PHYSIOLOGY OF LIVER BLOOD FLOW AND HEPATIC MACROHEMODYNAMICS
Hepatic blood flow and hepatic pressures
The liver has the most complicated circulation of any organ. According to the anatomical peculiarity of the double afferent blood supply of the liver, 75%-80% of the blood entering the liver is partially deoxygenated venous blood supplied by the portal vein, which collects all the blood that leaves the spleen, stomach, small and large intestine, gallbladder and pancreas[1-3]. The hepatic artery accounts for the remaining 25% with well-oxygenated blood. Total hepatic blood flow ranges between 800 and 1200 mL/min, which is equivalent to approximately 100 mL/min per 100 g liver wet weight[4]. Although the liver mass constitutes only 2.5% of the total body weight, the liver receives nearly 25% of the cardiac output.
The valveless portal vein is a low pressure/low resistance circuit, while the hepatic artery supplies the liver with arterial blood in a high pressure/high resistance system[4]. The mean pressure in the hepatic artery is similar to that in the aorta, while portal vein pressure has been reported to range between 6 and 10 mmHg in humans when determined by direct cannulation[5] or by splenic puncture[6]. Portal pressure depends primarily on the degree of constriction or dilatation of the mesenteric and splanchnic arterioles and on intrahepatic resistance. Both afferent systems merge at the sinusoidal bed, where the pressure is estimated to be slightly, namely, 2-4 mmHg above that in the smallest collecting veins or the inferior vena cava.
Hepatic blood volume
Although only limited data exist, it appears that hepatic blood volume ranges from 25 to 30 mL/100 g liver weight, and accounts for 10%-15% of the total blood volume[7]. Estimations of hepatic blood volume are highly variable, as indirect calculations of hepatic blood volume from red blood cell content of the liver and arterial hematocrit are inaccurate, and hepatic venous pressure largely influences hepatic blood volume[4]. Furthermore, rough estimation suggests that more than 40% of the hepatic blood is held in large capacitance vessels (portal vein, hepatic artery and hepatic veins), while the sinusoids accommodate up to 60% as small vessel content[4]. Of note is the high compliance of the hepatic vascular bed, calculated as the change in blood volume per unit change in venous pressure[8]. In cats, the hepatic blood volume increases in response to elevated venous pressure and is doubled when hepatic venous pressure is elevated to 9.4 mmHg[8]. Hepatic blood volume may expand considerably in cardiac failure and, in turn, serves as an important blood reservoir in case of bleeding episodes, and compensates up to 25% of the hemorrhage by immediate expulsion of blood from the capacitance vessels[9].
Hepatic oxygen consumption
As in any other artery of the body, oxygen saturation of the hepatic artery usually exceeds 95%. Oxygen saturation of portal blood during the fasting state ranges up to 85%, which is greater than that of other systemic veins; however, it substantially drops after food ingestion. It is generally accepted that 50% of the oxygen requirements of the liver are provided by portal venous blood and the other half derives from the hepatic artery[1]. If oxygen demand is increased, the liver simply extracts more oxygen from the blood in order to maintain oxygen uptake. In line with this, alterations of hepatic oxygen supply, attained by isovolemic hemodilution or stimulation of hepatic enzymes, lead to reduced oxygen content in the inflow and outflow vessels, but do not cause dilatation of the hepatic artery, which disproves the view that the hepatic artery might be regulated by the metabolic activity of the liver cell mass[10].
Hepatic blood flow control
Liver blood flow is controlled by mechanisms that are independent of extrinsic innervation or vasoactive agents that regulate (1) hepatic arterial inflow; (2) portal venous inflow; and (3) the interrelationship between hepatic arterial and portal venous inflow circuits. The relationship between arterial pressure and hepatic arterial blood flow has been analyzed in several species. However, there is disagreement as to whether the hepatic arterial vasculature exhibits autoregulation of blood flow. The term autoregulation is specifically used to describe the non-linearity of the arterial pressure-to-arterial flow relationship and comprises the tendency for local blood flow to remain constant in the face of pressure changes in the arteries that perfuse a given organ. Some studies have revealed evidence of pressure-dependent autoregulation of blood flow in the hepatic arterial bed[11-15]. In denervated dog liver preparations, Hanson and Johnson have shown that a step-wise reduction of hepatic artery pressure from 90 to 30 mmHg was accompanied by a substantial reduction in hepatic artery resistance[15]. Comparably, livers with intact peri-arterial nerve plexi showed a 60% decrease in arterial resistance upon a 63% pressure reduction[11]. Overall, however, the degree of autoregulation is considered small[11] and present in only about 60% of all preparations[15]. The fact that papaverine infusion can abolish hepatic artery dilatation indicates that the observed effects are primarily mediated by myogenic adaptation of the vasculature to changes in transmural pressure[11]. Besides that, a metabolic washout hypothesis is also tenable, where the hepatic artery washes out the endogenous adenosine, thereby completely accounting for autoregulation of the hepatic artery[16].
