Published online Dec 28, 2010. doi: 10.3748/wjg.v16.i48.6145
Revised: September 26, 2010
Accepted: October 3, 2010
Published online: December 28, 2010
AIM: To investigate the effect of pegylated interferon (IFN) α-2b on specific CD8+ T lymphocytes in patients with chronic hepatitis B (CHB).
METHODS: Twenty-one patients with CHB were treated with pegylated IFN α-2b. Periphery blood mononuclear cells were isolated from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation (density: 1.077 g/L, Pharmingen) at weeks 0, 4, 8, 12, and 24, respectively. Frequency of circulating hepatitis B virus (HBV) epitope-specific CD8 T cells was detected by flow cytometry. Cytokines were detected by cytometric bead assay.
RESULTS: The frequency of circulating HBV core or env-specific CD8 T cells was higher (P < 0.05), the number of HBV core specific CD8 T cells was greater at week 24 (P < 0.05), the level of Th1-type cytokines [interleukin (IL)-12, tumor necrosis factor-α, and IFN-γ] was higher, while that of Th2-type cytokines (IL-4, IL-6, and IL-10) was lower in responders than in non-responders (P < 0.05) after pegylated IFN α-2b treatment. The IL-6 level was correlated with HBV DNA (r = 0.597, P = 0.04), while the inducible protein-10 (IP-10) level was correlated with serum alanine aminotransferase (ALT) (r = 0.545, P = 0.005). The IP-10 level at week 8 after pegylated IFN α-2b treatment could predict the normalization of ALT in CHB patients (positive predict value = 56%, negative predict value = 92%).
CONCLUSION: Pegylated IFN α-2b can enhance the immune response of CHB patients by increasing the frequency of HBV specific CD8+ T cells and regulating the Th1/Th2 cytokines.
- Citation: Chen J, Wang Y, Wu XJ, Li J, Hou FQ, Wang GQ. Pegylated interferon α-2b up-regulates specific CD8+ T cells in patients with chronic hepatitis B. World J Gastroenterol 2010; 16(48): 6145-6150
- URL: https://www.wjgnet.com/1007-9327/full/v16/i48/6145.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i48.6145
More than two billion people have been infected with hepatitis B virus (HBV) and chronic HBV infection affects about 400 million people worldwide[1,2]. Chronic hepatitis B (CHB) is a chronic inflammatory liver disease, which can progress to end-stage liver diseases, such as cirrhosis and hepatocellular carcinoma.
Adaptive immunity plays a central role in the pathogenesis of chronic HBV infection, and it is crucial to understanding the behavior of T cell response for the design of effective strategies for the control of HBV infection[3-5]. Different studies in chronic and early acute phases of HBV infection suggested that the functional impairment of HBV-specific cell-mediated immune response plays an important role in HBV persistence[6-14]. Moreover, recent studies showed that both positive and negative signals regulate the antigen-specific T cell function and are important for the better outcome of patients with HBV infections[15-17].
Pegylated interferon (IFN) α-2b can modulate and reduce antiviral function of CHB patients by enhancing their immune responses. However, the exact effect of pegylated IFN α-2b on the immune responses of patients with HBV infections remains unclear. The present study was designed to investigate the effect of pegylated IFN α-2b on HBV specific CD8+ T cells and secretion of cytokines in CHB patients.
Twenty-one consecutive CHB patients (17 males and 4 females) at the age of 20-39 years (mean 25 years), admitted to our hospital from January 2008 to May 2009 were included in this study. Diagnosis of HBV infection was established as previously described[18]. Clinical data and characteristics of the patients are summarized in Table 1. The patients were treated with pegylated IFN α-2b (PegIntron from Schering-Plough), at the dose of 0.5-1 μg/kg of body weight, once a week for 24 wk. Clinical and laboratory data about the patients were detected before treatment, or at weeks 4, 8, 12, and 24 after treatment. Patients co-infected with HBV and HCV or with detectable antibodies against hepatitis delta virus or against human immunodeficiency virus were excluded, as were those with other causes of liver disease, including alcohol abuse. No patient had decompensated liver disease (evidence or history of ascites, variceal bleeding, hepatic encephalopathy or jaundice).
