Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5759
Revised: July 3, 2010
Accepted: July 10, 2010
Published online: December 7, 2010
AIM: To investigate the effects of Lactobacillus plantarum (L. plantarum) in the intestinal permeability and expression of tight junction (TJ) using the normal human colon cell line NCM460.
METHODS: Paracellular permeability of NCM460 monolayers was determined by transepithelial electrical resistance and dextran permeability. Expression of TJ proteins in NCM460 cell monolayers was detected by Western blotting and quantitative real-time polymerase chain reaction.
RESULTS: L. plantarum played an important role in increasing transepithelial electrical resistance and decreasing the permeability to macromolecules of NCM460 monolayers against the disruption caused by enteropathogenic Escherichia coli (E. coli) or enteroinvasive E. coli. L. plantarum also prevented the decrease in the expression of TJ proteins and F-actin in NCM460 cells.
CONCLUSION: L. plantarum can protect against dysfunction of NCM460 intestinal epithelial barrier caused by enteropathogenic E. coli or enteroinvasive E. coli, and thus can be a potential candidate of therapeutic agents for the treatment of intestinal diseases.
-
Citation: Liu ZH, Shen TY, Zhang P, Ma YL, Moyer MP, Qin HL. Protective effects of
Lactobacillus plantarum against epithelial barrier dysfunction of human colon cell line NCM460. World J Gastroenterol 2010; 16(45): 5759-5765 - URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5759.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5759
The human intestinal system included a group of viable microorganisms, which exceed the total number of somatic and germ cells[1-3]. Therefore, the human colon is confronted with the highest bacterial load in the digestive tract with enormous bacteria per gram of feces. Growing evidence showed that bacteria closely adherent to the mucosa are more relevant to human body, compared with those evacuated in the feces[4,5]. There is a homeostasis between probiotics and pathogens in the intestinal systems of healthy individuals[6,7], which, if broken, may lead to an imbalanced ecological microenvironment and subsequent intestinal barrier dysfunction[8,9]. Thereafter, the accumulation of pathogens and secretory products, such as the exotoxins and secretory antigens, can also directly or indirectly initiate and amplify the local and systemic inflammatory responses[10,11]. Probiotics of the genus lactobacillus that reside in the human intestine play an important part in maintaining the homeostasis of gut flora by adhering and colonizing to the intestinal mucosa and competing with pathogenic bacteria, such as pathogenic Escherichia coli (E. coli)[12,13]. Enteric diseases with flora disequilibrium have been treated with lactobacillus over the past decades[14-16]. There is evidence indicating that the modulation of the gut flora by lactobacillus can improve the intestinal epithelial barrier function[17].
Adhesion of lactobacillus to the intestinal epithelium initially involves the activation of specific binding between bacterial ligands and their corresponding surface receptors on the intestinal cells of the host, following the non-specific physical interactions[18,19]. Generally, these ligands are adhesive molecules either existing on the surface layer of the bacteria or secreting from the mycelium of the bacteria. Furthermore, these ligands could interact with the corresponding receptors on the surface of the intestinal epithelial cells. Thereafter, these adhesins activate specific signal transduction pathways in both the bacteria and host cells. The interaction between lactobacillus and the intestinal epithelial cells can also block the adhesion of other pathogenic bacteria to the receptors of the intestinal epithelial cells, such as enteropathogenic E. coli (EPEC) and enteroinvasive E. coli (EIEC). As a widespread member of the genus lactobacillus, Lactobacillus plantarum (L. plantarum) is commonly found in many fermented food and anaerobic plant products. Our previous studies demonstrated that L. plantarum was able to prevent colonic damage caused by EIEC or inflammation in vitro, in vivo and in patients with acute pancreatitis[20-24]. The normal human colon cell line NCM460, which is derived from the normal human colon mucosal epithelium and expresses colonic epithelial cell-associated antigens such as cytokeratins and villin, has been applied exclusively in various intestinal research areas, including the infectious diseases[25-27].
Our previous studies indicated that L. plantarum exerted its therapeutic effects by adhering to the intestinal epithelial cells, restoring tight junction (TJ) structure and function, and reducing paracellular permeability. However, studies about the interaction between lactobacillus and the human intestine were limited in the cancer cell line and the animal models, and further researches based on the normal human intestinal cells are still needed. Therefore, our study aims to investigate the protective effects of L. plantarum against epithelial barrier dysfunction of the normal human colon cell line NCM460 caused by EIEC and EPEC.
