Published online Nov 21, 2010. doi: 10.3748/wjg.v16.i43.5474
Revised: April 19, 2010
Accepted: April 26, 2010
Published online: November 21, 2010
AIM: To evaluate duodenal polyps, divided into non-neoplastic and neoplastic lesions. In addition, the clinical characteristics of duodenal hyperplastic polyps are determined.
METHODS: We analyzed medical records of 50 114 consecutive patients submitted to for first diagnostic esophago-gastroduodenoscopy between January 2004 and December 2009. We excluded lesions on the ampulla of Vater and submucosal tumors. We studied 510 cases that were diagnosed endoscopically with duodenal polyps and enrolled a total of 221 cases that had undergone tissue biopsy. We analyzed the differences between non-neoplastic and neoplastic lesions, and determined the clinical features of duodenal hyperplastic polyps.
RESULTS: Non-neoplastic lesions were found in 196 patients and neoplastic lesions in 25 patients. On univariate analysis, there were significant differences in shape, location, and size. Polyps more than 10 mm in diameter or polyps in the second portion had independent risk factors for being neoplastic lesions, as identified by multivariate analysis. In 23 cases of hyperplastic polyps (79.3%), they were accompanied by gastro-duodenal pathology, which was possibly associated with Helicobacter pylori.
CONCLUSION: Polyps of more than 10 mm or polyps in the second portion of the duodenum should be evaluated by histological examination.
- Citation: Jung SH, Chung WC, Kim EJ, Kim SH, Paik CN, Lee BI, Cho YS, Lee KM. Evaluation of non-ampullary duodenal polyps: Comparison of non-neoplastic and neoplastic lesions. World J Gastroenterol 2010; 16(43): 5474-5480
- URL: https://www.wjgnet.com/1007-9327/full/v16/i43/5474.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i43.5474
The prevalence of duodenal polyps is estimated to be 0.3%-1.5% in patients referred for upper endoscopy, on the basis of extensive retrospective studies[1,2], and 4.6% reported from a prospective study[3]. Although duodenal polyps are rare, the diagnosis appears to be increasing, possibly due to the wide use of diagnostic esophago-gastroduodenoscopy. Duodenal polyps are commonly sessile, but may also be pedunculated in nature. Most duodenal polyps are inflammatory polyps and have ectopic gastric mucosa[1,2]. Multiple and small polyps in the duodenal bulb are always benign and need neither biopsy nor treatment; except in patients with familial polyposis, where routine upper gastrointestinal surveillance endoscopy and biopsy of the duodenal polyps are mandatory[3]. Cases requiring clinical treatment are rare; however, a biopsy occasionally shows an adenoma or carcinoid tumor. These lesions require treatment due to their potential of malignant transformation. Until now, the clinical findings associated with non-neoplastic and neoplastic duodenal lesions have not been well described.
Hyperplastic (metaplastic) polyps have rarely been described in the duodenum, and published data on these lesions are limited to case reports or small case series[4-6]. They almost always occur in the setting of ectopic gastric mucosa and therefore have an appearance that more closely mimics hyperplastic polyps of the gastric type, rather than the colonic type. Moreover, when hyperplastic polyps are found in the duodenum, they can be associated with colonization by Helicobacter pylori (H. pylori)[7], and appear to occur most commonly in the setting of peptic ulcer disease or with other gastric disorders[5].
This study is the largest single-center study to date that reports on the clinical characteristics of non-ampullary duodenal elevated lesions. We aimed to evaluate the frequency, endoscopic findings, and histological characteristics of non-neoplastic and neoplastic lesions. We also analyzed a spectrum of patients with duodenal hyperplastic polyps.