| Patient | Age (yr)/sex | HBV DNA (IU/L) | ALT (U/L) | Total bilirubin (mg/dL) | Albumin (g/dL) | Platelets (× 109/L) | HBeAg | HBeAb | HBsAg | HBsAb | Genetype |
| 1 | 27/M | 201 000 000 | 143 | 9.1 | 43.7 | 113 | + | - | + | - | C |
| 2 | 25/M | 160 000 000 | 147 | 12.9 | 47.4 | 167 | + | + | + | - | C |
| 3 | 30/M | 471 000 000 | 205 | 11.9 | 44.3 | 127 | + | - | + | - | C |
| 4 | 21/M | 32 200 000 | 123 | 10.8 | 45.8 | 181 | + | - | + | - | C |
| 5 | 21/M | 186 000 000 | 148 | 14.2 | 44.1 | 110 | + | - | + | - | C |
| 6 | 38/F | 30 800 000 | 347 | 18.1 | 45.5 | 126 | + | - | + | - | C |
| 7 | 20/F | 29 000 000 | 171 | 10.0 | 44.9 | 284 | + | - | + | - | C |
| 8 | 20/M | 143 000 000 | - | 13.6 | 48.0 | 248 | + | - | + | - | B |
| 9 | 20/F | 621 000 000 | 112 | 13.4 | 48.5 | 170 | + | - | + | - | C |
| 10 | 23/M | 597 000 000 | 196 | 18.9 | 43.2 | 201 | - | + | + | - | C |
| 11 | 20/F | 2 910 000 | 98 | 15.6 | 48.0 | 137 | + | + | + | - | C |
| 12 | 38/M | 63 700 000 | 206 | 11.1 | 51.2 | 142 | + | + | + | - | C |
| 13 | 28/M | 134 000 000 | 138 | 9.0 | 45.9 | 174 | + | + | + | - | C |
| 14 | 25/M | 237 000 000 | 93 | 16.9 | 51.3 | 130 | + | - | + | - | B |
| 15 | 39/M | 1 190 000 000 | 122 | 18.2 | 50.6 | 166 | + | - | + | - | C |
| 16 | 36/M | 8 820 000 | 170 | 22.9 | 47.1 | 169 | + | - | + | - | C |
| 17 | 25/M | 655 000 000 | 90 | 20.9 | 46.8 | 161 | + | - | + | - | B |
| 18 | 25/M | 157 000 000 | 164 | 24.3 | 47.3 | 209 | - | + | + | - | C |
| 19 | 20/M | 290 000 000 | 124 | 11.9 | 47.8 | 140 | + | - | + | - | B |
| 20 | 23/M | 655 000 000 | 19.9 | 44.6 | 194 | + | - | + | - | B | |
| 21 | 28/M | 15 300 000 | 237 | 14.5 | 45.3 | 154 | + | - | + | - | C |
Peripheral blood monouclear cells (PBMC) were isolated from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation (density: 1.077 g/L, Pharmingen). Blood was two-fold diluted with RPMI 1640 medium containing 300 μg/mL L-glutamin, 100 U/mL penicillin, 100 μg/mL streptomycin and 10% fetal calf serum, then added into the isovolumic Ficoll, centrifuged for 400 ×g at 21°C for 35 min. The cells were washed twice with phosphate buffered saline (PBS).
One hundred microliters of fresh heparinized blood (100) was incubated with human leukocyte antigen-A2 primary antibody for 30 min. Erythrocytes were lysed with an erythrocyte lysate at 37°C, washed with PBS, and then incubated with secondary antibody, washed again and analyzed on Becton Dickinson FACS (Becton Dickinson, USA).
Frequency of HBV epitope-specific CD8 T cells was detected by flow cytometry after incubated with HBV core18-27 tetramers (ProImmune, Oxford, UK) and HBV env 335-343 pentamers (ProImmune, Oxford, UK). Freshly isolated PBMC were incubated with PE-labeled tetramer or pentamer in PBS (10% FCS) for 15 min at 37°C, washed once with PBS (1% FCS) and then incubated on ice for 30 min with FITC-anti-CD8 (ProImmune, Oxford, UK), washed twice with PBS, adjusted to 1 × 106 cells/vial, and fixed in 2% paraformaldehyde for analysis. About 1 × 106 PBMC were harvested and analyzed within the CD8 gate on Becton Dickinson FACS using the CELLQuest™ software.
Serum levels of interleukin (IL)-2, IL-4, IL-5, IL-6, IL-10, IL-12, IFN-γ and inducible protein-10 (IP-10) in CHB patients were measured by cytometric bead assay (BD, USA) according to its manufacturer’s instructions.
Fasting serum levels of liver enzymes [alanine aminotransferase (ALT), aspartate aminotransferase] were measured with a Hitachi-7180 automatic biochemistry analyzer (Hitachi Inc., Japan) following the standard laboratory methods. HBV DNA was detected by real time polymerase chain reaction (Amplicor, Roche).
All data were analyzed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Descriptive baseline data were expressed as mean ± SD for continuous variables. Differences between groups were assessed using Kruskal-Wallis H for continuous variables. Spearman P test was performed for correlation analysis. The accuracy of serum factors for predicting virologic response was assessed using the receiver operating characteristic curve. P < 0.05 was considered statistically significant.
