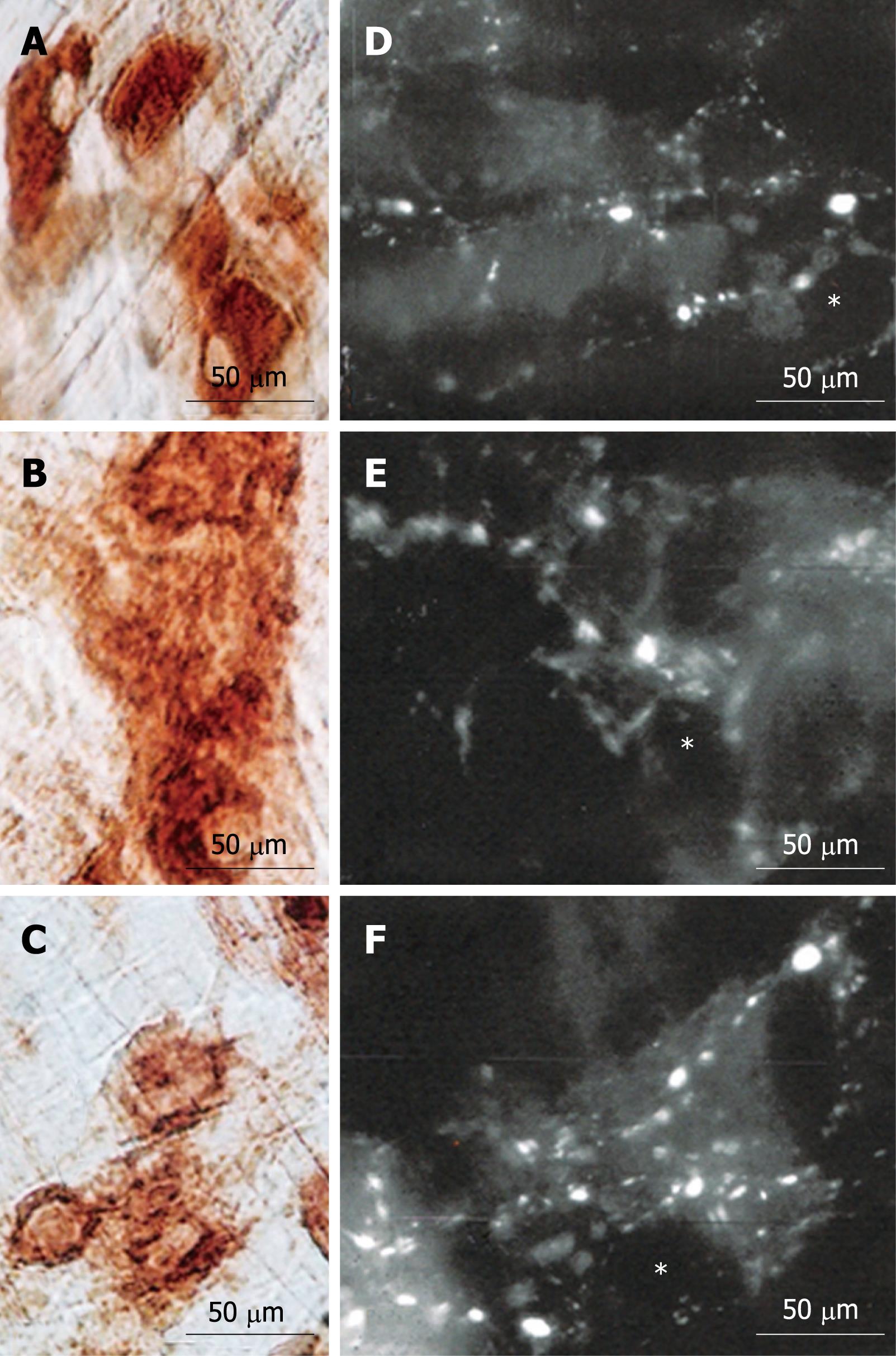
Vasoactive intestinal peptide (VIP) staining[19] was performed on three fresh segments of esophagus (approximate area: 3 mm2). Segments were removed from three animals each of the N42, D42 and R42 groups and placed in PBS (0.15 mol/L NaCl in 0.01 mol/L sodium phosphate buffer, pH 7.2) that contained nicardipine (10-6 mol/L; Sigma) to inhibit tissue contraction. The specimens were then pinned out tautly, mucosa side down, onto a balsa-wood board and fixed overnight at 4°C in a paraformaldehyde solution (0.2 mol/L, pH 7.3). The specimens were then cleared with three 10-min cycles in 100% dimethysulfoxide, followed by three 10-min washes in PBS. All samples were stored at 4°C in PBS that contained sodium azide (0.1%). After removing the mucosa, submucosa, and circular muscular layers, the whole-mount preparations were pre-incubated in 10% normal horse serum in PBS that contained 1% Triton X-100 for 30 min at room temperature to reduce nonspecific binding and to permeabilize the tissue. Rabbit anti-VIP antibody was then used at a concentration of 1:2000. This antibody was diluted in antibody diluent (1.8% NaCl in 0.01 mol/L sodium phosphate buffer that contained 0.1 sodium azide). Following incubation in the primary antibody for two nights in a humid chamber at 4°C, the specimens were washed three times in PBS (10-min cycles) and then incubated for 1 h at room temperature with a fluorescence-labeled secondary antibody (Donkey anti-rabbit IgG Alexa 488 at 1:500; Molecular Probes, Eugene, OR, USA). After washing in PBS, the specimens were mounted in 0.5 mol/L sodium carbonate-buffered glycerol (pH 8.6). The specimens were finally examined for florescence on a Leica Microscope equipped with the appropriate filter for Alexa 488 fluorescence (set to 00 for Alexa 488). The images were captured using an Image Proplus System.