L. plantarum CGMCC 1258 (generously provided by Dr. Xiao-Min Hang, the Onlly Institute of Life Science, Shanghai Jiao Tong University, Shanghai, China) was inoculated in 5% fresh De Man, Rogosa and Sharpe broth at 37°C for 24 h, harvested by centrifugation (3500 ×g) at 4°C for 20 min, and washed with 50 mL 0.01 mol/L phosphate buffered saline (PBS) (pH 7.4). The EIEC strain ATCC 43893 (O124:NM) and EPEC strain ATCC 43887 (O111:NM) (both from Shanghai Municipal Center for Disease Control and Prevention, Shanghai, China) were grown in static Dulbecco’s modified eagle media (DMEM) at 37°C for 24 h. Quantification of bacterial density was measured at 600 nm (Beckman DU-50 spectrophotometer) with the colony forming units.
NCM460 cells were purchased from INCELL Corporation (San Antonio, TX, USA) and cultured in M3 media supplemented with 10% FBS, 100 U/mL penicillin and 100 g/mL streptomycin at 37°C in a 95% humidified atmosphere with 5% CO2, as previously described[25].
NCM460 cells were grown on filters (Millicell culture plate inserts; 0.4 μm pore size; 0.6 cm2 surface area) at 37°C in a 95% humidified atmosphere, with 5% CO2. At full confluence (10-14 d) (i.e. a monolayer was formed), a transepithelial electrical resistance (TER) of > 450 Ω.cm2 monolayer was achieved as measured using a voltmeter (Millicell-ERS; Millipore, MA, USA). The intestinal epithelial monolayers were treated with EIEC or EPEC in the presence or absence of L. plantarum. In infection groups, 100 μL EIEC ATCC43893 (O124:NM) and EPEC ATCC43887 (O111:NM) at 1.0 × 108/mL were, respectively, added to the apical side of the cell culture insert for rapid infection of the monolayer, with an inoculation ratio of EIEC/EPEC to NCM460 cells of 100:1, and the insert was placed in a 50-mL tube and centrifuged at 200 ×g for 4 min. In L. plantarum groups, L. plantarum (100 μL of 1.0 × 108/mL) was added onto the monolayer of NCM460 cells simultaneously with the EIEC/EPEC infection. NCM460 cells cultured under the same conditions but without the infection of EIEC/EPEC, and addition of L. plantarum served as the control group. Two experiments were performed separately for EIEC and EPEC.
The integrity of the confluent polarized monolayers was verified by measuring TER at different time intervals. TER (Ω.cm2 monolayer) = (Total resistance - Blank resistance) (Ω) × Area (cm2 monolayer). Because TER values often vary among individual NCM460 cultures, the electrical resistance value was recorded for each monolayer before and after the treatment, and the percentage in the decrease of TER from the baseline (%TER) was calculated.
DMEM (0.2 mL) containing conjugated dextran was added to the apical compartment of Transwells (Corning Costar Co., MA, USA), and 0.4 mL DMEM alone added to the basolateral compartment. After treatment as described above, samples (0.5 mL) collected from the basolateral compartment were placed into a 96-well plate (Corning Costar Co., MA, USA) and analyzed to determine their fluorescent intensity using the Odyssey infrared imaging system (LI-COR Biosciences, NE, USA) at a wavelength of 700 nm. Relative intensity (RI; the integrated intensities of treated samples relative to the integrated intensity of untreated samples) was calculated to indicate the effect of the treatment.