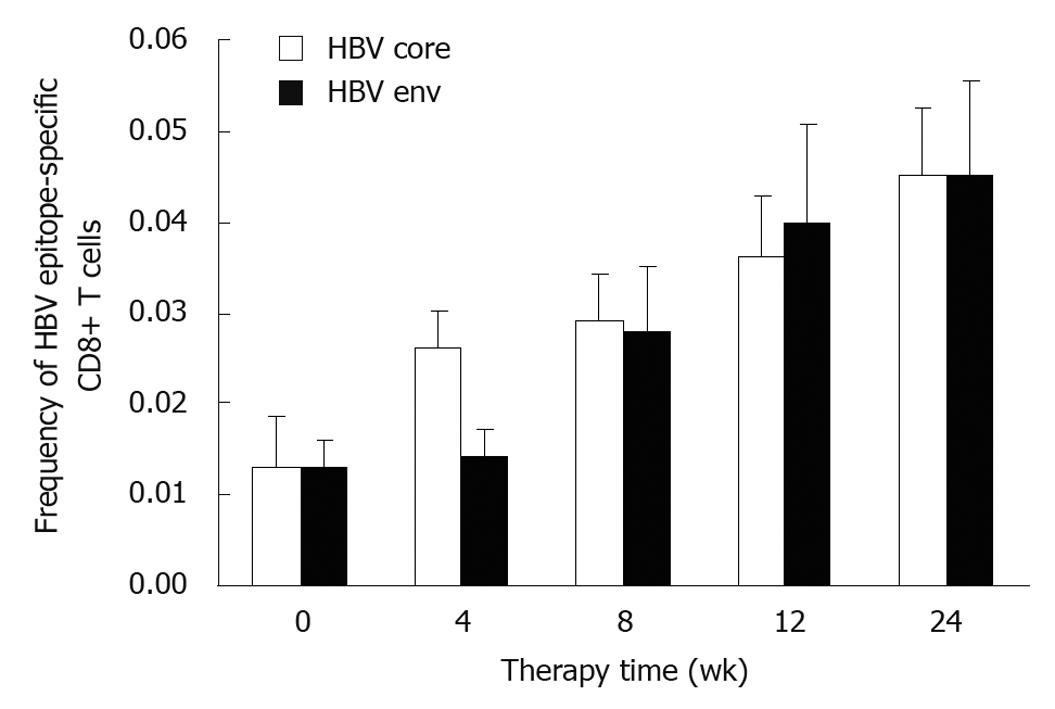
Circulating HBV epitope-specific CD8 T cells were detected 13 out of the 21 CHB patients (Table 1). The frequency of HBV core 18-27 tetramers+/CD8+ T cells at week 0 was 0.013 ± 0.002, which increased to 0.026 ± 0.015, 0.029 ± 0.019, 0.036 ± 0.025, and 0.045 ± 0.027, respectively, at weeks 4, 8, 12, and 24 after IFN α-2b treatment (Figure 1), with a significant difference between weeks 8 and 0, and between weeks 24 and 0 (P < 0.05). The frequency of HBV env 335-343 pentamers+/CD8+ T cells began to increase at week 8 with a significant difference between weeks 24 and 0 (P < 0.05). No significant difference was observed in frequency of HBV core and HBV env specific CD8 T cells.
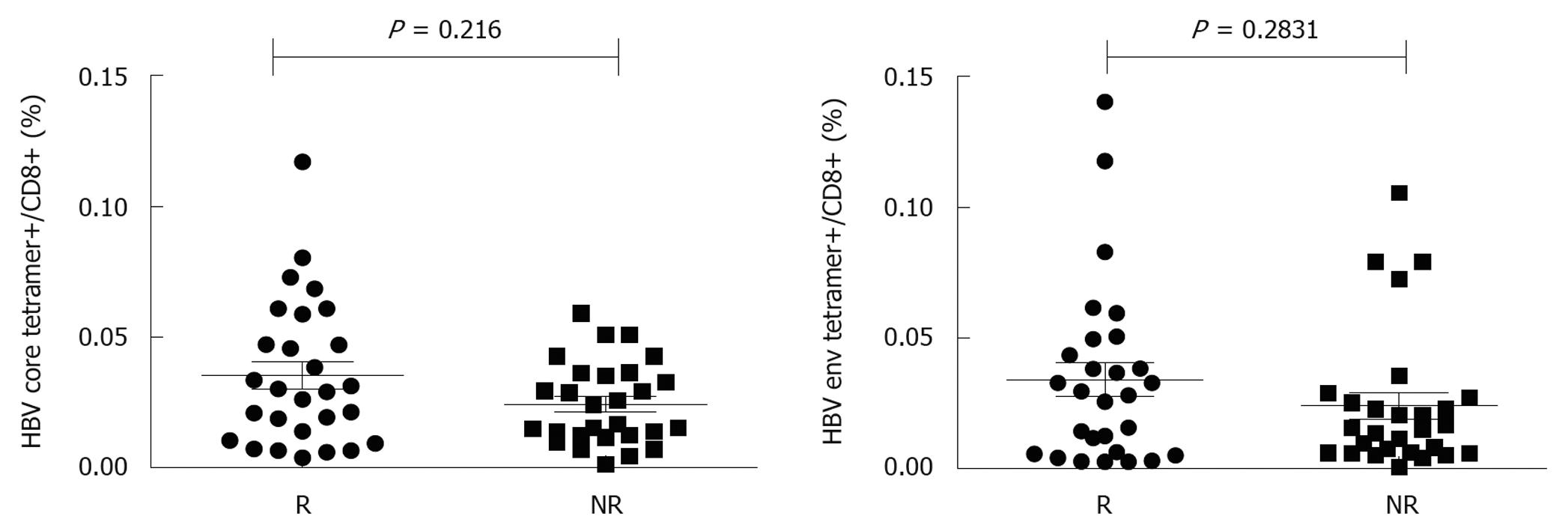
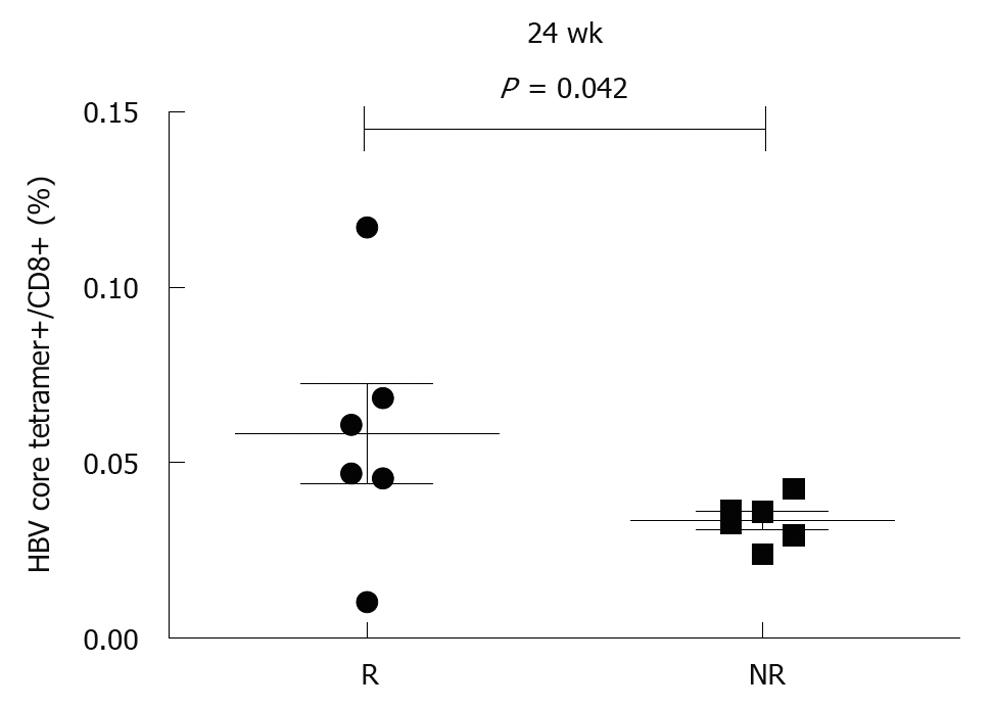
To further analyze the effect of pegylated IFN α-2b on HBV-specific CD8 T cells, 13 patients were divided into responders (n = 7) and non-responders (n = 6). Responders were defined as their ALT returned to its normal level and their HBV DNA was decreased to over 2log, and/or their serum HBeAg was conversed. The frequency of HBV core18-27 tetramers+/CD8+ T cells was 0.014 ± 0.011, 0.029 ± 0.022, 0.029 ± 0.021, 0.067 ± 0.029, and 0.05 ± 0.025, respectively, in responders at weeks 0, 4, 8, 12 and 24 after treatment, which was higher than that in non-responders (0.012 ± 0.007, 0.018 ± 0.009, 0.028 ± 0.019, 0.025 ± 0.021 and 0.030 ± 0.01, respectively). No significant difference was found in frequency of HBV core specific CD8 T cells between responders and non-responders at baseline, even at weeks 4, 8, and 12 after treatment (Figure 2), with a significant difference observed at week 24 (P < 0.05, Figure 3). The frequency of HBV env specific CD8 T cells was higher in responders than in non-responders (P < 0.05, Figure 2).