Less controversy exists concerning pressure-to-flow autoregulation of the portal venous vascular bed. Only a few studies have indicated autoregulation of portal venous blood flow[13], while the majority of studies have revealed a linear pressure-to-flow relationship with constant or increased portal venous resistance at low pressure gradients. In fact, there is even evidence for an opposite effect with (1) a partial passive collapse of the portal vascular bed taking place upon reduction of portal pressure; and (2) a reciprocal decrease in resistance upon a step-wise increase in portal venous pressure[15].
REGULATION OF LIVER BLOOD FLOW BY THE HEPATIC ARTERIAL BUFFER RESPONSE
Besides the intrinsic regulation of the hepatic artery by the classical arterial autoregulation, that is, the myogenic constrictive response of the hepatic artery if the arterial pressure rises, there is a second form of intrinsic regulation, termed the hepatic arterial buffer response (HABR). This unique mechanism represents the ability of the hepatic artery to produce compensatory flow changes in response to changes in portal venous flow. Although Burton-Opitz observed an increase in hepatic arterial blood flow upon reduced portal venous inflow in 1911[17], this intimate relationship between these two vascular systems was termed HABR for the first time in 1981 by Lautt[18]. If portal blood flow is reduced, the hepatic artery dilates, and the hepatic artery constricts, if portal flow is increased[19,20]. Using transit-time ultrasonic volume flowmetry, intraoperative measurement of the hepatic artery and portal venous flows in anesthetized patients with carcinoma of the splanchnic area has revealed a sharp and significant increase in hepatic arterial flow of about 30% after temporary occlusion of the portal vein, while temporary occlusion of the hepatic artery did not have any significant effect on portal venous circulation[20]. The HABR seems to operate in each individual under physiological conditions regardless of age. In addition, by establishing a method for measuring fetal hepatic arterial blood velocity, it has been reported that HABR even operates prenatally[21].
The increase in hepatic arterial blood flow is capable of buffering 25%-60% of the decreased portal flow[22,23]. The physiological role of this response is to minimize the influence of portal venous flow changes on hepatic clearance and to maintain adequate oxygen supply to tissues[24]. The latter function, however, may be of minor importance, since the liver normally receives more oxygen than it requires, and it can extract more oxygen to compensate for reduced delivery[25]. Thus, metabolic activity of the hepatic parenchymal cells does not directly control the hepatic arterial flow[22,25]. Instead, hepatic arterial flow subserves the hepatic role as a regulator of blood levels of nutrients and hormones by maintaining blood flow and thereby hepatic clearance as steadily as possible[24,26]. Because the portal vein cannot control its blood flow, which is simply the sum of outflows of the extrahepatic splanchnic organs, there is no reciprocity of the HABR, that is, alterations of the hepatic arterial perfusion do not induce compensatory changes of the portal vascular flow[18,20] or resistance[27].
The current view is that the HABR can be accounted for by the adenosine washout hypothesis[23]. This hypothesis states that adenosine is released at a constant rate into fluid in the space of Mall that surrounds the hepatic resistance vessels and portal venules. The space of Mall is contained within a limiting plate that separates this space from other fluid compartments. The concentration of adenosine is regulated by washout into the portal vein and the hepatic artery. If portal blood flow is reduced, less adenosine is washed away from the space of Mall, and the elevation in adenosine levels leads to dilation of the hepatic artery with a subsequent increase in hepatic arterial flow[10].
There are several lines of evidence that adenosine mediates the HABR: (1) adenosine produces hepatic arterial dilation[23]; (2) portal venous application of adenosine exerts one-half to one-third the effect of the same dose infused directly into the hepatic artery, which indicates that portal blood has some access to the arterial resistance vessels[10]; (3) adenosine uptake antagonists potentiate the HABR[23]; and (4) pharmacological antagonists of adenosine produce competitive blockade of the buffer response[16,28-30]. However, it has been suggested that adenosine itself does not diffuse from the portal venous to hepatic arterial bed to elicit the arterial response[31,32]. Rather ATP is released from the portal venous vasculature as a response to hypoxia associated with portal flow reduction, and diffuses into the hepatic arterial vasculature. No difference in the degree of inhibition of HABR by an adenosine antagonist has been observed between intra-arterial and intraportal injection of ATP in a rabbit model. This suggests that only the adenosine produced from ATP catabolism in the hepatic arterial vasculature contributes to arterial dilation. The adenosine produced from ATP in the portal venous vasculature is taken up effectively by the endothelium and vascular smooth muscle cells as soon as it is formed, and it does not diffuse to the hepatic arterial vasculature[30]. Mathie and Alexander have pointed out that adenosine is unlikely to be the sole regulator of HABR[33]. Other vasoactive compounds, such as nitric oxide and carbon monoxide, may be potential candidates to affect hepatic arterial flow and contribute to the HABR. Nitric oxide participates in regulation of hepatic arterial blood flow with changes in portal venous blood flow via ATP-dependent stimulation of endothelial purinergic receptors in the hepatic artery, which results in vasodilation[30,34,35]. Although nitric oxide is an important regulator of hepatic arterial resistance[36], it does not mediate the HABR and it is not found to play any significant role in total hepatic capacitance regulation[37].