Following systemic perfusion with a fixative solution (3% glutaraldehyde in Millonig’s buffer (pH 7.2-7.4) with 0.25% tannic acid)[20], esophagi of three animals from each group (N42, D42 and R42) were removed. Two fragments (approximately 2 mm in length) from the Or, Me and Ab sections were obtained from each specimen and incubated for 6 h in the same fixative solution. Specimens were subjected to post-fixation treatment in a 2% osmium tetroxide solution and embedded in adhesion medium. Ultra-thin sections were stained with uranyl acetate/lead citrate[21] and examined with a JEOL JEM-1010 (Jeol, Tokyo, Japan) electron microscope.
A semiautomatic device (Axioskop 40, Axiocam HRc, Axiovision software 4.5; Zeiss, Germany) was utilized for morphometric neuronal examination. The cross-sectional (profile) areas of 20 neuronal perikarya from each Or, Me and Ab circular fragment stained with NADH and NADPH were determined in each specimen at 400 × magnification.
mean ± SE were calculated and compared by analysis of variance (ANOVA), Student’s t test, and Duncan’s test for multiple comparisons as appropriate, with the level of significance set at P < 0.05[22].
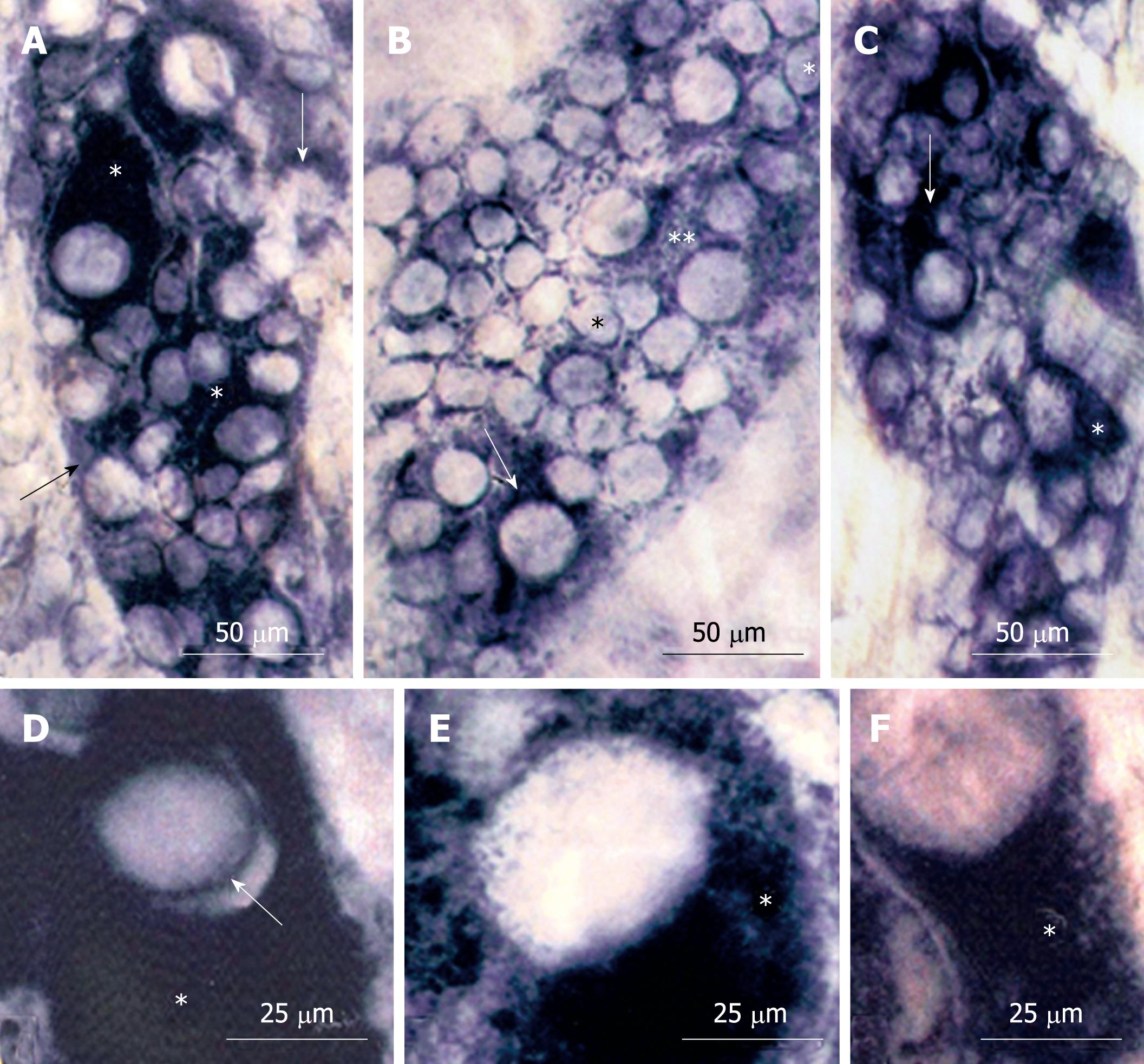
No significant differences were detected among the Or, Me and Ab esophageal segments in all groups. The NADH-diaphorase-stained whole-mount preparations of esophagi from all groups (N42, D42, R42) had myenteric neurons of varying size and reactivity (Figure 1). Most of the large and medium neurons in animals from the N42 and R42 groups had intense staining of the cytoplasm; an aspect detected in only a few large neurons of the D42 group (Figure 1A-C). Under high magnification, the dark and homogeneous aspects of the cytoplasm from N42 neurons were partially observed in R42 neurons. In contrast, the neurons from the D42 group had cytoplasm with granular aspects (Figure 1D-F).
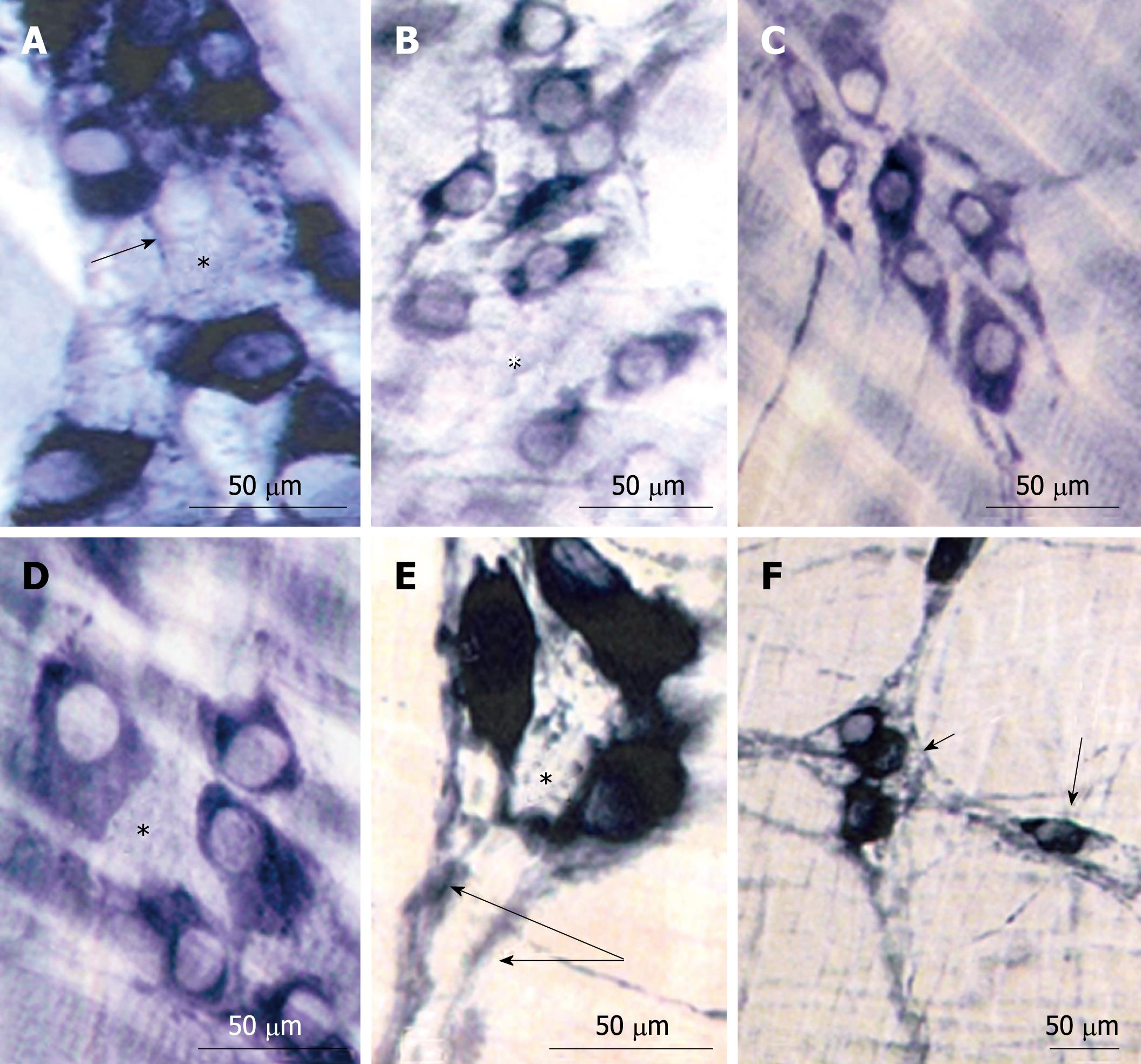
The reactivity for NADPH-diaphorase was detected in both neurons and meshes from the MP. The space inside the myenteric ganglia of all groups (N42, D42, R42) was occupied by non-nitrergic neurons (Figure 2). The cytoplasm from most of the neurons from the N42 group