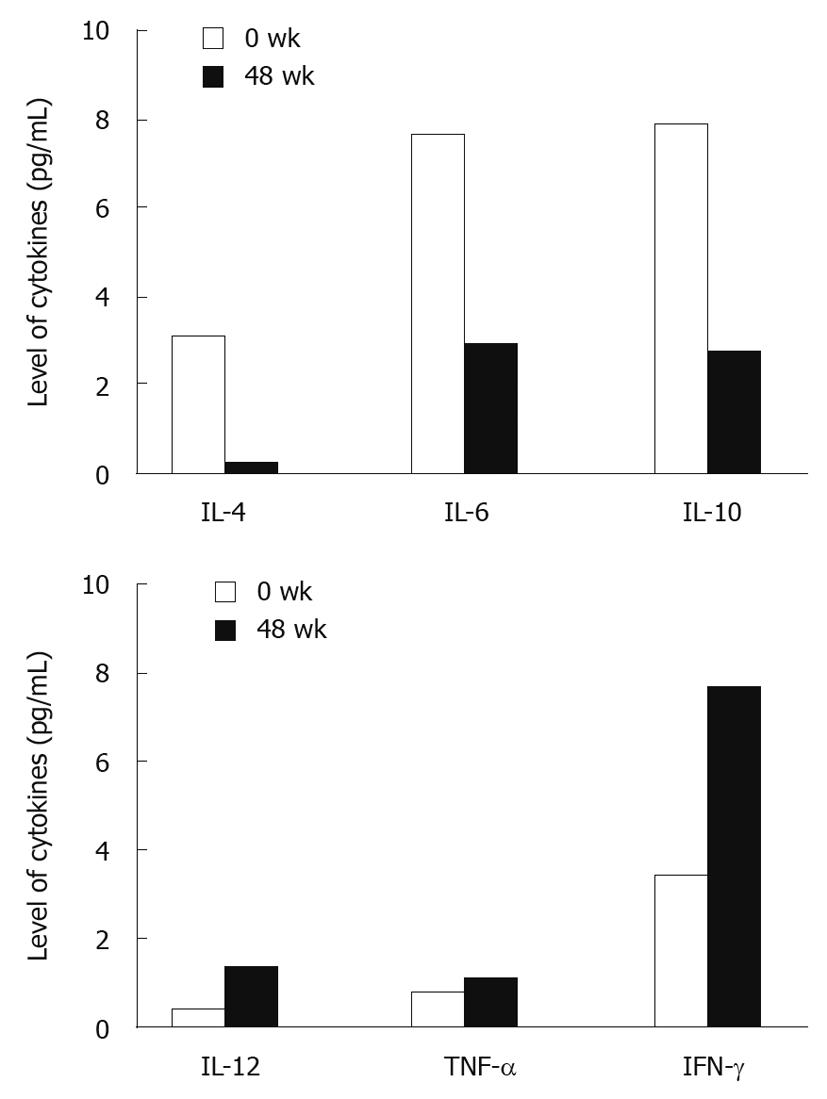
The serum levels of IL-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, IFN-γ, IL-12, and IP-10 were measured at baseline, during the treatment and follow-up. The serum IL-2 level was very low in CHB patients, which was almost undetectable. The levels of Th1-type cytokines including IL-12, TNF-α and IFN-γ were increased while those of Th2-type cytokines including IL-4, IL-6 and IL-10 were decreased at week 48 after treatment (Figure 4). The baseline IP-10 level was increased from week 4 and decreased from week 48 after treatment.
The baseline IL-6 level was correlated with HBV DNA in responders (r = 0.597, P <0.05) but not with HBV DNA in non-responders. IL-10 was correlated with IL-6 (r = 0.762, P = 0.002), and IL-12 was correlated IFN-γ (r = 0.485, P = 0.026).
