INTRODUCTION
Despite many advances in diagnostics and multimodal treatment (surgery, radiotherapy, chemotherapy), esophageal cancer is still generally associated with poor prognosis. The resectability rate in esophageal cancer is reported to be 60%-90%, but the resulting 5-year overall survival rate of resectable disease ranges from 10%-25%[1].
The incidence rate of esophageal cancer varies considerably according to geographical location. The highest rates occur in northern China and northern Iran, where incidence exceeds 1 in 1000 individuals[1]. By contrast, the incidence of esophageal cancer in most European countries and United States does not exceed 1/10 000[2,3].
Histologically, the main tumor types are squamous cell carcinomas and adenocarcinomas. Adenocarcinomas usually occur in the lower third of the esophagus, and this histological type has greatly increased in the last decade in several european countries and in the United States[4].
Currently, radiotherapy has a well-defined role in the management of esophageal cancer. Together with surgery and chemotherapy, it represents the main treatment modality in esophageal cancer. However, recent technological advances in radiation treatment (e.g. intensity-modulated radiotherapy, image guided-radiotherapy, positron emission tomography based radiotherapy planning, etc.) have progressively changed the practice, particularly in esophageal cancer. The main goal of these new approaches is the precise irradiation of the tumor while minimizing the risk of damage to healthy tissues.
CURRENT STRATEGIES
Multidisciplinary strategies for treatment
The choice of treatment strategy depends primarily on the patient‘s performance status, stage and extent of the disease, histology, and location of the primary tumor. The main curative treatment modalities are surgery and concurrent radiotherapy and chemotherapy. In most resectable thoracic esophageal carcinomas (stage IIA-IVA), concurrent chemoradiotherapy is provided as a preoperative treatment or as definitive treatment[5]. Meta-analyses confirmed that preoperative chemoradiotherapy substantially downstages the tumor[6], and the combination of preoperative chemoradiotherapy and surgery significantly increases 3-year overall survival and reduces the local-regional recurrence rate compared to surgery alone[7]. A recent meta-analysis by Gebski et al[8] evaluated ten randomized trials of preoperative chemoradiotherapy compared with surgery alone and suggested a 13% absolute difference in survival at 2 years with the combined treatment. Definitive chemoradiotherapy is the preferred modality for cervical esophageal cancer[5].
Recently, perioperative chemotherapy ECF (combination of epirubicin, cisplatin, and 5-fluorouracil) has become available for adenocarcinomas of the lower esophagus and the gastroesophageal junction, based on the results of the MAGIC trial. This randomized trial compared perioperative chemotherapy plus surgery to surgery alone in patients with adenocarcinoma of the stomach (74%), gastroesophageal junction (11%), and lower esophagus (14%). The 5-year survival rates were 36.3% for the perioperative-chemotherapy group compared to 23.0% for the surgery alone group[9].
The current standard scheme of concurrent chemoradiotherapy is radiotherapy (RT) in a dose of 50-50.4 Gy in 5-5.5 wk and 5-fluorouracil (5-FU) and cisplatin (cDDP) based concurrent chemotherapy. This recommended scheme is based on the results of two randomized trials. RTOG 8501 compared chemoradiotherapy in a dose of 50 Gy plus cisplatin and 5-FU vs radiotherapy alone in a dose of 64 Gy. This trial demonstrated the survival advantage of combined treatment (26% vs 0% at 5 years, P < 0.001), despite the lower dose of radiotherapy[10]. The intergroup study 0123 (RTOG 94-05) compared two arms with the same concurrent chemotherapy regimen (cDDP, 5-FU) and radiotherapy in doses of 64.8 Gy vs 50.4 Gy. There was no benefit from the higher dose with respect to the local failure rate (56% vs 52% at 2 years) or overall survival (31% vs 40% at 2 years)[11].
Standard of radiotherapy planning
The current standard of radiation treatment is a three-dimensional conformal radiotherapy (3D-CRT) based on three-dimensional computer tomography (CT) planning, with volumes delineated according to the International Commision on Radiation Units and Measurements (ICRU) reports 50[12] and ICRU Supplement 62[13].
The gross tumor volume (GTV) is defined on CT slices as a macroscopic primary tumor and involved lymph nodes. Endoscopic evaluation, endoscopic ultrasonography (EUS), and/or barium swallow are helpful. The endoscopic marking of the upper and lower extension of the visible tumor with metallic clips improves the definition of the GTV[14].