exhibited intense NADPH-diaphorase staining with only a few showing weaker staining (Figure 2A and D). The variability of intensity distinguished the cytoplasm of the myenteric neurons from the D42 and R42 groups, however, more neurons from the R42 group exhibited darker staining (Figure 2B and C). The intra- and interganglionar meshes were detected in all groups (Figure 2A, E and F).
The myenteric ganglia from all groups contained neurons either strongly or weakly reactive for AChE. Qualitatively, the reactivity was diffuse in the neurons from the D42 and R42 groups (Figure 3A, C and E).
The immunohistochemical reaction for VIP revealed the presence of large and small varicosities around the shadows of the neuron perikarya. The low density of these structures in ganglia of D42 animals was evident. No apparent differences were observed between the N42 and R42 groups concerning these structures (Figure 3B, D and F).
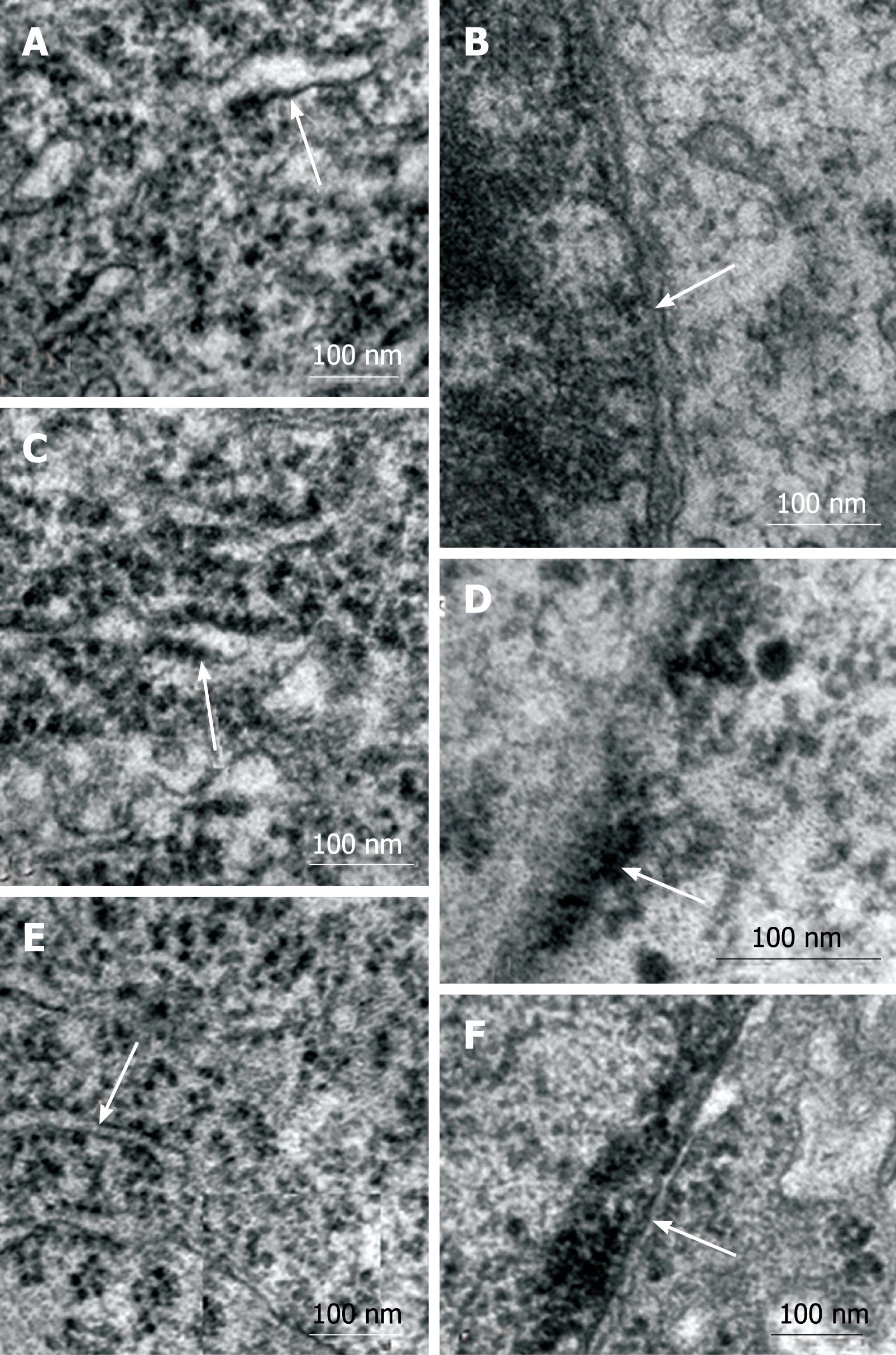
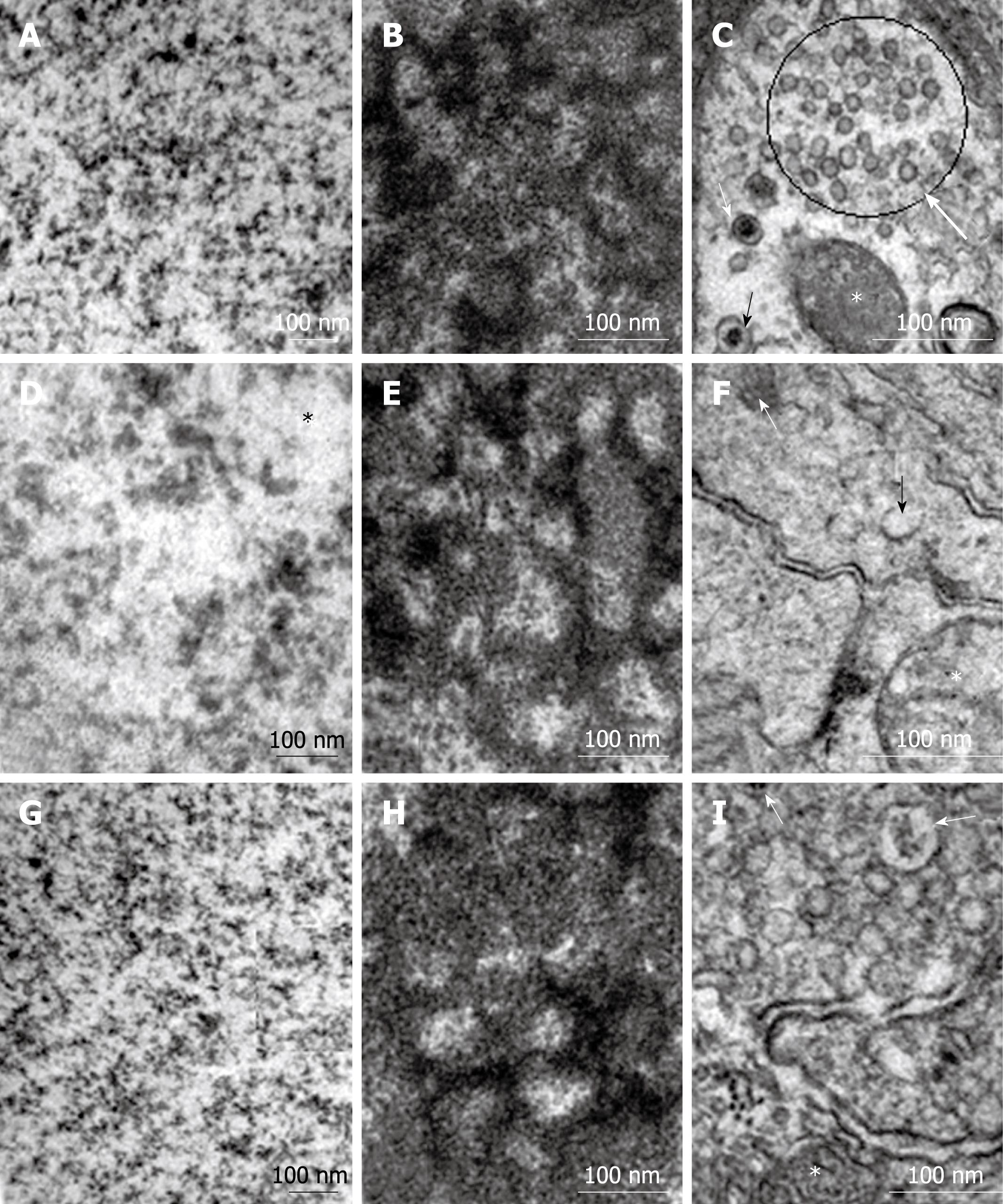
Specific organelles of the myenteric neurons were selected for comparative analysis using TEM. The granular reticulum from all groups exhibited ribosomes aligned on the outer surface of the regularly arranged membrane of the cisternae (Figure 4A, C and E). The nuclear double membrane and the perinuclear cisterna were well defined in the myenteric neurons from the N42 and R42 groups and the electron-dense nuclear chromatin was detected on the inner membrane of the nuclear envelope (Figure 4B and F). In several neurons of the D42 group, the double membrane of the nuclear envelope and the perinuclear cistern were not distinguishable (Figure 4D). The euchromatin was homogeneously dispersed in the nucleus of myenteric neurons from all groups (Figure 5A, D and G). No group-specific differences in the granular and fibrillar parts of the nucleoli were detected (Figure 5B, E and H). Agranular, large, and small granular vesicles were observed in the neurons from all groups. However, large granular vesicles were only delineated in the N42 group. In the D42 and R42 groups, many of the neurons had ill-defined membranes and showed a weak electron-dense content (Figure 5C, F and I).