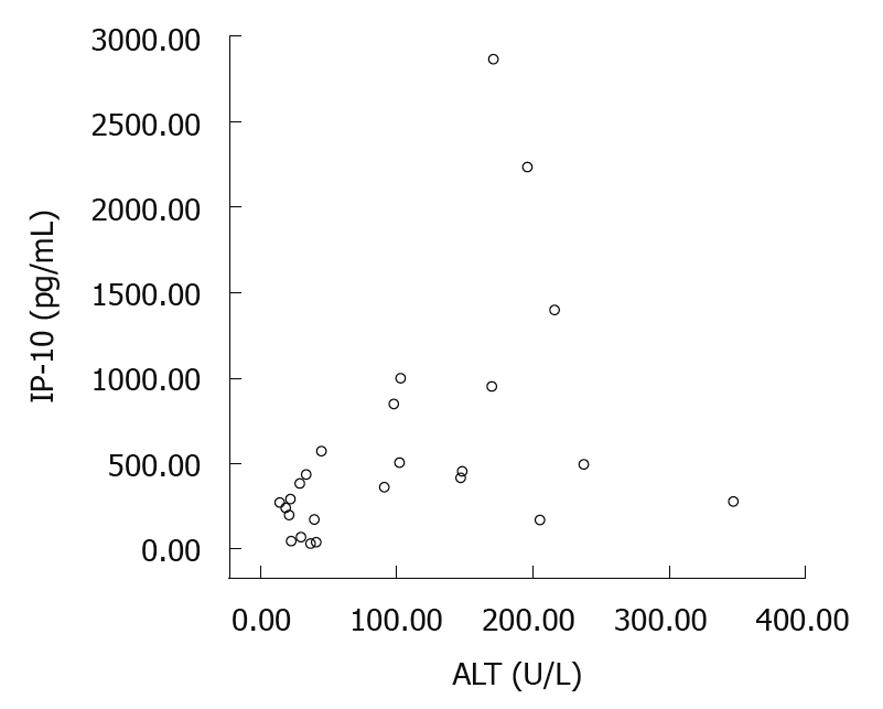
The IP-10 level was closely correlated with the serum ALT level not only in responders but also in non-responders (r = 0.545, P = 0.005, Figure 5), indicating that IP-10 level fluctuates with serum ALT level. The baseline IP-10 level was lower in patients with their ALT < 40 U/L than in those with their ALT > 40 U/L.
To determine whether IP-10 can predict the normalization of ALT (< 40 U/L) after peg-IFN α-2b treatment, receiver operating characteristic curve was plotted for IP-10. The IP-10 level at week 8 after treatment was predictable. The area under the curve was 0.741 (P = 0.065). A cutoff value of 437.78 was chosen. Correspondingly, the positive and negative predictive value was 56% and 92%, respectively (Table 2).
| IP-10 (pg/mL) | ALT < 40 U/L | ALT ≥ 40 U/L | Predictive value |
| < 437.78 | 5 | 4 | PPV = 56% |
| ≥ 437.78 | 1 | 11 | NPV = 92% |
HBV has a high propensity to persist and several strategies have been developed for control of its evading from T cell responses, including the direct inhibitory effect of viral proteins on T cell responses and the emergence of escape mutations[19-21]. Moreover, HBV infection is more common in immune deficient individuals, such as infants, patients with cancer and those treated with steroid hormone, thereby can interfere with viral clearance by the innate immune system[22,23]. Inefficient innate responses and rapid spread of HBV may in turn delay and impair adaptive responses because of inefficient promotion of T cell priming by innate immunity and through T cell exhaustion induced by a rapidly increased viral load. However, the actual impact of exhaustion by persistent exposure to high antigen concentrations on virus persistence has only been partially defined.
Furthermore, two kinds of drugs (nucleoside analogs and IFN) are usually used in antiviral treatment of CHB patients. IFN is involved in numerous immune interactions during viral infection, as an inducer, regulator, and effector of both innate and adaptive antiviral systems. IFN-α and beta are produced rapidly due to viral factors, such as envelope glycoprotein, CpG DNA or dsRNA, and interact with cellular pattern-recognition receptors, such as mannose receptors, toll-like receptors, and cytosolic receptors[24]. In addition, IFN modulates both innate and adaptive immunity, ultimately resulting in an enhanced antiviral effector function.
In the present study, the frequency of HBV epitope-specific CD8+ T cells in peripheral blood was persistently increased at weeks 4, 8, 12 and 24 after peg-IFN α-2a treatment, while the number of HBV epitope-specific CD8 T cells in HBV core 18-27 tetramers and HBV env 335-343 pentamers was greater in responders than in non-responders after pegylated IFN α-2b treatment, suggesting that the therapeutic effect of pegylated IFN α-2b on HBV infection may be attributed to the elevated HBV-specific CD8 T cells, and that the immune response mediated by HBV-specific cells plays an important role in control of HBV. However, the frequency of HBV core 18-27 tetramers+/CD8+ T cells was higher than that of HBV env 335-343 pentamers+/CD8+ T cells after pegylated IFN treatment, suggesting that the HBV core epitope plays a more critical role in induction of a stronger immune response to HBV infection than to HBV env epitope. Pegylated IFN α-2b could enhance specific immune response of CHB patients. Further study should be performed with a large sample size.