Although nitric oxide serves as a potent vasodilator in the hepatic arterial circulation and exerts only a minor vasodilatory effect in the portal venous vascular bed, carbon monoxide is reported to maintain portal venous vascular tone in a relaxed state and to exert no vasodilation in the hepatic artery[38]. Recently, a third gaseous mediator, H2S, has been recognized as an important endogenous vasodilator and neuromodulator[39]. There is now major evidence that H2S also contributes to the HABR and partly mediates the vasodilatory response of the hepatic artery. This conclusion is based on the fact that supplementation of H2S increases hepatic arterial conductance and almost doubles the buffer capacity. In turn, inhibition of the H2S function by application of a selective inhibitor of KATP channels, which mainly mediate the ability of H2S to relax vascular smooth muscle cells, markedly decreases buffer capacity[40].
Next to vasoactive mediators, there is evidence that sensory innervation and sensory neuropeptides are, at least to some extent, involved in the HABR. Accordingly, sensory denervated rats[41] and pigs[42] have revealed a reduced HABR upon partial occlusion of the portal vein. Furthermore, pretreatment with antagonists of calcitonin gene-related peptide (CGRP) and neurokinin (NK)-1 receptors significantly reduce the hepatic arterial blood flow, which indicates that the observed vasodilation in the vascular bed of the hepatic artery is due to stimulation of CGRP and NK-1 receptors[41].
IMPLICATIONS OF THE HABR IN LIVER DISEASES
HABR in liver resection, transplantation and laparoscopic surgery
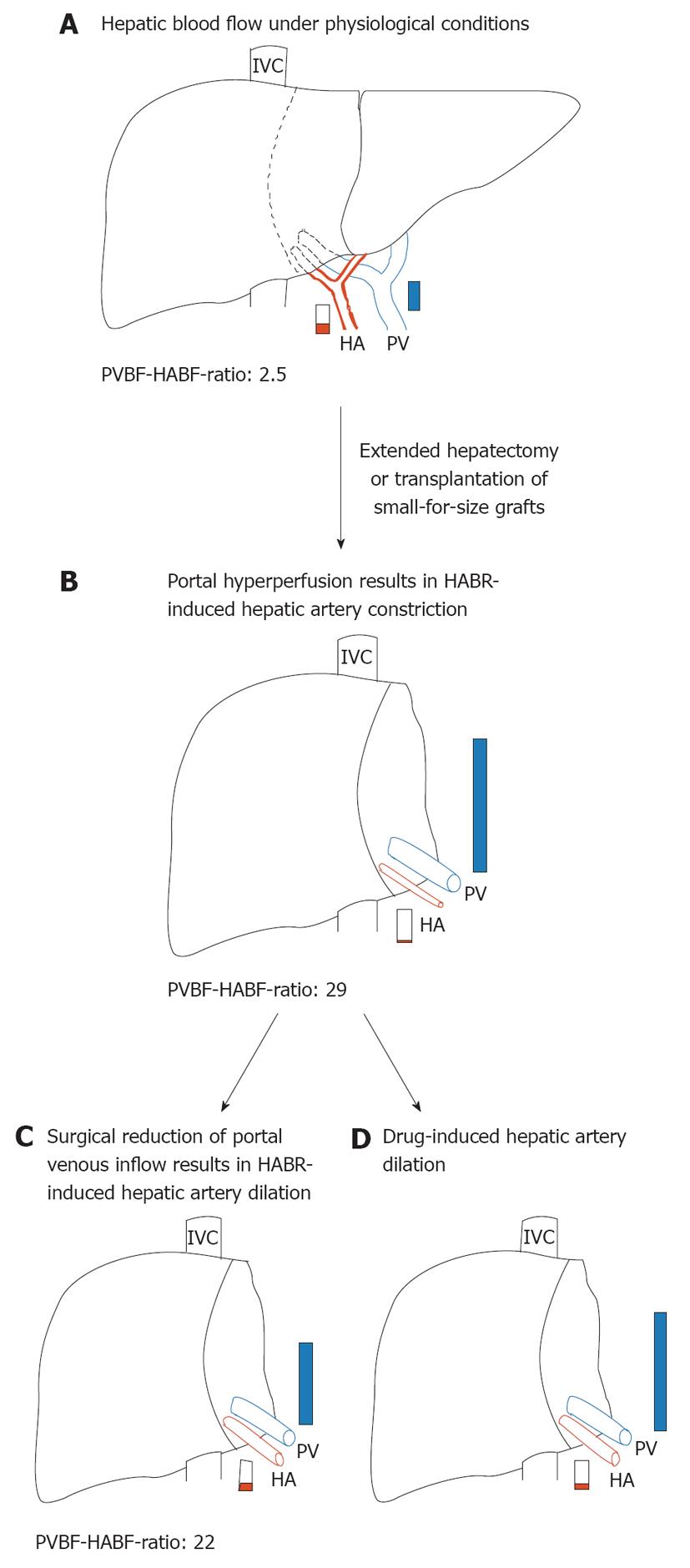
The ability of the liver to regenerate after major resection has been studied extensively, but the factors responsible for regeneration are not fully understood[43]. Although a clear association between flow and regenerative response has been suggested, the exact role of hepatic blood flow in liver regeneration is still a matter of intense debate. The increased blood flow to liver mass ratio immediately after partial hepatectomy (pHx) and the resultant increased intrahepatic shear stress have been proposed to stimulate and regulate liver regeneration[44-47]. On the other hand, the failure of the liver to control directly the portal venous blood flow has the consequence of portal hyperperfusion of the reduced-size liver (Figure 1A and B), which has been shown to impair seriously postoperative recovery of patients who are undergoing living donor liver transplantation or extended pHx[48,49]. In humans, 60% pHx results in a doubling of the portal flow in the 40% of remnant liver tissue[50]. This extent of pHx is followed by a transient and minor degree of small-for-size syndrome that usually resolves spontaneously within a few days. In contrast, major liver resection (> 75%) is followed by a more pronounced and long lasting small-for-size syndrome with much higher morbidity and mortality[50].
Figure 1 Hepatic hemodynamics in normal and reduced-size livers.