In all groups, most of the NADH-stained neurons had perikarya that ranged in cross-sectional area from 200 to 400 μm2 (percentage of neurons in that range: 62%, 78% and 59%, respectively) with the following respective mean ± SD: 375 ± 169, 323 ± 134 and 393 ± 133 μm2. The perikarya of nitrergic neurons (NADPH+) also ranged from 200 to 400 μm2 for the N42, D42 and R42 groups (66%, 67%, 62%, respectively), with the following respective mean ± SD: 376 ± 118, 366 ± 132 and 382 ± 117 μm2. No statistically significant differences were detected among any of the groups with respect to the neuronal area profiles for the NADH and NADPH reactions.
In our previous work, we examined the effects of malnutrition on the MP of diverse regions of the gut in 21-d-old weanling rats subjected to pre- and postnatal protein deprivation using diverse time-honored histochemical methods[1-3,23-25]. We observed a decrease of approximately 15% in the cross-sectional areas of neurons in the colon of malnourished rats relative to normal-fed controls[1]. Average perikaryon area of myenteric neurons in the small intestine of malnourished animals was approximately half that observed for the normally fed controls. Moreover, most of the neurons that were reactive to NADH-diaphorase from malnourished animals showed weaker cytoplasmic staining[2]. In the esophagus, perikaryon area of myenteric neurons did not differ significantly between normally fed and protein-deprived animals. Nevertheless, the reactivity for AChE was clearly reduced in neurons from protein-deprived animals[3].
At a later stage of MP development (42 d old), we observed less NADH-diaphorase staining in large intestinal neurons from protein-deprived rats than in the controls, although the average size of the nerve cell perikaryon area was not significantly different from that of normally fed animals[1]. In the small intestine of rats of the same age, there was no detectable difference in the average perikaryon area of myenteric neurons between normally fed and protein-deprived animals. However, AChE histochemical activity and choline acetyltransferase (ChAT) immunoreactivity showed that most ganglionic neurons from the protein-deprived animals were unstained or moderately stained[4]. Similar aspects were detected in the MP from the duodenum of 90-d-old rats given protein and vitamin B complex. The effects did not alter the mean perikaryon area but did considerably reduce the reactivity of the myenteric neurons for NADPH-diaphorase (nitrergic neurons)[5].
The absence of neuron cell profile reduction in protein-deficient animals (D42) shown in the present study is in line with previously described data using the esophagus of 21-d-old protein-deficient animals[3]. This observation also fits with data from other viscera of the digestive tract, such as the small intestine, where the neuron cell profiles were similar in 42-d-old nourished, malnourished and protein-recovered rats[4]. However, the myenteric neurons from the large intestine of protein-deprived 42-d-old rats had a smaller mean perikaryon area than that normally found in fed and protein-recovered animals[4]. This finding corroborates data from 3-mo-old rats subjected to inadequate nutrition over a long time period. A decrease in the perikaryon area of myenteric neurons has been verified in the duodenum and ascending colon of these animals[26,27]. Mean area of neuronal perikarya does not change with age[28,29]. However, exposure of the myenteric neurons to other factors, such as dietary sodium intake and chagasic infection, effectively causes an irreversible hypertrophy of the neuron perikaryon area[30,31]. Although myenteric neurons may react differently depending on the extrinsic (i.e. dietary) and/or intrinsic (i.e. aging) factors they are exposed to, early protein recovery can reestablish their mean perikaryon area[1,4]. This has also been recently confirmed in the CNS (personal communication from Dr. Liberti EA).