Cytokines play an important role in immune modulation. Clearance of HBV infection is mediated by a strong polyclonal cellular response of both CTL and Th1 cells. Chronic HBV infection is caused mainly by an increased response of Th2 cells and impaired production of type 1 cytokines. IL-10, a Th2-type cytokine secreted by T-cells, activated B cells and monocytes, is a powerful inhibitor of Th1 activation and suppresses cell-mediated immunity in mice and humans[25,26]. Of the detected cytokines, Th2-type cytokines such as IL-4, IL-6 and IL-10, were altered conspicuously. After treatment, the level of Th2-type cytokines (IL-4 and IL-10) was down-regulated, thus confirming the immune recover potential of pegylated IFN α-2b, the level of IL-12 which can promote the differentiation of Th1-type cytokines was low, and the production of Th1-type cytokines was increased, indicating that the immune function of pegylated IFN α-2b can be achieved by regulating the balance of Th1/Th2 cytokines.
IL-6 is a multifunctional cytokine with both differentiation and growth-promoting effects for a variety of target cells. IL-6 is generally considered an important cytokine in the network of cytokines that regulate immune reactions and acute phase responses[27]. It was reported that IL-6 is correlated with liver fibrosis/cirrhosis[28] and is a cell attachment site for HBV[29]. In the present study, the IL-6 level was correlated with HBV DNA plasma only in responders.
IP-10, a chemotactic CXC chemokine of 77 aa in its mature form[30,31], can be produced by a variety of cells, including hepatocytes[32,33]. The correlation between IP-10 levels and necroinflammatory activity, as well as the high and low IP-10 levels before and after pegylated IFN α-2b treatment, may imply that IP-10 plays a role in the natural pathogenesis of HBV-induced liver damage[34]. It was reported that the baseline IP-10 level can predictive the response of CHB patients to HCV treatment, and is correlated with liver inflammation and fibrosis[35,36]. In this study, the baseline IP-10 level in CHB patients could predict the normalization of ALT after pegylated IFN α-2b treatment.
In conclusion, given the importance of protective T cell responses in control of HBV, the correlation between immunomodulatory molecules and pegylated IFN α-2b treatment in restoration of the immune responses of antiviral T cells are highly desirable. Pegylated IFN α-2b therapy can enhance the immune response of CHB patients by influencing the production of cytokines. IP-10 can potentially predict the normalization of ALT, which is correlated with liver damage. Further study is needed with a large sample size.
More than two billion people have been infected with hepatitis B virus (HBV) and chronic HBV infection affects about 400 million people worldwide. Two kinds of drugs [nucleoside analogs and interferon (IFN)] are mainly used in treatment of chronic hepatitis B (CHB) patients. IFN is involved in numerous immune interactions as an inducer, regulator, and effector in treatment of viral infections. Cytokines play an important role in immune modulation. Clearance of HBV infection is mediated by a strong polyclonal cellular response of both CTL and Th1 cells. Chronic HBV infection is caused mainly by an increased response of Th2 cells and impaired production of type 1 cytokines. Inducible protein 10 (IP-10) is a chemotactic CXC chemokine of 77 aa in its mature form.
IFN-α and β are produced rapidly due to viral factors, such as envelope glycoproteins, CpG DNA or dsRNA, and interact with cellular pattern-recognition receptors, such as mannose receptors, toll-like receptors, and cytosolic receptors. IP-10 can be produced by a variety of cells, including hepatocytes. The results of this study show that the baseline IP-10 level can predict the response of patients with HBV infection to its treatment with pegylated IFN α-2b.
The present study demonstrated the correlation between pegylated IFN α-2b treatment and HBV-specific T lymphocytes. In addition, the effect of pegylated IFN α-2b on HBV infection could be achieved by balancing the production of Th1/Th2 cytokines and IP-10 could predict the outcome of patients with HBV infection after pegylated IFN α-2b treatment.
In this study, pegylated IFN α-2b could up-regulate HBV epitope specific CD8+ T cells. The specific cellular immune response could control HBV. IP-10 serum level could predict the outcome of patients with HBV infection after pegylated IFN α-2b treatment, thus providing a new index for the treatment of HBV infection. Pegylated IFN α-2b may be used as a novel strategy for the treatment of HBV infection by regulating the cytokines.
Human leukocyte antigen (HLA) typing is a method to define the HLA+ and HLA+ blood for studied subjects. Flow cytometry is used to define the HBV epitope specific CD8+ T lymphocytes. Cytometric bead assay is a new technique for detecting serum concentration of cytokines.
This is a very interesting study, showing that pegylated IFN α-2b therapy can increase the frequency of specific CD8+ T lymphocytes in CHB patients. This may contribute to the better control of HBV replication and to the recovery of CHB patients, thus having a promise for therapeutic interventions. The experiments support the claim of the authors.