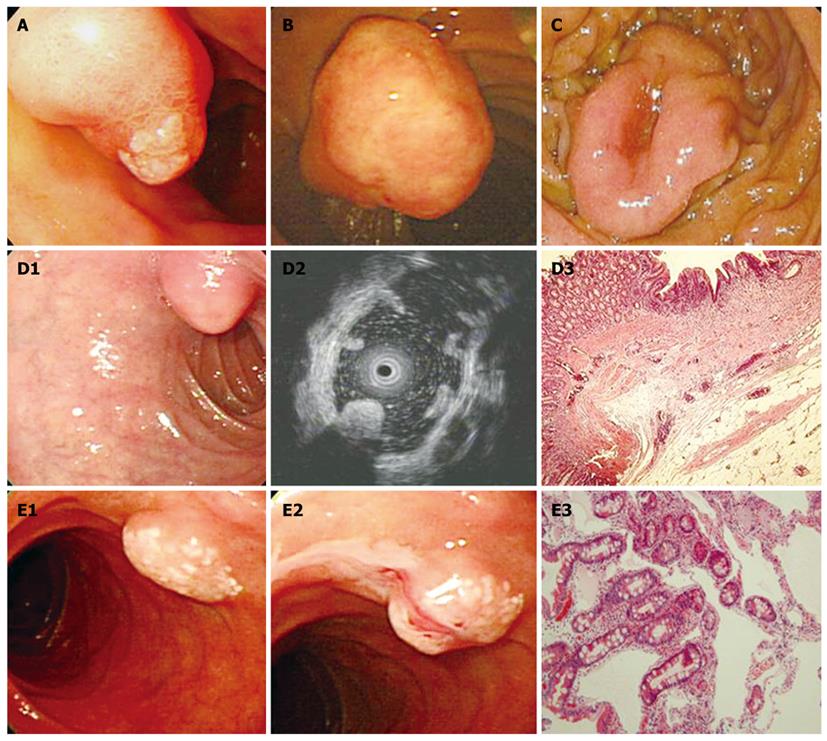
We searched the medical records of 50 114 consecutive patients that had a first diagnostic esophago-gastroduodenoscopy between January 2004 and December 2009. Most of patients had visited the gastrointestinal department and had dyspeptic symptoms. We used the electronic database of St. Vincent’s Hospital, the Catholic University of Korea and searched the keywords of “duodenal polyp” (K317; International Statistical Classification of Disease, the 10th Revision, ICD-10) or “benign neoplasm of duodenum” (D132). Cases were accrued by searching the database. Among 510 cases diagnosed endoscopically with duodenal polyps, 221 cases that underwent tissue biopsy were enrolled. Lesions of the ampulla of Vater (D135) and submucosal tumors (D372) - cysts, lipomas, lymphangiectasia, and gastrointestinal stromal tumors, were excluded and were traditionally recognized as submucosal lesions (Table 1). Endoscopic and histological features of the ampullary tumors and submucosal tumors are shown in Figure 1. This study protocol was approved by the Ethics Committee of the Catholic University of Korea.
| Histologic diagnosis | No. (n = 44) |
| Lesions in the ampulla of vater | |
| Adenoma | 12 |
| Focal carcinomatous change | 1 |
| Inflammatory myofibroblastic tumor | 1 |
| Carcinoid | 1 |
| Submucosal lesions | |
| Lipoma | 14 |
| Cyst | 9 |
| Lymphangioma | 3 |
| Gastrointestinal stromal tumor | 2 |
Data collected on the study subjects included age, gender, family history of polyposis, and location, size, and histology of the duodenal polyp. All tissue specimens were obtained using biopsy forceps (FB-19K-1, Olympus, Tokyo, Japan), polypectomy, or endoscopic mucosal resection. The size of the polyp was measured endoscopically using the “open biopsy forceps method”. Biopsy forceps with a diameter of 4 mm when fully opened were used. The forceps were withdrawn in the open position toward the endoscope tip as far as possible, until both cups were fully visualized. The open forceps was then advanced until they were aligned against the largest diameter of the polyp, with the tip of the endoscope still placed at approximately 3 to 4 cm from the polyp[8].