The clinical target volume (CTV) includes the GTV and areas at risk of a microscopic spread of the disease. In general, it is recommended to use the cranial and caudal margin of 4 cm due to submucosal spread and 1 cm radially. The same margins were used in the latest Radiation Therapy Oncology Group trials for esophageal cancer[15]. The lymph nodes at risk are included in the CTV according to the location of the primary tumor. For instance, the supraclavicular lymph nodes are included in the CTV in cervical esophageal cancer[16]. The PTV includes the CTV plus a margin for internal movements, mainly respiratory movements, (internal margin - IM; internal target volume - ITV) and for setup uncertainties (setup margin - SM). A margin of 1-2 cm beyond the CTV is used[15].
Dosimetric parameters of toxicity
Modern radiotherapy approaches have to evaluate the probability of organ-specific radiation toxicity. Organs at risk for esophageal cancer radiotherapy include lungs, heart, spinal cord, and for distal esophageal cancer, the liver and kidneys. Current knowledge of radiation toxicity derives from conventional and 3D-CRT data.
Lung toxicity: The dosimetric parameters of lung injury risk were studied mainly on lung cancer irradiation, but a few studies evaluated the risk of lung toxicity in esophageal cancer. In these studies, the increased risk of radiation pneumonitis correlated with heterogeneous parameters, such as mean lung dose (Dlung mean), the percentage of lung volume receiving at least 20 Gy (V20), 13 Gy (V13), 10 Gy (V10) or 5 Gy (V5) [17-22].
Graham et al[17] found a strong correlation between parameter V20 and the severity of pneumonitis in lung cancer patients. They reported the incidence of grade ≥ 2 pneumonitis as 7%, 13% and 36% for patients with V20 in the range of 22%-31%, 32%-40%, and > 40%, respectively. Kwa et al[18] considered the mean lung dose to be the most useful predictor of radiation pneumonitis in thoracic tumor radiotherapy. Based on pooled data from 540 patients irradiated for thoracic malignancy, the calculated risk of grade ≥ 2 pneumonitis was 43%, 18%, and 11% for mean lung doses of 24-36, 16-24 and 8-16 Gy, respectively. Schallenkamp et al[19], in a retrospective study, found the strongest predictors of pneumonitis to be the parameters V13 and V10. Lee et al[20] found, in 61 patients irradiated preoperatively with concurrent chemotherapy for esophageal cancer, a significant increase of postoperative pulmonary complications in cases with V10 > 40%. A more recent study from the same institution suggested that the factor most strongly associated with postoperative pulmonary complications is the volume of lung spared to doses of ≥ 5 Gy[21]. Tucker et al[22] analyzed 110 patients with preoperative chemoradiotherapy for esophageal cancer and, in that cohort, the mean lung dose and absolute volume of lung volume receiving < 5 Gy were similar predictors of postoperative pulmonary complications.
Other potential risk factors for radiation pneumonitis, besides the heterogenous dosimetric parameters, have been proposed, such as concurrent chemotherapy, age, pretreatment pulmonary functions, presence of chronic pulmonary disease, and others[23-25]. Radiation oncologists have to judge all dosimetric and non-dosimetric risk factors for pulmonary complications of radiotherapy before approval of the treatment plan.
Heart toxicity: The most common manifestation of late radiation injury to the heart is pericardial disease. It may present as acute pericarditis, as chronic pericardial effusion, or can be asymptomatic. The myocardium is involved less frequently, but it develops into more serious cardiomyopathy, usually characterized pathologically as diffuse fibrosis. From long term surviving patients after radiotherapy for Hodgkin‘s lymphoma or left sided breast cancer, there is evidence that radiotherapy can substantially increase the risk of acute myocardial infarction after radiotherapy. The reason is probably an acceleration of coronary artery disease as a late effect of the radiotherapy[26].
Wei et al[27] evaluated the influence of definitive radiochemotherapy on pericardial toxicity. They reported, in 101 patients, a development of pericardial effusion in 27.7 % at a median of 5.3 mo. The pericardium volume irradiated by a dose of 30 Gy or higher (V30) was identified to be a significant predictor of pericardial effusion risk. Tripp et al[28] reported a significant reduction of ejection fraction in 20 patients after neoadjuvant chemoradiotherapy (59% vs 54 %, P = 0.01), but without significant clinical morbidity. Inferior left ventricle ischemia is commonly found in patients having received radiotherapy for distal esophageal cancer. The ischemic segments usually occurr in volumes irradiated to a dose of 45 Gy or more[29].
Spinal cord toxicity: The recommended maximum dose on the spinal cord is 45 Gy[5]. Current data suggests that the probability of myelopathy at 45 Gy is 0.03%, and at 50 Gy, is 0.2%[30]. In this case, the risk of radiation myelopathy in esophageal cancer is low, with a standard prescribed dose of 50.4 Gy as it is easy to comply to the recommended dose limits for the spinal cord. An increased risk of myelopathy can be expected in connection with a dose escalation approach.