For Western blotting, NCM460 cells were cultured and the monolayers were treated as described above, and the protein samples from NCM460 cells was prepared for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to the previous studies[20]. SDS-PAGE was performed using the standard laboratory techniques with a discontinuous gradient, 5% (w/v) stacking gel and a 10% (w/v) separating gel, in a Mini-PROTEAN II (Bio-Rad Laboratories, CA, USA). Briefly, samples were mixed with loading buffer containing SDS and mercaptoethanol, boiled for 3 min, centrifuged, and loaded onto the SDS-PAGE gel for separation. Molecular weights of samples were determined by comparing mobility with known marker proteins. Gel was then transferred to PVDF membrane (Millipore, MA, USA) in a semidry electroblotter (Bio-Rad Laboratories) for 120 min at 100 V. The membrane was washed three times (20 min each) with PBS containing 0.1% Tween-20 (PBS-T buffer). After blocking overnight in Tris-buffered saline containing 0.05% Tween-20 (TBS-T) and 5% dry powdered milk, membranes were washed three times for 5 min each with TBS-T and incubated with corresponding primary antibodies against TJ proteins (claudin-1, occludin, JAM-1, and ZO-1) and a cell cytoskeleton element F-actin (all from Abcam, MA, USA) for 2 h at room temperature. After three washes with TBS-T, the membranes were incubated for 1 h with corresponding HRP-conjugated secondary antibodies. The membrane was washed three times (60 min each) with PBS-T buffer. The TJ proteins were tested using enhanced chemiluminescence (ECL kit; Pierce, IL, USA) according to the manufacturer’s instructions.