The endoscopic, histological, and clinical characteristics of the duodenal polyps were recorded; subsequently, the duodenal polyps were divided into non-neoplastic and neoplastic lesions. The non-neoplastic lesions included Brunner’s gland hyperplasia, Brunner’s gland hamartoma, ectopic gastric mucosa, ectopic pancreas, hyperplastic polyps, and inflammatory polyps. The neoplastic lesions included adenomas, carcinoid tumors, and miscellaneous lesions. We analyzed the differences of age, sex, size, shape, location and multiplicity between the non-neoplastic and neoplastic lesions. In addition, we evaluated the clinical features of the duodenal hyperplastic polyps. Each case was classified as H. pylori positive or negative according to the histological results (CLO test or silver stain) using two pieces of biopsy specimen taken from the antrum and the body of the stomach.
All data were recorded on standard forms and computer analyzed. The Mann-Whitney U-test was used to compare the continuous variables between the two groups. Differences between dichotomous variables were evaluated with the Pearson Chi-square test. Calculations were performed with the SPSS package software (SPSS version 12.0, Chicago, IL, USA). P values less than 0.05 were considered significant.
The frequency of duodenal polyps was 1.02% (510 of 50 114 patients). Among 221 patients, forceps tissue biopsy, and endoscopic or surgical resections were performed and a histological diagnosis was obtained. We divided them into non-neoplastic and neoplastic lesions. Non-neoplastic lesions were found in 196 patients and neoplastic lesions in 25 patients. For the non-neoplastic lesions, Brunner’s gland hyperplasia/hamartoma, ectopic gastric mucosa, ectopic pancreas, hyperplastic polyps and inflammatory polyps were found. Adenomas, carcinoid tumors, and metastatic cancer were found in neoplastic lesions (Table 2). The mean age and gender ratio were not significantly different between patients having non-neoplastic and neoplastic lesions.
| Non-neoplastic lesions | No. (n = 196) | Neoplastic lesions | No. (n = 25) |
| Brunner’s gland hyperplasia | 17 | Adenoma | 14 |
| Brunner’s gland hamartoma | 4 | Serrated adenoma | 1 |
| Focal cancer change in adenoma | 1 | ||
| Ectopic gastric mucosa | 12 | Originated in Brunner’s gland | 1 |
| Ectopic pancreas | 1 | In familial adenomatosis polyposis | 1 |
| Hyperplastic polyp | 29 | Carcinoid tumor | 6 |
| Inflammatory polyp | 33 | ||
| Normal pathology | 100 | Metastatic cancer | 1 |
The characteristics of the non-neoplastic and neoplastic lesions are shown in Table 2. The size of the neoplastic lesions was larger than the non-neoplastic lesions (P < 0.01). Non-neoplastic duodenal polyps were more frequently localized in the bulb (156/196, 79.6%) than in the second portion. In the bulb, approximately 90% of duodenal polyps were non-neoplastic. Neoplastic duodenal polyps had a tendency to be located in the second portion. Twelve (48%) out of 25 neoplastic lesions were encountered, whereas 42 (21.4%) out of 196 non-neoplastic lesions were noted (P < 0.01). In the bulb, neoplastic lesions comprised six cases of carcinoid tumor and seven cases of adenoma. The endoscopic findings for duodenal carcinoid tumors revealed a smooth and round elevation with an erythematous change or central depression. In one case of carcinoid tumor, a pedunculated type polyp was observed. Except for carcinoid tumors, only one case of adenoma was smaller than 10 mm in diameter. In the second portion of the duodenum, most of the neoplastic lesions were adenomas.