Liver toxicity and kidney toxicity: The liver and kidneys are considered to be organs at risk mainly in distal esophageal cancer when the irradiated volume involves the upper abdomen. The risk of radiation injury is minimal when the mean liver dose is < 30 Gy[31] and if at least 50% of the functional kidney parenchyma is spared doses > 20 Gy, which is considered to be the tolerance dose for the human kidney[32].
NEW TECHNOLOGIES IN RADIOTHERAPY
Positron emission tomography/CT based radiation therapy planning
In esophageal cancer there is a high interobserver variability in target volume delineation among tumor sites[33]. The precise definition of the primary tumor and involved lymph nodes is crucial for RT planning.
Positron emission tomography (PET) with 18F-fluoro-2-deoxyglucose (FDG) as a tracer, was documented in several centres as a highly effective diagnostic modality for the initial staging in patients with esophageal cancer, especially in revealing lymph node involvement or distant metastases[34], although the sensitivity for regional lymph node involvement of FDG-PET is controversial[35-38]. FDG-PET imaging became beneficial in radiotherapy planning in several tumor types, mainly in lung cancer, because of the significant impact on target volume delineation[39]. Therefore, the concept of integration of PET into the RT planning process was rational.
Vrieze et al[40] added FDG-PET imaging to the RT planning process based on conventional CT and EUS status in 30 patients. In six patients, eight positive lymph nodes were identified on FDG-PET alone, but not on CT/EUS imaging. In three of these patients the target volumes would be enlarged. By contrast, in eight patients, nine positive lymph nodes were identified only on CT/EUS, but not on PET. Therefore, the authors recommended not to reduce the CTV based on negative lymph node FDG-PET status.
In another study, Konski et al[41] evaluated the impact of PET and EUS compared with CT alone in radiotherapy planning in 21 patients. Their results showed a low sensitivity of FDG-PET alone to determine regional lymph node metastases. EUS detected significantly more patients with periesophageal and celiac lymphadenopathy compared to PET and CT. Patients with periesophageal lymphadenopathy on PET had a higher primary tumor standard uptake value. The authors also found that the length of the primary tumor (GTV) was significantly longer when determined on CT scans compared with PET scans. The mean length of the GTV, as determined on PET, CT and endoscopy was 5.4, 6.77 and 5.1 cm, respectively.
The sensitivity of FDG-PET alone is considered by some authors to be low[37,38]. Compared to FDG-PET alone, integrated PET/CT imaging improves the sensitivity and accuracy in the assessment of locoregional lymph nodes in esophageal cancer[42,43]. In a retrospective study by Muijs et al[44], target volumes were independently defined based on CT only and based on coregistered PET/CT in 21 patients. In that study, PET/CT-based planning of target volumes was inadequately covered by CT only-based treatment plans in eight patients (38%). In the study of Gondi et al[45], PET/CT-based target volumes were compared to CT only-based target volumes in 16 patients with esophageal cancer. In ten of these patients the addition of PET to the planning led to a reduction of the GTV volume.
Leong et al[46] evaluated the impact of PET/CT-based planning in a prospective trial. The target volumes based on CT alone and PET/CT were compared in 21 patients. The addition of PET information altered the clinical stage in eight patients (38%). Four patients had a distant metastatic disease and four had an unsuspected regional nodal disease. The PET findings led to a change in the management from radical chemoradiation to treatment with palliative intent in five of these patients (24%). In 16 patients (69%) the PET avid disease was not included in the GTV.
In a similar study by Moureau-Zabotto et al[47], the target volumes based on CT alone and subsequently on PET/CT were defined and compared in 34 patients. Unknown metastatic disease was detected by PET/CT in two patients. The GTV was modified in 19 patients (56%); in 12 of these patients the GTV was decreased and in seven patients it was increased by PET/CT. In 18 patients it led to the modification of the PTV. The influence of PET/CT based RT planning on the total lung volume receiving > 20 Gy was also shown.
Recently, Shimizu et al[48] published a study of 20 patients who were examined before surgery by PET/CT and EUS. Based on these preoperative diagnostic modalities, the target volumes for radiotherapy were defined and these volumes were compared to the histopathologic findings. Although hybrid PET/CT was used, the CTV was inadequately covered in seven cases out of 20. When EUS was added to PET/CT, inadequate CTV cover occurred in five cases compared to eight cases with CT only- based target volumes.