mRNA expression of TJ proteins, including claudin-1, occludin, JAM-1, and ZO-1, was determined by quantitative real-time polymerase chain reaction (RT-PCR). After the treatment as described above, total RNA was isolated from NCM460 cells using the Trizol reagent (Gibco Brl, USA)[28], followed by DNase I treatment. The quantity and quality of RNA were verified with the ratio of absorbance values at 260 and 280 nm, and by visualization of the bands on agarose gels. For each sample, 600 ng mRNA was used in reverse transcription reaction (iScript kit from BioRad Laboratories) according to the manufacturer’s specifications. Further analysis of mRNA levels of each group was performed by RT-PCR with a light-cycling system (LightCycler; Roche Diagnostics GmbH, Mannheim, Germany). Sequences of the primers used are listed in Table 1. The mRNA expression level was described as the ratio of the mean reading of the experimental group over that of the control group for NCM460 cells.
| Gene | Upstream primer | Downstream primer |
| Occluding | GCAGCTACTGGACTCTACG | ATGGGACTGTCAACTCTTTC |
| Claudin-1 | GTGCCTTGATGGTGGTTG | TGTTGGGTAAGAGGTTGT |
| JAM-1 | GATGTGCCTGTGGTGCTG | GCTCTGCCTTGAGATAAGAA |
| ZO-1 | AAGAGTGAACCACGAGAC | TCCGTGCTATACATTGAG |
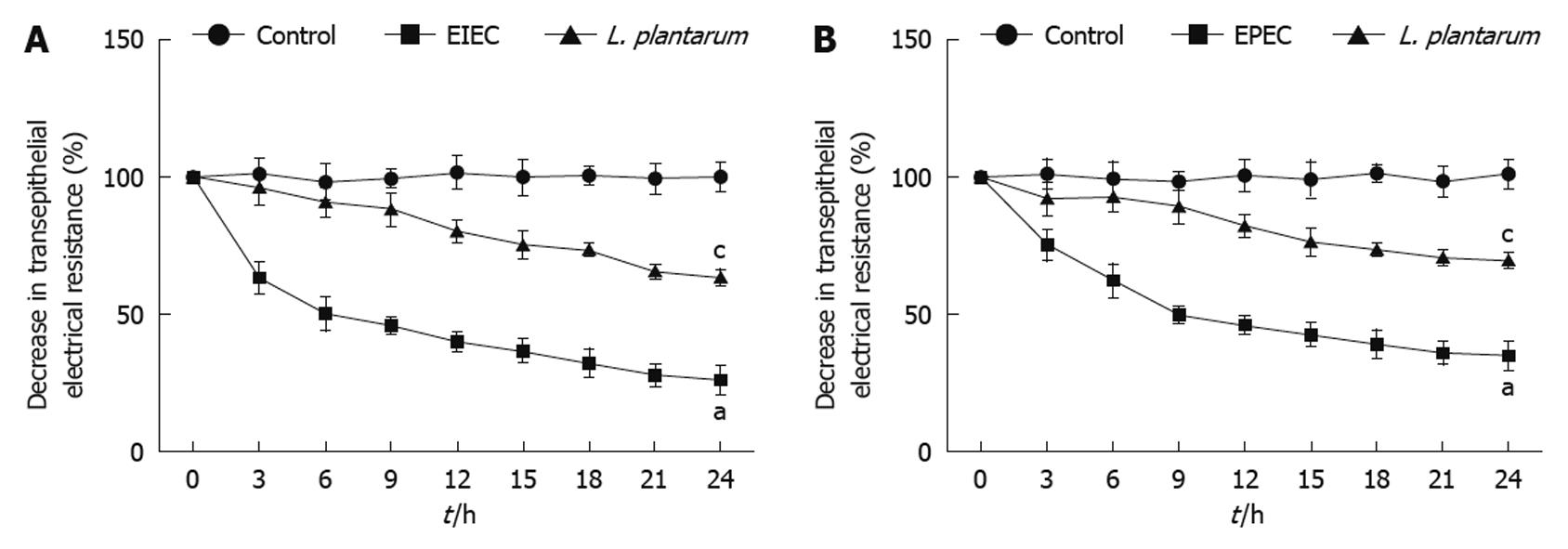
TER in the NCM460 cell monolayers was decreased significantly in response to infection with EIEC/EPEC compared with uninfected control cells. However, decrease of TER induced by EIEC/EPEC was prevented by the simultaneous treatment of L. plantarum (Figure 1A and B).
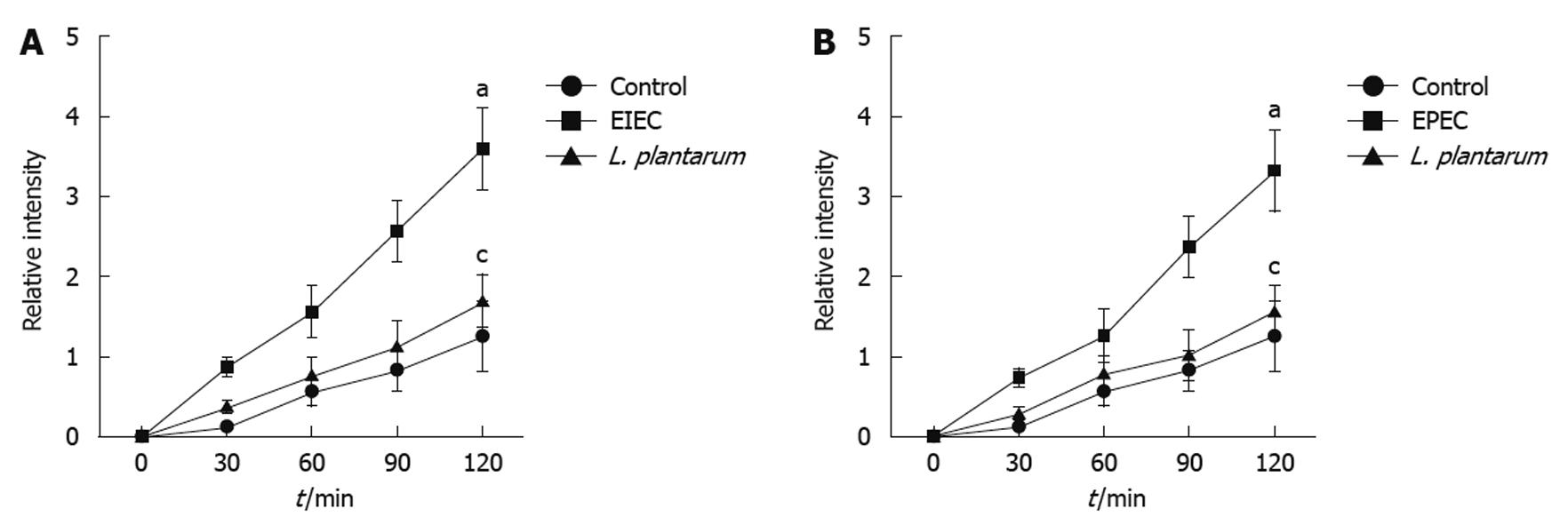
EIEC/EPEC had an obvious enhancing effect on permeability of NCM460 cell monolayers, as compared with the uninfected control cells. However, this effect was inhibited by the co-treatment of L. plantarum (Figure 2A and B).