A: Preoperative hepatic blood flow in a donor liver or before extended hepatectomy representing a normal portal vein blood flow-hepatic artery blood flow (PVBF/HABF) ratio of 2.5; B: As a consequence of portal hyperperfusion, hepatic arterial buffer response (HABR) leads to hepatic arterial hypoperfusion of reduced-size liver that is characterized by a dramatically increased PVBF/HABF ratio of 29; C: Surgical reduction of the portal venous inflow, for example, by splenectomy or hepatic artery ligation, leads to HABR-induced dilation of the hepatic artery and results in a reduced PVBF/HABF ratio of 22; D: Possible effects of pharmacological interventions to preserve hepatic artery supply. PVBF/HABF ratios are adopted from[59]. HA: Hepatic artery; IVC: Inferior vena cava; PV: Portal vein.
With the increasing practice of living-donor liver transplantation and the enlargement of the resectable limit, the small-for-size syndrome has emerged as an important clinical problem[51]. Although the pathogenetic causes of the small-for-size syndrome are still debated, it is assumed that the syndrome is primarily linked to portal hyperperfusion with high intravascular shear stress[52-54]. As a consequence of portal venous hyperperfusion, however, HABR may lead to hepatic arterial hypoperfusion of reduced-size livers (Figure 1B). In line with this, Smyrniotis et al[55] have shown in a porcine study that portal flow to split grafts with a graft-to-recipient liver volume ratio of 2:3 and 1:3 showed an inverse relationship to graft size, for example, the smaller the graft, the higher portal blood flow. By contrast, arterial flow decreased proportionately to graft size. In addition, HABR, which is present in all split-liver transplanted pigs, has been found to be increase as the graft-to-recipient liver volume ratio decreases[55].
A comparable hemodynamic pattern of hepatic blood flow has been observed in living related liver transplantation, in which size disparity between graft and native liver is the rule and almost universal[56]. In patients with living right lobe living donor transplantation, the grafts are subjected to impressive, more than double, increases of portal blood flow (Figure 1A and B). In the absence of active regulation, arterial flow might be expected to double as well. On the contrary, striking decreases in arterial flow have been seen in right lobe grafts[57], which represent the HABR as a reflexive response to changes in portal blood flow, to maintain total blood flow within an acceptable physiological range[58]. Troisi et al[59] have reported mean recipient portal venous flow values in small liver grafts (graft-to-recipient body weight ratio < 0.8) at least three times higher than those recorded in donors. Simultaneously, hepatic artery flow is significantly reduced and results in a decreased contribution to the liver from 30% in donors to only 6% in the recipients. In a porcine small-for-size liver transplantation model, the portal-to-arterial flow ratio remains increased until 5 d after surgery, which is poorly tolerated by transplanted livers[60].