Therefore, despite the growing popularity of PET/CT based RT planning in esophageal cancer, the recently published International Atomic Energy Agency expert report 2006-2007 classifies esophageal cancer as a diagnosis where the use of PET or PET/CT for RT planning should be cautiously considered, as there is still limited supporting data[49].
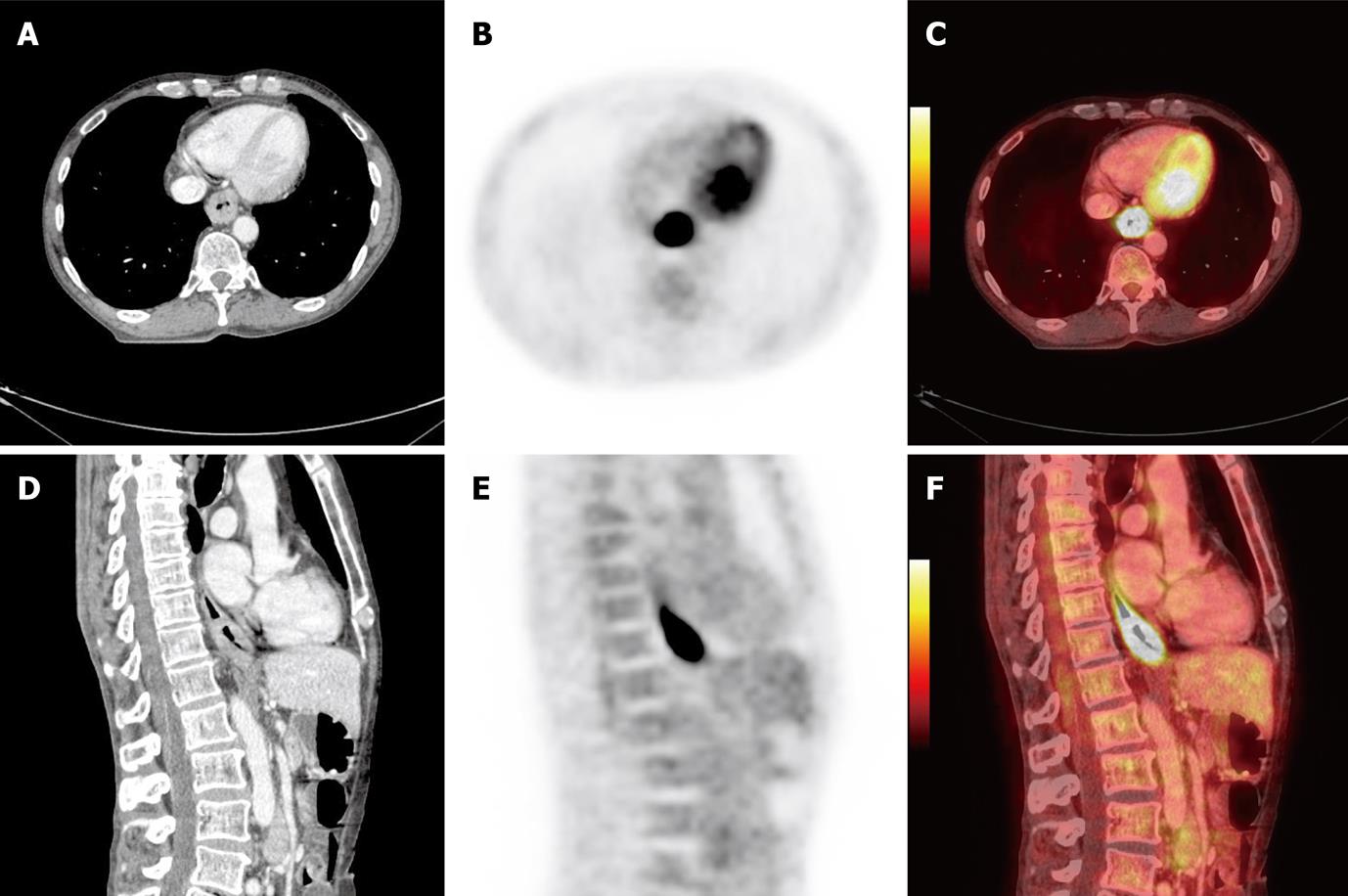
An example of PET/CT images performed in treatment position for radiotherapy for esophageal cancer is shown in Figure 1.
Figure 1 Positron emission tomography/computed tomography images of 61-year-old man with primary squamous cell carcinoma in distal part of the esophagus T3N0M0 prepared in treatment position for radiotherapy planning.