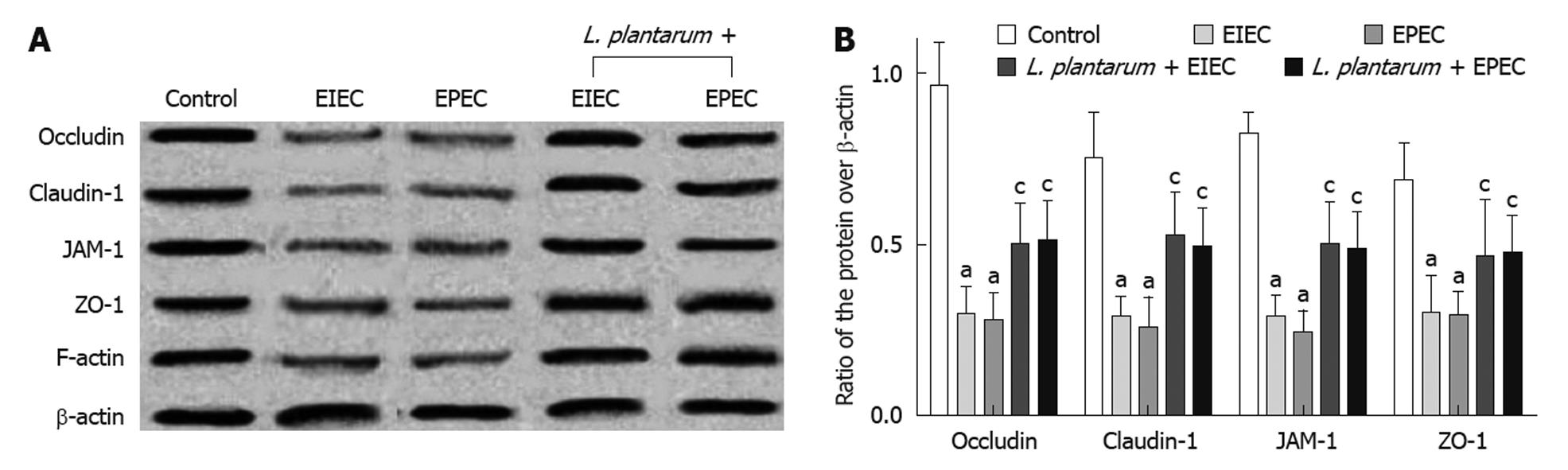
The expression of TJ proteins, including claudin-1, occludin, JAM-1 and ZO-1, and the cytoskeleton element F-actin, was decreased in NCM460 cells infected with EIEC or EPEC (Figure 3A and B) compared with the control cells, as detected by Western blotting of epithelial whole cell protein extracts of NCM460 cells (P < 0.001). However, after the pre-treatment of L. plantarum, the expression of TJ proteins and F-actin remained at similar levels to the control cells.
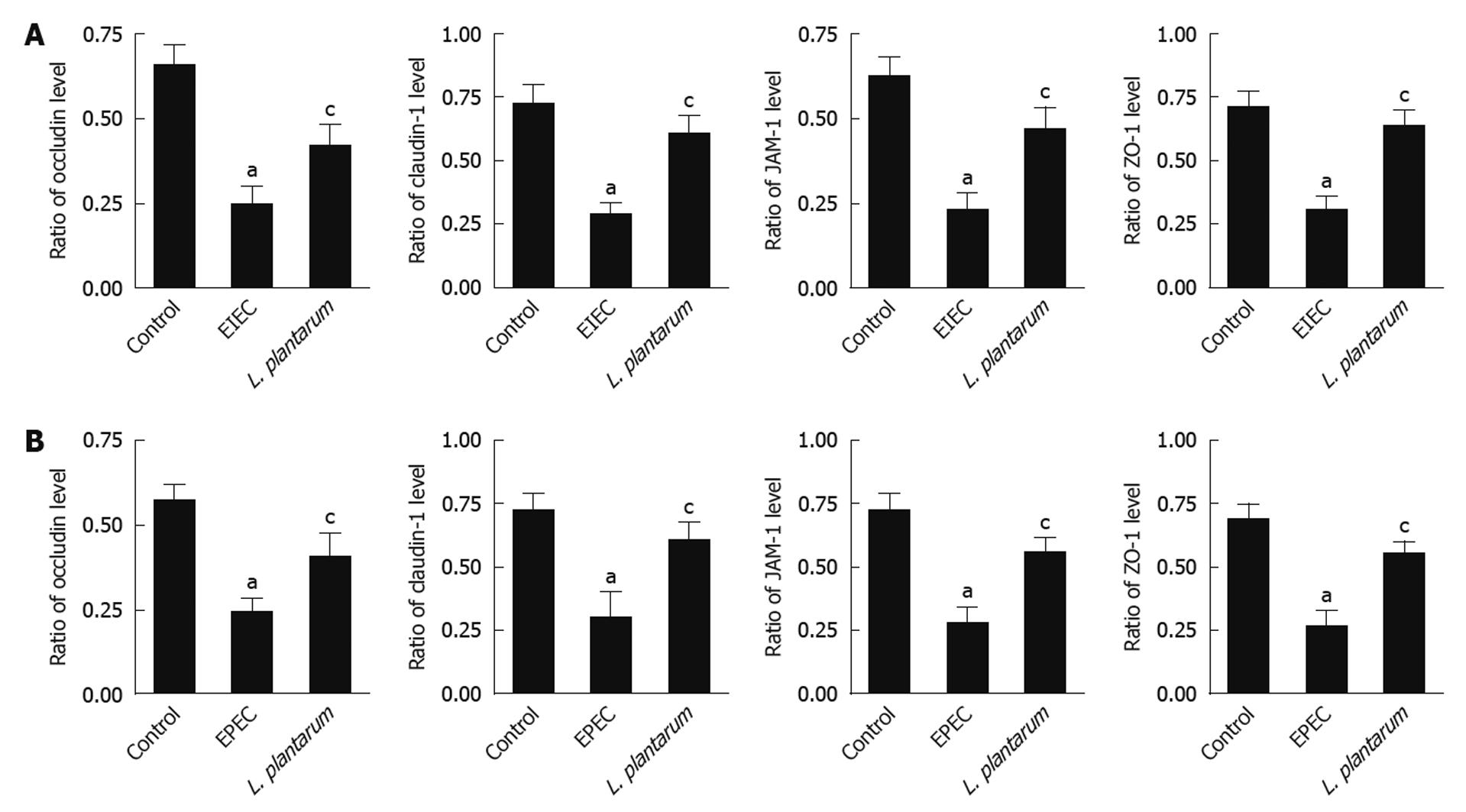
mRNA expression of TJ proteins, including claudin-1, occludin, JAM-1 and ZO-1, was significantly decreased in the NCM460 cells infected with EIEC or EPEC, as compared with the uninfected control cells (Figure 4A and B). However, treatment with L. plantarum raised mRNA expression levels similar to those in the uninfected control cells (Figure 4A and B).
It has been reported that probiotics, such as L. plantarum, have beneficial effects on the human intestinal barrier function in patients with intestinal diseases[29]. Our previous studies also found that L. plantarum adhered to the intestinal epithelial cells, restored TJ structure and function, reduced paracellular permeability, and then showed the therapeutic effects[20,23]. However, the studies about the interaction between lactobacillus and the human intestine were only limited in the cancer cell line and the animal models. The present study investigated the protective effects of L. plantarum against epithelial barrier dysfunction of the normal human colon cell line NCM460.
L. plantarum played an important role in increasing TER and decreasing the permeability to macromolecules of NCM460 monolayers against the disruption caused by EIEC or EPEC. EIEC and EPEC had the ability to decrease TER and increase the permeability to macromolecules[20]. In the present study, we further observed that L. plantarum protected the epithelial barrier of NCM460 monolayers against the disruption caused by EIEC or EPEC. In other words, L. plantarum is able to attenuate the pathogen-induced decrease in TER, and inhibit the increase in the macromolecular permeability of dextran. Similar results were also found in other studies. Johnson-Henry reported that probiotics attenuated enterohemorrhagic E. coli O157:H7-induced drop in electrical resistance, and increased the corresponding intestinal barrier permeability[30].