The HABR has been clearly demonstrated to be present also in patients after cadaveric liver transplantation[61]. Measurement of hepatic arterial and portal venous flow using ultrasound transit-time flow probes over the first 3 h after reperfusion has revealed a mean total liver blood flow of 2091 ± 932 mL/min, with a disproportionately high mean portal flow of 1808 ± 929 mL/min, which represents approximately 85% ± 10% of total liver blood flow. Correlation analysis has shown a positive correlation between cardiac output and portal venous flow, and a trend toward negative correlation between cardiac output and hepatic arterial flow[61]. In patients with a 50% reduced portal flow, Henderson and colleagues have reported a significant increase in hepatic artery flow, which indicates an intact HABR after cadaveric liver transplantation[61]. In line with this, Payen et al[62], by measuring hepatic arterial and portal venous blood flow during alternative clamping of both vessels every 12 h during 7 d in patients after orthotopic liver transplantation, have reported reciprocal increases of hepatic arterial flow only during selective clamping of the portal vein. By analysis of patients transplanted for liver cirrhosis, a high portal flow was present, together with an early increase of hepatic arterial resistance, which agrees with the HABR theory[63]. The presence of HABR in the transplanted liver is unequivocal[61,62,64], and because of liver denervation, it might be the only active mechanism that regulates liver arterial flow.
The consequences of inadequate hepatic arterial flow range from mild cholestasis to rapidly progressive graft failure[65,66]. In a porcine model of small-for-size syndrome, histological examinations of the grafts consistently confirm hepatic artery vasospasm and its consequences; namely, cholestasis, centrilobular necrosis and biliary ischemia[67]. In severe cases of small-for-size grafts, poor hepatic arterial flow and vasospasm lead to functional de-arterialization, ischemic cholangitis, and parenchymal infarcts[54,68]. Michalopoulos has concluded that the failure to regenerate is not different from the situation in which pHx is accompanied by ligation of the hepatic artery, which also results in failure to regenerate[68].
Prolonged CO2 pneumoperitoneum in laparoscopic surgery reduces substantially the portal venous flow in humans, and the extent of the flow reduction is related to the level of intraperitoneal pressure[69,70]. HABR may serve for maintenance of total liver blood supply during laparoscopy-associated portal venous flow reduction. However, controversial data exist on the maintenance of HABR during high-pressure pneumoperitoneum. Yokoyama et al[71] have reported on activation of HABR in a rat model using fluorescent microspheres to measure splanchnic flow. Although portal venous flow decreased, the hepatic arterial flow was relatively preserved throughout all levels of intraperitoneal pressure studied. In contrast, Richter et al[72] have used ultrasonic flow probes in a rat model, and have shown reduced portal venous flow paralleled by a linear reduction of hepatic arterial flow during CO2-pneumoperitoneum. HABR is also markedly impaired in cirrhotic rats undergoing CO2 pneumoperitoneum[73]. Studies in large animals have revealed intact HABR with doubled hepatic arterial flow in neonatal lambs during abdominal distension[74], as well as loss of HABR with reduced hepatic arterial flow in pigs[75], or unchanged hepatic arterial flow in dogs upon CO2 pneumoperitoneum[76]. In particular, head up body position leads to reduction in portal venous and arterial hepatic blood flow during elevated abdominal pressure[77]. Thus, head up position and intraperitoneal pressure elevation above 15 mmHg should be avoided during laparoscopic surgery to preserve hepatic blood flow[77,78].
HABR in inflammatory liver diseases
Owing to the scarcity of clinical studies on this subject, one must turn to experimental data, with reservations concerning their extrapolation to humans. In models of continuous intravenous infusion of Escherichia coli in rats, portal venous flow was reduced, and increased hepatic arterial flow resulted in unchanged total hepatic blood flow[79,80]. The increased hepatic arterial flow could be a result of an active HABR, although, in parallel, reports exist to demonstrate an increased hepatic artery flow without a reduction in portal venous flow during endotoxemia[81-83]. In a porcine model, it has been shown that endotoxin shock leads to time-dependent impairment of liver inflow beds, which results in increased portal venous back pressure and incremental resistance. The hepatic artery bed is dilated in the early phase of endotoxic shock but, over time, it is constricted[84]. There is ongoing discussion as to whether excessive production of nitric oxide is the cause of the endotoxin-induced alterations in hemodynamic homeostasis. While nitric oxide induces arterial hypotension and hepatic arterial vasodilation during endotoxic shock[85], ablation of the HABR has been shown to be independent of nitric oxide or an α-adrenergic-receptor agonist[84]. On the contrary, early administration of the nitric oxide donor sodium nitroprusside can reverse the negative effects on hepatic arterial flow induced by endotoxin[86,87]. Moreover, sodium nitroprusside partially reverses the detrimental effect of the nitric oxide synthase inhibitor L-NAME in experimental endotoxemia, which implies that the endotoxin-induced dysfunction of the HABR may be due to a selective inhibition of vascular endothelial function[87]. Furthermore, nitroprusside maintains mRNA levels of constitutive nitric oxide synthase in liver tissue that are decreased by endotoxin shock and tempers the burst in inducible nitric oxide synthase expression, thereby reestablishing the autoregulatory response of the hepatic artery following reduction of portal venous blood flow[86].
In turn, application of the vasopressin analog terlipressin during long-term hyperdynamic porcine endotoxemia significantly decreases portal venous flow, whereas hepatic arterial flow is markedly increased, which presumably reflects a restored HABR[88]. Furthermore, terlipressin attenuates the endotoxin-induced increase in exhaled nitric oxide[88], which points to the interaction between the vasopressin and the nitric oxide system in septic shock[89].