A: Computed tomography (CT), axial slice; B: Positron emission tomography (PET), axial slice; C: PET/CT fusion, axial slice; D: CT, sagital slice; E: PET, sagital slice; F: PET/CT fusion, sagital slice.
Intensity-modulated radiation therapy
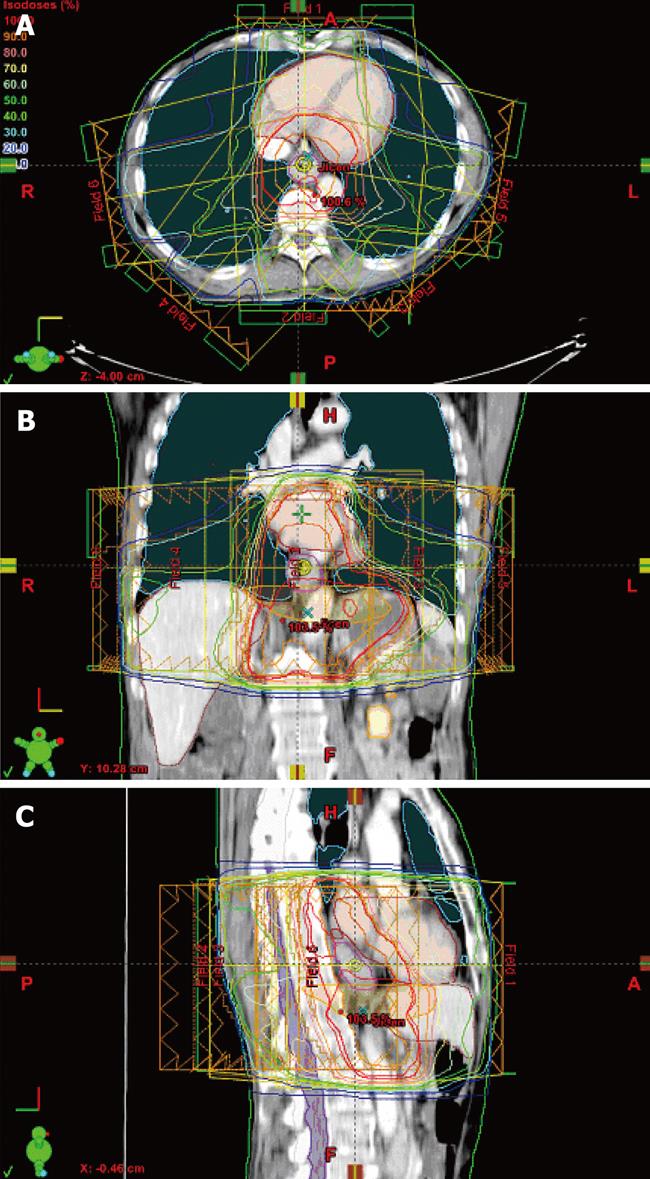
Intensity-modulated radiotherapy (IMRT) is an advanced form of conformal radiotherapy that utilizes computer-controlled linear accelerators to deliver precise radiation doses to the PTV (Figure 2). The principal of IMRT is the use of variable radiation fluence patterns from multiple beam angles. The beam fluences are calculated by automated computer-assisted optimization and the combination of optimal beam fluences results in an optimal dose distribution. Current commercial treatment planning software for optimization usually requires only a definition of beam configuration, PTV(s), and dose-volume constraints of organs at risk with varying penalty weights - so called inverse planning.
Figure 2 Intensity-modulated radiotherapy plan prepared on a positron emission tomography/computer tomography dataset (Figure 1).
Delineated target volumes and organs at risk, beam arrangement and dose distribution in axial (A), coronal (B), and sagittal slices (C).
The main dosimetric advantage of IMRT is the possibility of better sparing of healthy tissues and organs at risk, including shape concavities of the PTV. Therefore, IMRT facilitates a lower risk of late adverse effects of radiotherapy and better local control of the tumor, due to the possibility of safe dose escalation. There has already been much data published worldwide demonstrating dosimetric, and even clinical, improvement of radiation treatment by IMRT, mainly in prostate cancer and head and neck cancer[50,51]. For these reasons, there has been a massive expansion of IMRT worldwide in the last few years.
In addition to the sparing of organs at risk and the possibility of dose escalation in the whole PTV, IMRT offers a dose escalation in every fraction in the subregion with a high risk of local recurrence (primary tumor or tumor bed). This principle is called simultaneous integrated boost (SIB), and it is used mainly in head and neck cancer. The advocates of SIB-IMRT techniques emphasize a better conformality of irradiation in comparison to shrinking volumes technique[52,53].