Peer reviewer: Dr. Jeff Butterworth, MB, FRCP, Department of Gastroenterology, Shrewsbury and Telford Hospital NHS Trust, Mytton Oak Road, Shrewsbury, Shropshire, SY3 8XQ, United Kingdom
S- Editor Sun H L- Editor Wang XL E- Editor Lin YP
| 2. | Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486-1500. |
| 3. | Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117-1124. |
| 4. | Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68-76. |
| 5. | Grady GF, Lee VA, Prince AM, Gitnick GL, Fawaz KA, Vyas GN, Levitt MD, Senior JR, Galambos JT, Bynum TE. Hepatitis B immune globulin for accidental exposures among medical personnel: final report of a multicenter controlled trial. J Infect Dis. 1978;138:625-638. |
| 6. | Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, Cavalli A, Petit MA, Fiaccadori F. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442-3449. |
| 7. | Jung MC, Spengler U, Schraut W, Hoffmann R, Zachoval R, Eisenburg J, Eichenlaub D, Riethmüller G, Paumgartner G, Ziegler-Heitbrock HW. Hepatitis B virus antigen-specific T-cell activation in patients with acute and chronic hepatitis B. J Hepatol. 1991;13:310-317. |
| 8. | Penna A, Artini M, Cavalli A, Levrero M, Bertoletti A, Pilli M, Chisari FV, Rehermann B, Del Prete G, Fiaccadori F. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest. 1996;98:1185-1194. |
| 9. | Penna A, Del Prete G, Cavalli A, Bertoletti A, D'Elios MM, Sorrentino R, D'Amato M, Boni C, Pilli M, Fiaccadori F. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology. 1997;25:1022-1027. |
| 10. | Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047-1058. |
| 11. | Penna A, Chisari FV, Bertoletti A, Missale G, Fowler P, Giuberti T, Fiaccadori F, Ferrari C. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J Exp Med. 1991;174:1565-1570. |
| 12. | Jung MC, Hartmann B, Gerlach JT, Diepolder H, Gruber R, Schraut W, Grüner N, Zachoval R, Hoffmann R, Santantonio T. Virus-specific lymphokine production differs quantitatively but not qualitatively in acute and chronic hepatitis B infection. Virology. 1999;261:165-172. |
| 13. | Böcher WO, Herzog-Hauff S, Schlaak J, Meyer zum Büschenfeld KH, Löhr HF. Kinetics of hepatitis B surface antigen-specific immune responses in acute and chronic hepatitis B or after HBs vaccination: stimulation of the in vitro antibody response by interferon gamma. Hepatology. 1999;29:238-244. |
| 14. | Sobao Y, Tomiyama H, Sugi K, Tokunaga M, Ueno T, Saito S, Fujiyama S, Morimoto M, Tanaka K, Takiguchi M. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J Hepatol. 2002;36:105-115. |
| 15. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. |
| 16. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. |
| 17. | Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682-687. |
| 18. | Chinese Society of Infectious disease and Parasitology and Chinese Society of Hepatology of Chinese medical association. The programme of prevention and cure for viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |
| 19. | Baumert TF, Barth H, Blum HE. Genetic variants of hepatitis B virus and their clinical relevance. Minerva Gastroenterol Dietol. 2005;51:95-108. |
| 20. | Tong S. Mechanism of HBV genome variability and replication of HBV mutants. J Clin Virol. 2005;34 Suppl 1:S134-S138. |
| 21. | Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology. 2003;38:1075-1086. |
| 22. | Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215-229. |
| 23. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. |
| 24. | Malmgaard L. Induction and regulation of IFNs during viral infections. J Interferon Cytokine Res. 2004;24:439-454. |
| 25. | Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. |
| 26. | Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131-136. |
| 27. | Le JM, Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989;61:588-602. |
| 28. | Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77:221-225. |
| 29. | Neurath AR, Strick N, Sproul P. Search for hepatitis B virus cell receptors reveals binding sites for interleukin 6 on the virus envelope protein. J Exp Med. 1992;175:461-469. |
| 30. | Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672-676. |
| 31. | Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207-219. |
| 32. | Hua LL, Lee SC. Distinct patterns of stimulus-inducible chemokine mRNA accumulation in human fetal astrocytes and microglia. Glia. 2000;30:74-81. |
| 33. | Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, Marinos G, Lloyd AR. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360-369. |
| 34. | Wang J, Zhao JH, Wang PP, Xiang GJ. Expression of CXC chemokine IP-10 in patients with chronic hepatitis B. Hepatobiliary Pancreat Dis Int. 2008;7:45-50. |
| 35. | Romero AI, Lagging M, Westin J, Dhillon AP, Dustin LB, Pawlotsky JM, Neumann AU, Ferrari C, Missale G, Haagmans BL. Interferon (IFN)-gamma-inducible protein-10: association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895-903. |
| 36. | Zeremski M, Petrovic LM, Chiriboga L, Brown QB, Yee HT, Kinkhabwala M, Jacobson IM, Dimova R, Markatou M, Talal AH. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440-1450. |