Most of the reactive neurons in the D42 and R42 groups exhibited a diffuse pattern of AChE staining with a predominance of weakly reactive cells in the D42 group. Similar findings were verified in myenteric neurons from the colon of a chagasic mouse[32] and this fits with our previous observations from esophagi of 21-d-old malnourished animals. In that study, we only observed a few myenteric neurons that showed intense AChE staining in protein-deprived animals[3]. In the present study, although the myenteric neurons from the N42 and R42 animals had similar mean perikaryon areas assessed with AChE staining, the pattern of reactivity of neurons from the R42 group resembled that of the D42 group. This result is not in accordance with observations from the small intestine. In the small intestine, we have detected the same pattern of reactivity for nourished and protein-recovered animals when the myenteric neurons are immunohistochemically stained for ChAT[4].
We observed similar patterns of VIP immunoreactivity in R42 and N42 cells. In addition, the pattern of NADH and NADPH reactivity of myenteric neurons was similar to that of AChE. However, the homogeneity of the reaction for NADH detected in the cytoplasm of most neurons from the N42 group was not totally recovered in neurons of the R42 group. Moreover, although the R42 group exhibited many intensely staining neurons for NADPH, their presence was far less frequent than that for the N42 group. The ultrastructural aspects of the large granular vesicles support this assertion. Although some neurons from the R42 group exhibited electron-dense areas, most of them clearly had less dense content. In consideration of the current study, it is possible that different enteric neurotransmitter systems respond differently to the protein-recovery regimen.
The deleterious effect of protein deficiency on myenteric neurons of the esophagus has not been previously considered. Our data from protein-deprived (D42) and protein-recovered (R42) animals suggest that protein deficiency retards neuronal maturation in a manner that is to some extent, but far from completely recovered with 20 d of protein reinstatement.
Malnutrition affects the myenteric plexus (MP) of the gastrointestinal tract in different manners according to location. Definite changes in neuronal population, morphometry and morphology have been described.
It is not known whether morphometrical and morphological parameters return to normal after adequate refeeding, that is, whether modifications induced by malnutrition are permanent.
The present study shows that postnatal refeeding of perinatally malnourished rats restores almost all of the normal characteristics of the neurons of the MP of the esophagus.
The present study suggests that adequate nutrition can restore almost all of the changes induced by malnutrition on MP neurons. Changes in malnourished individuals should thus be reverted with adequate nutrition with hopefully significant clinical impact.
The manuscript by Dr. Greggio and co-workers describes the evaluation of myenteric neurons from esophagi of young rats. They investigated normal, protein-deprived and protein-recovered groups of rats. This is an extension to studies previously done by this group and others, and an important aspect previously not done is the investigation of whether the structural and ultrastructural characteristics of the MP of the esophagus are reversible. The manuscript is in general very clearly written and the data are convincing.
Peer reviewer: Dr. Claudia Zwingmann, PhD, Professor, Department of Medicine, University of Montreal, Centre de Recherche, 264 Rene-Levesque Est, Montreal, QC, H2X 1P1, Canada
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH
| 1. | Castelucci P, de Souza RR, de Angelis RC, Furness JB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat large intestine: a quantitative morphological study. Cell Tissue Res. 2002;310:1-7. |
| 2. | Brandão MCS, De Angelis RC, De-Souza RR, Fróes LB, Liberti EA. Effects of pre- and postnatal protein energy deprivation on the myenteric plexus of the small intestine: a morphometric study in weanling rats. Nut Res. 2003;23:215-223. |
| 3. | Liberti EA, Fontes RB, Fuggi VM, Maifrino LB, Souza RR. Effects of combined pre- and post-natal protein deprivation on the myenteric plexus of the esophagus of weanling rats: a histochemical, quantitative and ultrastructural study. World J Gastroenterol. 2007;13:3598-3604. |
| 4. | Gomes OA, Castelucci P, de Vasconcellos Fontes RB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat small intestine: a quantitative morphological study. Auton Neurosci. 2006;126-127:277-284. |