Several dosimetric studies have been published in esophageal cancer. Nutting et al[54] compared IMRT plans with various beam angles and 3D-CRT plans in five patients. They concluded that IMRT using conventional beam angles (two opposed antero-posterior fields and two posterior oblique fields) can provide an acceptable dose homogeneity within the PTV and reduces lung irradiation compared to 3D-CRT. The mean values of the mean lung dose and relative mean lung volume within a dose of 18 Gy were 11.0 Gy and 9.5 Gy (P = 0.001), and 18.8% and 14.1% (P = 0.001), for 4-field IMRT and 3D-CRT, respectively. IMRT plans using nine equispaced fields were not superior to 3D-CRT plan due to the increased lung volume involved with low doses of irradiation.
The reduction of lung dose by IMRT inverse planning in esophageal cancer was subsequently reported by Wu et al[55] They prepared IMRT plans using 3-9 equispaced beams and 3D-CRT plans in CT data of 15 patients. The percentage volume of the lung receiving 25 Gy or above (V25) was used as the main dosimetric parameter for the lungs. There was a considerable reduction in V25 in the IMRT plans compared with the inverse planned 3D-CRT (24.6% and 18.2% for the left and right lungs, respectively). The average mean heart dose was not statistically different, but the calculated normal tissue complications probability (NTCP) was significantly lower using IMRT.
The comparison of IMRT vs 3D-CRT plans in cervical esophageal cancer was the goal of the dosimetric study in five patients of Fenkell et al[56] IMRT plans provided better PTV coverage, higher conformality, a reduction of the maximum dose to the spinal cord and brain stem, and a lower mean dose to the parotid glands.
In another study for upper esophageal cancer, Fu et al[57] reported a dosimetric comparison of conformal radiotherapy and SIB-IMRT technique with 3, 5, 7 and 9 beams. The prescribed doses were 67.2 Gy and 50.4 Gy in the primary lesions and electively treated regions; the dose per fraction was 2.4 and 1.8 Gy, respectively. The authors concluded that IMRT-SIB shortens the total treatment time and the primary tumor can receive a higher equivalent dose by SIB. The five equispaced coplanar SIB-IMRT technique produced desirable dose distribution. This regimen is under clinical evaluation in this center.
Chandra et al[58] retrospectively compared 4-9 beam IMRT and 3D-CRT plans for distal esophageal cancer in ten patients. The IMRT improved PTV heterogeneity. There was a reduced total lung volume irradiated above 10 Gy and 20 Gy (V10 and V20), and the mean lung dose using the IMRT plans. However, at low dose levels (below 7 Gy), there was a tendency of increased volume V7 with more beams for IMRT plans.
Mayo et al[59] described a technique of combined static and intensity-modulated beams for lung and esophageal cancer radiotherapy. This hybrid IMRT technique reduced the V5, V13, and V20 volumes in a subgroup of esophageal cancer compared to the IMRT only plans. The largest differences were in V13. However, there was an increase in dose to the heart in the hybrid IMRT plans.
The risk of larger volumes of lungs irradiated by lower doses of radiotherapy (≥ 5 Gy; V5) was mentioned above. The IMRT plans can be associated with higher V5 because of the high number of beams and their configuration, but it can also be partially caused by a leakage of the multileaf collimator during IMRT[60].
Although the dosimetric studies demonstrated the advantage of IMRT in tumors of all parts of esophagus, the clinical results of IMRT for esophageal cancer are still limited to a few small studies. Wang et al[61] retrospectively analysed six patients treated by 5-9 beam IMRT with concurrent chemotherapy for locally advanced cervical and upper thoracic esophageal cancer. The prescription dose was 59.7-66 Gy in 28-33 fractions; in five patients a simultaneous integrated boost technique was used. In this study, all six patients achieved complete remission. However, two patients developed local recurrence; one patient had distant metastases and one patient had both regional and distant metastases. Three of the four surviving patients developed an esophageal stricture or fistula.
At Stanford University, 30 patients were treated with preoperative or definitive chemoradiotherapy using IMRT in a median dose of 50.4 Gy (range 34.2-58.8 Gy) for esophageal cancer. 67% of tumors were adenocarcinomas. The 2-year local-regional control was 83% in patients treated preoperatively and 51% in definitively treated patients. One patient died because of a complication following the placement of a gastrostomy tube during the irradiation course (after a dose 34.2 Gy). One patient developed postoperative acute respiratory distress syndrom. Eight patients (27%) developed late esophageal stricture requiring dilatations[62].
Tomotherapy
Helical tomotherapy is a form of intensity-modulated radiotherapy using a helical radiation delivery system. The beam delivery is similar to that of helical computed tomography.