Almost no data exist on hepatic hemodynamics during conditions of acute or chronic viral hepatitis[90]. In addition, only a few studies have addressed hepatic hemodynamics under low-flow conditions, such as hemorrhagic shock. However, the data so far are consistent in that HABR is not abolished during sustained low abdominal blood flow[91-93]. In critically ill patients, mechanical ventilation has been found to decrease splanchnic perfusion. However, importantly, HABR is preserved and increased hepatic arterial blood flow compensates the decrease in portal blood flow under conditions of ventilation-associated positive end-expiratory pressure[94].
HABR in liver fibrosis and cirrhosis
The pathogenesis of liver fibrosis and cirrhosis is characterized by initial hepatocyte necrosis and inflammatory response, with subsequent activation of hepatic stellate cells and their transformation into myofibroblasts, which is responsible for excessive extracellular matrix synthesis and deposition. As a consequence, distinct alterations of the hepatic microvasculature, that is, rarefaction of sinusoids and structural changes of sinusoidal endothelia[95,96], result in deteriorated nutritive blood supply, increased total hepatic vascular resistance, and hence, portal hypertension and portosystemic collateralization[97]. Due to this increase in sinusoidal resistance, the capillarization of the hepatic microvasculature and the development of portocaval collaterals[98], portal venous blood flow progressively decreases in patients with cirrhosis[99,100]. An increase of hepatic arterial blood flow, that is, a decrease of hepatic arterial resistance, if it occurs, would indicate an activated HABR.
Studies in cirrhotic rats have underlined this hypothesis by demonstrating that, under baseline conditions, cirrhotic animals have higher hepatic arterial blood flow compared to controls[101]. Moreover, induction of HABR by a stepwise reduction of portal venous inflow causes a disproportionate increase in hepatic arterial flow in cirrhosis, which is further reflected by the significantly higher buffer capacity[101,102].
Although this concept has been well established, analyses in cirrhotic patients have produced conflicting results. Several studies have shown an increased hepatic arterial resistance in patients with cirrhosis. This is related to the degree of portal hypertension[103,104], portal resistance[104,105] and Child-Pugh score[104]. In contrast to these observations, a considerable body of evidence exists to indicate that, in cirrhosis, hepatic arterial vasodilatation occurs in response to reduced portal venous blood flow[106-109]. Accordingly, intraoperative measurements in patients with end-stage liver cirrhosis, who underwent living-donor liver transplantation, have revealed a continuously activated HABR under baseline conditions[109]. In these cirrhotic patients, the reduced portal venous blood flow is associated with an increased hepatic arterial blood flow (hepatic arterial to portal venous flow ratio = 0.88), which is in contrast to the relationship in healthy volunteers (hepatic arterial to portal venous flow ratio = 0.58)[109]. However, total clamping of the portal vein provokes a blunted response, as evaluated by the absolute and relative changes in hepatic arterial blood flow and by the buffer capacity[109]. In line with this, Iwao et al[110] have reported that the hepatic artery buffer index is significantly lower in cirrhotic than in control subjects. They have analyzed portal venous blood flow and hepatic artery pulsatility index as measures of hepatic artery resistance upon a 500-kcal mixed liquid meal consumption, which increases portal venous blood flow. They found an increase in hepatic artery resistance in all subjects, however, it was less pronounced in cirrhotic than control subjects[110,111]. Vice versa, the vasopressin-induced decrease of portal venous blood flow was met by a fall in hepatic arterial pulsatility index, which again was significantly lower in cirrhotic than control subjects[110]. In a large series of patients with advanced cirrhosis, who are undergoing transjugular intrahepatic portosystemic shunt (TIPS), Gülberg et al[108] have demonstrated that patients with hepatofugal blood flow show significantly lower resistance index before TIPS placement than patients with antegrade portal flow direction, and TIPS placement induces a significant decrease in the resistance index in patients with hepatopedal flow, but not in patients with hepatofugal flow. The fact that some degree of HABR is preserved even in patients with advanced cirrhosis and significant portal hypertension may further underline the biological need for this intrinsic mechanism. Although one might argue that the drop in resistance and thus increase in hepatic arterial flow may not fully compensate for the TIPS-induced reduction in portal blood flow, it has been shown that hepatic arterial vasodilatation provides substantial functional benefit in patients with cirrhosis, and that this effect does not depend directly on hepatic arterial microperfusion and is observed preferentially in patients with decompensated disease[107]. Thus, it is reasonable to state that the change in the ratio of portal venous to hepatic arterial blood flow in favor of the hepatic artery may sustain oxygen delivery and exert a protective effect on organ function and integrity. In line with this, portal vein occlusion does not cause deterioration in hepatic tissue pO2 in the presence of HABR, although maximum buffer capacity of the hepatic artery was limited to 50%-60% in both cirrhosis and control animals, and total liver blood flow was found to be restored to only 71%-76% of baseline values[102].