Furthermore, we found that L. plantarum prevented the decrease in the expression of TJ proteins and F-actin in NCM460 cells. The expression of TJ proteins, including claudin-1, occludin, JAM-1 and ZO-1, and the cytoskeleton element F-actin were decreased in NCM460 cells infected with EIEC or EPEC compared with the control cells, as detected by Western blotting of epithelial whole cell protein extracts of NCM460 cells. However, after the pre-treatment of L. plantarum, the expression of TJ proteins and F-actin remained at similar levels to the control cells. Other studies also found that Lactobacillus rhamnosus GG protected epithelial monolayers against EHEC-induced redistribution of the claudin-1 and ZO-1 TJ proteins. Resta-Lenert suggested that probiotics and/or commensals also reversed the epithelial damage produced by cytokines, and prevented the deleterious effects of tumor necrosis factor-α and interferon-γ in epithelial function[31].
Lactobacillus is reported to exert its beneficial effects by either producing bacteriostatic or bactericidal agents[32,33], competitively excluding pathogenic bacteria[34], or regulating immunomodulatory effects[13,31]. Furthermore, special signal transduction pathway is involved in the protective effects of L. plantarum on the intestinal epithelial barrier. Janus kinase inhibitor synergistically potentiated the effects of lactobacillus acidophilus on TER and permeability, while p38, ERK1, 2, or PI3K had no effects. After treated by lactobacillus, epithelial cells exposed to cytokines reduced the activation of SOCS3 and STAT1, 3.
We believe that our study broadens our knowledge of effects of L. plantarum in intestinal epithelial function and its therapeutic effects in the cellular and molecular mechanisms of intestinal barrier dysfunction and intestinal inflammation and justifies the use in inflammatory disorders, which is significant to both biotechnical and clinical fields. L. plantarum can protect against intestinal epithelial barrier dysfunction of NCM460 caused by EIEC or EPEC. However, the bacterial protein and its exact mechanisms of action remain unknown. We are conducting a study in an attempt to identify the protein and the smallest active domain within the protein from L. plantarum strain CGMCC1258 that is responsible for the adhesion of the bacterium to the intestinal epithelium. And further functional characterization by determining the effects of smallest active domain on the intestinal barrier function and immune responses is also in progress.
Lactobacillus plantarum (L. plantarum) that resides in the human intestine plays an important part in maintaining the homeostasis of gut flora by adhering and colonizing to the intestinal mucosa and competing with pathogenic bacteria, which contributes to the protection of the human intestinal barrier function.
Although L. plantarum exerted its therapeutic effects by adhering to the intestinal epithelial cells, restoring tight junction structure and function, and reducing paracellular permeability, the studies about interaction between lactobacillus and the human intestine were just limited in the cancer cell line and the animal models, and further studies based on the normal human intestinal cell had been unavailable.
Using the normal human colon cell line NCM460, this study investigated the protective effects of L. plantarum against epithelial barrier dysfunction caused by enteropathogenic Escherichia coli (E. coli) or enteroinvasive E. coli.
L. plantarum can be a potential candidate of therapeutic agents for the treatment of intestinal diseases.
NCM460 cell line is a normal human colon cell line, which is derived from the normal human colon mucosal epithelium and expresses colonic epithelial cell-associated antigens such as cytokeratins and villin.
This is a straightforward study extending previous work of the authors showing that L. plantarum maintains a high resistance to permeability to enteropathogenic and enteroinvasive E. coli. The work is extended to NCM460 colon cell cells in culture. Transepithelial electrical resistance was maintained high, dextran permeability was low and TJ protein expression was normal.