Chen et al[63] compared three radiotherapy techniques diametrically - tomotherapy, step-and-shoot method of IMRT, and 3D-CRT - in the CT data of six patients. In the study, tomotherapy was superior to IMRT and 3D-CRT due to better dose conformity, dose homogeneity, and sparing of lung volume from doses ≥ 20 Gy (V20). Helical tomotherapy and IMRT compared to 3D-CRT spared the heart better (decreased V30 and V45). However, tomotherapy and IMRT plans resulted in a larger V10 of lungs compared to 3D-CRT plans. The same centre also reported the first clinical results of tomotherapy with concurrent chemotherapy in 20 patients with esophageal cancer at the American Society for Radiation Oncology Annual Meeting 2007. The prescribed dose was 50 Gy in the GTV and 45 Gy in the region of a possible subclinical disease. Ten patients were indicated after chemoradiotherapy for surgery, and in eight of them, downstaging was noted with two complete responses. There was a clinically complete response in six patients without surgery. Grade 3 acute toxicity was noted in nine patients (45%) without any specification by the authors. No grade 4 toxicity occured. Two patients developed pneumonitis after surgery[64].
Image guided radiation therapy
The risk of set-up uncertaintities known from megavoltage portal imaging led to a development of modern technological devices integrated into linear accelerators facilitating high precision positioning of a patient before each irradiation (minimizing of interfraction movements). Image-guided radiotherapy (IGRT) generally means the process of two-dimensional or three-dimensional imaging of the patient in the treatment position on the linear accelerator before an irradiation, with the aim of minimizing of the set-up error. Currently, a wide variety of online 2D and 3D imaging is used - megavoltage portal imaging, kilovoltage imaging, kilovoltage CT (commonly cone-beam), megavoltage CT (mainly on helical tomotherapy), and others[65]. The IGRT software allows an image acquisition, matching with the referrence images and individual setup corrections.
The IGRT can be practiced as a daily procedure before each fraction of radiotherapy to minimize random and systemic errors[66]. The second strategy uses an evaluation of random setup errors and, based on the result, the PTV is corrected. This concept, described by Yan et al[67,68], was first implemented in prostate cancer radiotherapy and was called by the authors adaptive radiotherapy.
Stroom et al[69] recommended the size of the CTV-PTV margin which ensures at least 95% of the prescribed dose to 99% of the CTV, to be equal to about 2Σ + 0.7σ, where Σ is the standard deviation of the distribution of systemic deviations and σ is the average standard deviation of the distribution of random deviations. Chen et al[70] calculated these parameters in ten patients who underwent helical tomotherapy for esophageal cancer. The suggested CTV-PTV margins were 5.0 mm in the anterior-posterior direction, 11.1 mm in the lateral direction and 12.7 mm in the superior-inferior direction. Therefore, the authors recommend a megavoltage CT on tomotherapy before each fraction to minimize setup errors when lesser CTV-PTV margins are used.
Hawkins et al[71] recently presented the concept of cone-beam CT-derived adaptive radiotherapy for esophageal cancer treatment at their center. In 14 cases, the standard plan with CTV-PTV margin of 1 cm was prepared. The cone-beam CT was obtained before the first four fractions and, based on this data, the composite CTV with 5 mm margin for the PTV was defined. The same process was subsequently repeated weekly and the plans were compared. The study demonstrated a significant reduction in the dose received by the heart and lungs because of lesser individualized CTV-PTV margins.
4D-computer tomography and respiratory control techniques
The limitation of the precise dose delivery by 3D-CRT or IMRT in the region of the thorax and upper abdomen are physiologic movements of tumor and organs, mostly due to respiratory or cardiac cycles (intrafraction movements). Therefore, radiation oncologists have to estimate the internal target margin to be adequate to these movements to cover target volumes sufficiently.
The respiratory motion of anatomic structures and target volumes has been investigated in several diagnoses, mainly in lung cancer, breast cancer, and upper abdomen malignancies. Currently, the optimal method for acquiring exact information on movements of structures during the respiratory cycle is respiratory-correlated computed tomography imaging (4D-CT). This technology allows the capture of CT data in separate phases of the respiratory cycle. Their co-registration gives precise information on the amplitude of the structure motion and the position of the structure in each phase of the cycle.
New technologies were evolved to compensate for tumor motion to lower the internal margin for the PTV and, hence, to reduce the volume of surrounding healthy tissues. These include active or passive respiratory gating (respiratory gated radiotherapy) and respiratory tracking techniques.