| 5. | Mello ST, Liberti EA, Sant'Ana DMG, Miranda Neto MH. The myenteric plexus in the duodenum of rats with deficiency of protein and vitamin B complex: a morpho-quantitative study. Acta Scientiarum. 2004;26:251-256. |
| 6. | Wiggins RC, Fuller GN, Brizzee L, Bissel AC, Samorajski T. Myelination of the rat optic nerve during postnatal undernourishment and recovery: a morphometric analysis. Brain Res. 1984;308:263-272. |
| 7. | Rana SV, Chopra JS, Mehta S, Chand UK, Sharma U. Electrophysiological and histopathological changes in peripheral nerves of postnatally undernourished and rehabilitated young rhesus monkeys. Indian J Exp Biol. 1991;29:385-390. |
| 8. | Andrade JP, Madeira MD, Paula-Barbosa MM. Effects of long-term malnutrition and rehabilitation on the hippocampal formation of the adult rat. A morphometric study. J Anat. 1995;187:379-393. |
| 9. | Cordero ME, Valenzuela CY, Rodriguez A, Aboitiz F. Dendritic morphology and orientation of pyramidal cells of the neocortex in two groups of early postnatal undernourished-rehabilitated rats. Brain Res Dev Brain Res. 2003;142:37-45. |
| 10. | Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939-1951. |
| 11. | Fontes RB, Froes LB, Omar ED, Liberti EA. The myenteric plexus of the rat colon after fecal stream diversion: a morpho-quantitative study. Auton Neurosci. 2004;114:39-46. |
| 12. | Gabella G. Detection of nerve cells by a histochemical technic. Experientia. 1969;25:218-219. |
| 13. | Gabella G. The number of neurons in the small intestine of mice, guinea-pigs and sheep. Neuroscience. 1987;22:737-752. |
| 14. | Christensen J, Fang S. Colocalization of NADPH-diaphorase activity and certain neuropeptides in the esophagus of opossum (Didelphis virginiana). Cell Tissue Res. 1994;278:557-562. |
| 15. | Santer RM. Survival of the population of NADPH-diaphorase stained myenteric neurons in the small intestine of aged rats. J Auton Nerv Syst. 1994;49:115-121. |
| 16. | Karnovsky MJ, Roots L. A "Direct-coloring" thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219-221. |
| 17. | Baker DG, McDonald DM, Basbaum CB, Mitchell RA. The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol. 1986;246:513-526. |
| 18. | Mizuno MS, Pompeu E, Castelucci P, Liberti EA. Age-related changes in urinary bladder intramural neurons. Int J Dev Neurosci. 2007;25:141-148. |
| 19. | Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147-161. |
| 20. | Cotta-Pereira G, Rodrigo FG, David-Ferreira JF. The use of tannic acid-glutaraldehyde in the study of elastic and elastic-related fibers. Stain Technol. 1976;51:7-11. |
| 21. | Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208-212. |
| 22. | Zar JH. Biostatistical analysis. 4th ed. New Jersey: Prentice-Hall 1999; 80-83. |
| 23. | Maifrino LB, Liberti EA, de Souza RR. Vasoactive-intestinal-peptide- and substance-P-immunoreactive nerve fibres in the myenteric plexus of mouse colon during the chronic phase of Trypanosoma cruzi infection. Ann Trop Med Parasitol. 1999;93:49-56. |
| 24. | Maifrino LB, Prates JC, De-Souza RR, Liberti EA. Morphometry and acetylcholinesterase activity of the myenteric plexus of the wild mouse Calomys callosus. Braz J Med Biol Res. 1997;30:627-632. |
| 25. | Liberti EA, Gaspar LP, de Carvalho CA, Fujimura I, de Souza RR. A morpho-quantitative study of the myenteric ganglia throughout the human digestive tract. Rev Hosp Clin Fac Med Sao Paulo. 1998;53:55-60. |
| 26. | Natali MR, Miranda-Neto MH. Effects of maternal proteic undernutrition on the neurons of the myenteric plexus of the duodenum of rats. Arq Neuropsiquiatr. 1996;54:273-279. |
| 27. | Sant’ana Dde M, Miranda Neto MH, de Souza RR, Molinari SL. Morphological and quantitative study of the myenteric plexus of the ascending colon of rats subjected to proteic desnutrition. Arq Neuropsiquiatr. 1997;55:687-695. |
| 28. | Gomes OA, de Souza RR, Liberti EA. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43:210-217. |
| 29. | Alves N, Liberti EA. Morphoquantitative study of aging of the guinea pig’s myenteric plexus colon. Rev Chil Anat. 2002;20:125-130. |
| 30. | De Souza RR, Gama EF, Silva RD, Heimann JC, Maifrino LB, Liberti EA. Dietary sodium intake induced myenteric neuron hypertrophy in Wistar rats. Braz J Med Biol Res. 2000;33:847-850. |
| 31. | Maifrino LB, Amaral SO, Watanabe I, Liberti EA, De Souza RR. Trypanosoma cruzi: preliminary investigation of NADH-positive and somatostatin-immunoreactive neurons in the myenteric plexus of the mouse colon during the infection. Exp Parasitol. 2005;111:224-229. |
| 32. | Maifrino LB, Liberti EA, Watanabe I, De Souza RR. Morphometry and acetylcholinesterase activity of the myenteric neurons of the mouse colon in the chronic phase of experimental Trypanosoma cruzi infection. Am J Trop Med Hyg. 1999;60:721-725. |