MODIFICATIONS OF THE HABR
Surgical interventions for modification of the HABR
With the development of partial liver transplantation, either as living donation or as deceased donor split graft, much effort has been spent on improvement of surgical techniques. Full-right full-left splitting for two adult recipients is associated with risk of small-for-size syndrome, which manifests as a pattern of liver dysfunction associated with portal hypertension, diminished arterial flow, delayed synthetic function, and prolonged cholestasis[112-115]. However, the present rates of splitting livers are too low in comparison with the calculated potential and it is to be expected that further improvement in the management of small-for-size grafts would bring splitting of the liver for two adult recipients within the reach of broad application[112]. Small-for-size syndrome is a clinical problem that is also observed after living donor liver transplantation and extended hepatectomy[52,116]. When the full portal vein flow has to transverse through a much reduced liver size, then the pressure building up in the portal vein effectively shuts down the flow through the hepatic arterioles and the liver becomes de-arterialized[68]. Arterial flow impairment appears as result of an active HABR, although in the past, reduced hepatic arterial flow has repeatedly been ascribed to the splenic artery steal syndrome[117-120]. This phenomenon describes the impaired hepatic artery flow by shifting of the main blood flow to the splenic or gastroduodenal artery in patients with hypersplenism. Quintini et al[121] have analyzed whole-organ liver recipients by Doppler ultrasonography, and have reported that hepatic artery vasoconstriction in response to portal hyperperfusion and exaggerated HABR produces a high resistive index with poor arterial perfusion. In all patients, splenic artery embolization reduces the resistance to distal hepatic artery flow by reducing flow in the splenic circulation and consequently in the portal vein. This has prompted the authors to revise the name of splenic artery steal syndrome to splenic artery syndrome, thereby underlining that the cause is portal hyperperfusion and not arterial siphon[121]. Most recently, a retrospective analysis of 650 orthotopic liver transplantations has revealed an incidence of 5.1% for splenic artery syndrome[122], which is well within the range of the estimated incidence of artery splenic syndrome of 3.1%-11.5% after orthotopic liver transplantation[117,118,123,124]. Prophylactic treatment with ligation of the splenic artery for all patients at risk for development of splenic artery syndrome is recommended and effectively prevents splenic artery syndrome[122]. In the case of postoperative diagnosis of splenic artery syndrome, coil embolization of the splenic artery can be recommended as the treatment of choice, with a low risk profile[122].
Clinical features of small-for-size syndrome are neither specific nor inevitable in low-weight livers, and many other factors than actual liver weight contribute to their occurrence. Among these, early elevation of portal venous pressure and persistent portal overperfusion most probably play a key role[48,49,125-128]. A reduction in the portal venous flow by means of splenic artery ligation, splenic artery embolization, or splenectomy has been shown to be efficient also in case of the small-for-size syndrome[48,59,127,129]. A case report by Lo et al[51] and prospective studies by Troisi et al[130] and Umeda et al[131] have shown that modulation of the recipient portal inflow by ligation or embolization of the splenic artery leads to an increase in recipient hepatic arterial inflow (Figure 1C), with improved liver function. The fact that splenic artery syndrome and small-for-size syndrome can be successfully treated by coil embolization of the splenic artery strongly underlines that both syndromes are pathophysiologically linked to the HABR. In line with this, detailed histopathological examination of sequential post-transplant biopsies and failed allografts with clinicopathological correlation has revealed that portal hyperperfusion, venous pathology, and the arterial buffer response make an important contribution to early and late clinical and histopathological manifestations of small-for-size syndrome[54]. In the most recent study of our group, we observed significantly increased survival of simultaneously splenectomized and hepatectomized rats compared to animals with 90% pHx alone[132]. It has been suggested that this effect is mainly caused by suppression of intrahepatic flow and less sinusoidal shear stress[49,55,133]. However, reduction of total hepatic inflow in simultaneously splenectomized and hepatectomized animals was marginal and not as pronounced as that required to improve survival by reduced shear stress. Instead, splenectomy before pHx caused a doubling of hepatic tissue pO2 due to a HABR-induced rise of hepatic blood flow during extended pHx, which led to high tissue pO2 values and reduced hypoxic stress. Supposedly, the increase of arterial inflow covers the oxygen demand and thereby improves organ regeneration and animal survival. Thus, improved arterial inflow rather than reduction of portal venous hyperperfusion is of great significance for the beneficial effect after inflow modulation in small-for-size livers[132].