The principle of active breathing control (ABC) is the monitoring of the respiratory cycle by a special mouth apparatus measuring the airflow (spirometric system). At a preset phase of the cycle (preset volume of expired air), the ABC apparatus temporarily blocks the airflow of the patient and during this period the irradiation is applied[72]. The Real-Time Position Management™ (RPM) System (Varian Medical System, Palo Alto, USA) uses infrared camera monitoring of the movements of a small plastic box with reflective markers placed on the skin in the patient’s upper abdomen during free breathing or active breathing.
Real-time respiratory tracking is a method that dynamically moves or shapes the radiation beam to follow the tumor‘s motion during irradiation. This method was first evolved for CyberKnife System (Accuray, Inc., Sunnyvale, USA), which features a linear accelerator mounted on a robotic arm (Synchrony Respiratory Tracking System).
The evaluation of esophageal tumor movements during the respiratory cycle was studied by Lorchel et al[73] in eight patients with various locations of the primary tumor in the esophagus. The cumulative distribution of the GTV and CTV motion in all three directions in absolute terms showed that 95% of the data ranged from 0 to 0.8 cm and from 0 to 1.0 cm, respectively. Therefore, the authors recommended the size of the internal target margin of 1 cm. Zhao et al[74] quantified the internal target motion in tumors near the gastroesophageal junction. They found that the tumors exhibited asymmetric and directional changes in shape and volume, mainly for large GTVs. The mean range of the aboral margin of the tumor was greatest in the caudal direction at 0.91 ± 0.36 cm. The mean range of the motion in anterior and posterior directions was 0.68 ± 0.23 cm and 0.36 ± 0.13 cm, respectively. The lateral motion was 0.27 ± 0.09 cm to the right and 0.63 ± 0.20 cm to the left because of movements of the stomach on the left side of the GTV. Based on these results, the authors suggested the use of asymmetric internal target margins: 1.0 cm to the left, 0.8 cm to the right, 1.1 cm anteriorly, 0.6 cm posteriorly, 1.0 cm superiorly, and 1.6 cm inferiorly. The study also evaluated the influence of the heart beat: the largest mean range of motion was 0.56 ± 0.18 cm for the esophageal wall adjacent to the heart.
The optimal approach to respiratory gated radiotherapy was studied by Lorchel et al[75] In a dosimetric study, four spiral scans were performed in eight patients with advanced esophageal cancer: one in the end of expiration, one in the end of inspiration, one in deep inspiration breathhold, and one acquisition was performed in free breathing. Based on the results, the authors suggested the irradiation of esophageal cancer patients in deep inspiration breathhold in the case of using spirometric system and in the inspiration phase in the case of free breathing gating system to reduce a dose to the lungs (reduction in V20) and heart (decreased V40).
Proton therapy
The idea of using proton beam therapy as an effective radiotherapy method was first proposed by Wilson et al[76] in 1946. The main advantage of proton radiotherapy is a better dose conformity due to proton beam characteristics. Proton beams are characterized by a narrow penumbra and maximal energy transfer at the end of the range in tissue (Bragg peak). The proton therapy of various malignancies has been discussed in recent years. A few studies on proton therapy for esophageal cancer are also available[77-80].
The dosimetric study of Isacsson et al[77] compared the treatment plans with proton beams to plans with photons and combined plans in five patients with esophageal cancer. There was an evident advantage of proton plans in the reduction of dose to organs at risk in all patients. The sparing of the lungs using proton radiotherapy compared to IMRT was noted by Zhang et al[78] in a dosimetric study for distal esophageal cancer.
The published clinical studies using proton beam therapy for esophageal cancer applied a dose escalation approach. Koyama et al[79] irradiated 30 patients (13 patients with superficial and 17 patients with advanced tumors) with proton beam therapy alone (median fraction dose of 3.2 Gy) or in combination with photons (median fraction dose of 3.1 Gy). Overall mean total doses were 77.7 Gy in superficial carcinomas and 80.7 Gy in advanced carcinomas, respectively. The rates of local recurrence at 5 and 10 years were 0% for superficial cancer, and 56.6% and 78.3%, respectively, for advanced cancer. The radiation-induced esophageal ulcer as a late effect occurred in 66.7% of patients.
Sugahara et al[80] reported the clinical results of proton beam therapy in 46 patients. Forty patients were treated by a combination of photons and protons as a boost to a median total dose of 76.0 Gy (median fraction dose 3.0 Gy), six patients were irradiated only by protons to a median total dose of 82.0 Gy (median fraction dose 3.1 Gy). The local control at 5 years was 57% (83% with stage T1 and 29% with stage T2-T4). Postradiation esophageal ulcers were developed in 48% of patients.