Peer reviewers: Dan L Dumitrascu, Professor, President, Romanian Society of Neurogastroenterology 2nd Medical Department University of Medicine and Pharmacy Iuliu Hatieganu Cluj, Romania; Dr. Adrian G Cummins, Department of Gastroenterology and Hepatology, (DX 465384), 28 Woodville Road, Woodville South, 5011, South Australia, Australia
S- Editor Wang YR L- Editor Ma JY E- Editor Zheng XM
| 1. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. |
| 2. | Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: a special focus on liver diseases. World J Gastroenterol. 2010;16:403-410. |
| 3. | Turroni S, Vitali B, Candela M, Gionchetti P, Rizzello F, Campieri M, Brigidi P. Antibiotics and probiotics in chronic pouchitis: a comparative proteomic approach. World J Gastroenterol. 2010;16:30-41. |
| 4. | Nuding S, Fellermann K, Wehkamp J, Stange EF. Reduced mucosal antimicrobial activity in Crohn's disease of the colon. Gut. 2007;56:1240-1247. |
| 6. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. |
| 7. | Fiocchi C. One commensal bacterial molecule--all we need for health? N Engl J Med. 2005;353:2078-2080. |
| 8. | Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med. 2003;31:598-607. |
| 9. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. |
| 10. | Ewaschuk J, Endersby R, Thiel D, Diaz H, Backer J, Ma M, Churchill T, Madsen K. Probiotic bacteria prevent hepatic damage and maintain colonic barrier function in a mouse model of sepsis. Hepatology. 2007;46:841-850. |
| 11. | Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010;16:69-75. |
| 12. | Matsuzaki T, Takagi A, Ikemura H, Matsuguchi T, Yokokura T. Intestinal microflora: probiotics and autoimmunity. J Nutr. 2007;137:798S-802S. |
| 13. | Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580-591. |
| 14. | Makharia GK, Sood A, Midha V, Ahuja V, Singal DK, Arora R, Sood S, Goswami P, De Simone C, Sahu AR. A randomized, double-blind, placebo-controlled trial of a probiotic preparation, Vsl#3, for the treatment of mild to moderate active ulcerative colitis. Gastroenterology. 2008;134 Suppl 1:A-99. |
| 15. | Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. 2009;104:437-443. |
| 16. | Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635-639. |
| 17. | Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300-310. |
| 18. | García-Lafuente A, Antolín M, Guarner F, Crespo E, Malagelada JR. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut. 2001;48:503-507. |
| 19. | Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522-1528. |
| 20. | Zhang M, Wang XQ, Zhou YK, Ma YL, Shen TY, Chen HQ, Chu ZX, Qin HL. Effects of oral Lactobacillus plantarum on hepatocyte tight junction structure and function in rats with obstructive jaundice. Mol Biol Rep. 2010;37:2989-2999. |
| 21. | Liu Z, Zhang P, Ma Y, Chen H, Zhou Y, Zhang M, Chu Z, Qin H. Lactobacillus plantarum prevents the development of colitis in IL-10-deficient mouse by reducing the intestinal permeability. Mol Biol Rep. 2010;Epub ahead of print. |
| 22. | Qin H, Zhang Z, Hang X, Jiang Y. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 2009;9:63. |
| 23. | Chu ZX, Chen HQ, Ma YL, Zhou YK, Zhang M, Zhang P, Qin HL. Lactobacillus plantarum prevents the upregulation of adhesion molecule expression in an experimental colitis model. Dig Dis Sci. 2010;55:2505-2513. |
| 24. | Qin HL, Zheng JJ, Tong DN, Chen WX, Fan XB, Hang XM, Jiang YQ. Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr. 2008;62:923-930. |
| 25. | Moss AC, Anton P, Savidge T, Newman P, Cheifetz AS, Gay J, Paraschos S, Winter MW, Moyer MP, Karalis K. Urocortin II mediates pro-inflammatory effects in human colonocytes via corticotropin-releasing hormone receptor 2alpha. Gut. 2007;56:1210-1217. |
| 26. | Kim H, Kokkotou E, Na X, Rhee SH, Moyer MP, Pothoulakis C, Lamont JT. Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology. 2005;129:1875-1888. |
| 27. | Koon HW, Zhao D, Zhan Y, Moyer MP, Pothoulakis C. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA. 2007;104:2013-2018. |
| 28. | Lelievre V, Hu Z, Byun JY, Ioffe Y, Waschek JA. Fibroblast growth factor-2 converts PACAP growth action on embryonic hindbrain precursors from stimulation to inhibition. J Neurosci Res. 2002;67:566-573. |
| 29. | Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658-672. |
| 30. | Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect Immun. 2008;76:1340-1348. |
| 31. | Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731-746. |
| 32. | Corr SC, Li Y, Riedel CU, O'Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci USA. 2007;104:7617-7621. |
| 33. | Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Komatsu A, Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunol Med Microbiol. 2004;41:219-226. |
| 34. | Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun. 2005;73:5183-5188. |