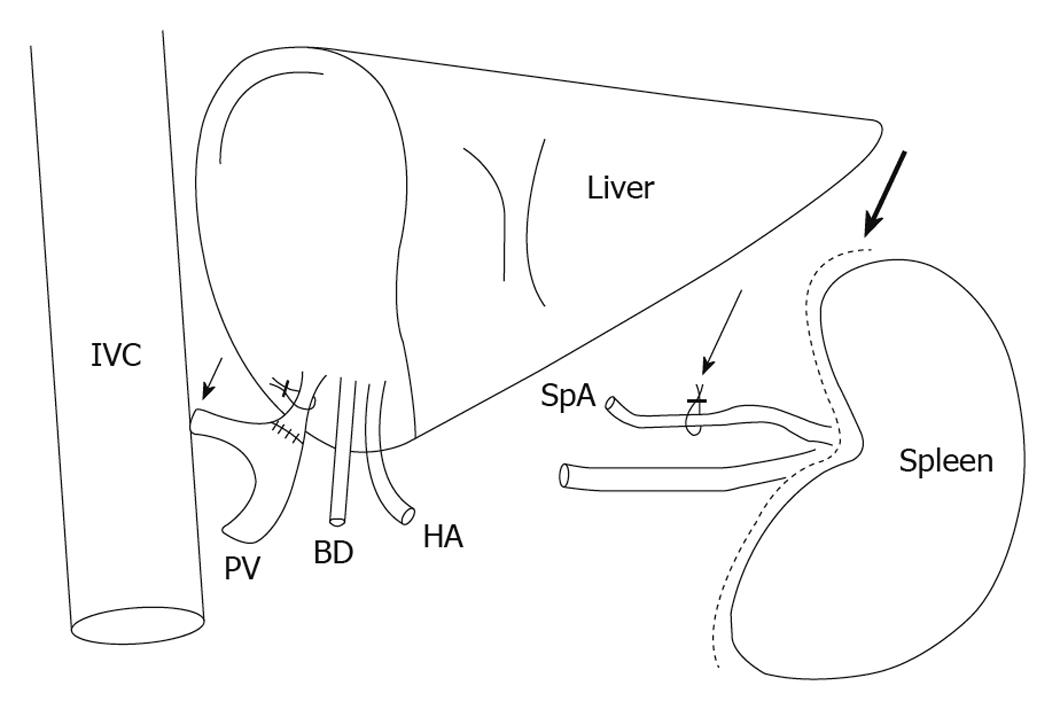
Besides splenic artery ligation, established techniques such as portocaval or mesocaval shunts (Figure 2) may cause not only reduction of portal hyperperfusion, but also an increase of hepatic arterial inflow by reversion of the HABR (Figure 1C)[130,134-136].
Figure 2 Surgical interventions for modulation of the hepatic inflow, showing the portocaval shunt (short arrow), ligation of the splenic artery (thin long arrow) and splenectomy (thick long arrow).
BD: Bile duct; HA: Hepatic artery; IVC: Inferior vena cava; PV: Portal vein; SpA: Splenic artery.
In conclusion, the intraoperative measurement of both hepatic blood flows is important to predict the risk of small-for-size syndrome. The better ability to regulate finely the hepatic inflows would be useful in the treatment of liver dysfunction in settings of small-for-size transplantation, as well as extended hepatectomy, and necessitates further studies.
Pharmacological modifications of the HABR
The responsiveness of the hepatic artery to changes in portal flow is undoubtedly a desirable homeostatic mechanism under most circumstances, that is, the increase of hepatic arterial blood flow in case of reduced portal venous inflow. In contrast, the opposite situation, in which the dramatic excess of portal flow due to a smaller-than-average organ causes hepatic arterial constriction and hypoperfusion, might harm the liver (Figure 1A and B). Both extended hepatectomy and split-liver transplantation by fashioning two transplantable grafts from one liver result in small-for-size livers[50,116,137]. The regenerating liver requires an enormous amount of oxygen for its increased metabolic load and for DNA synthesis[138,139]. Suboptimal arterial inflow may be poorly tolerated in the reduced-size liver and increase the risk of organ dysfunction[60,68,138]. Possible pharmacological interventions could aim to enhance the hepatic arterial supply (Figure 1D). In line with this, in a porcine model of small-for-size syndrome, hepatic arterial infusion of adenosine significantly restored hepatic artery flow, reversed pathological changes in the graft, and finally improved survival[67]. In addition, an imbalance of vasorelaxing and vasoconstricting mediators is considered to be an important pathogenetic feature in reduced-size livers[140]. Maintenance of endothelin-1/nitric oxide balance by blocking endothelin A receptor reduces small-for-size injury by protecting the liver microcirculation and reducing hepatocellular damage[140]. Vice versa, substitution of nitric oxide has been shown to counteract the decline in hepatic arterial inflow in rats with 85% hepatectomy and cause a significantly greater increase in cell proliferation, with improvement of liver function[141].
Several programs in Japan have started clinical trials to reduce injury in small-for-size grafts by direct infusion of drugs such as prostaglandin E1 and proteolytic enzyme inhibitors into the portal flow[126,142]. However, further experimental studies, including intraoperative measurement of both hepatic blood inflows, are warranted to clarify the precise strategies of pharmacological interventions and to select the appropriate drugs.