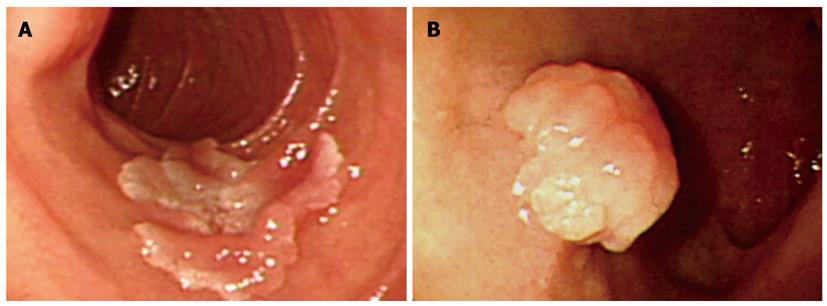
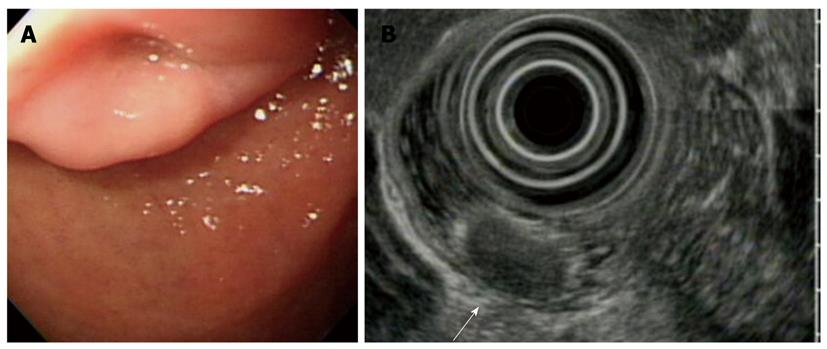
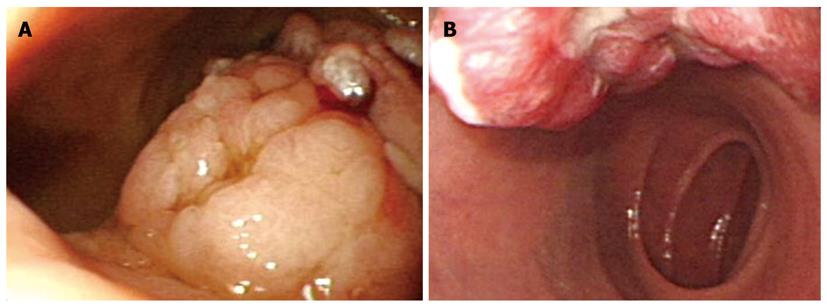
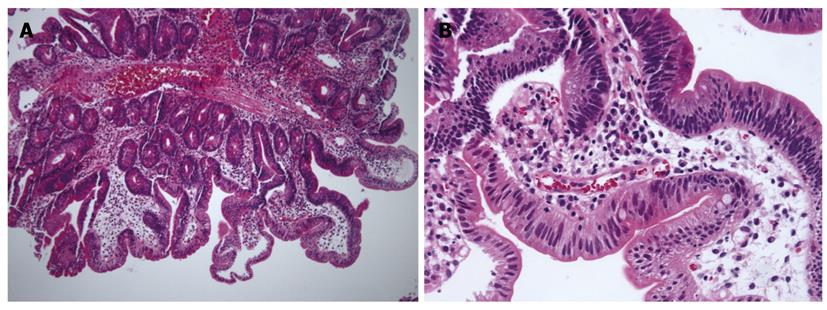
With regard to polyp shape, 9 (36%) of the 25 patients had neoplastic lesions with a semi-pedunculated or pedunculated appearance, whereas 25 (12.8%) patients had non-neoplastic lesions of similar shape (P < 0.01). Regarding the number of polyps in a single subject; 26 patients had multiple non-neoplastic lesions and only two patients had multiple neoplastic lesions. On multivariate analysis, lesions of more than 10 mm or located in the second portion of the duodenum had independent risk factors for being neoplastic lesions (Table 3). The endoscopic and histological features of the duodenal polyps are shown in Figures 2, 3, 4,5.
For patients with neoplastic lesions, 20 (80%) of 25 patients had therapeutic removal and 16 (64%) patients had successful endoscopic resection. Two of the remaining five patients have chosen surveillance endoscopy, because the lesion was small and the patients had co-morbid diseases. Two cases were lost to follow-up. One patient with a metastatic lesion from esophageal cancer has received radiation therapy.
Hyperplastic polyps were detected in 29 patients. Two cases had adenomatous changes or focal cancer changes with an adenomatous component. Gastroesophageal lesions identified with duodenal hyperplastic polyps are shown in Table 4. In 21 patients, the H. pylori status was evaluated according to the histological results and 12 (57.1%) patients had H. pylori infection. The clinical features accompanying duodenal hyperplastic polyps are shown in Table 5. Twenty-three patients (79.3%) were had associated gastroduodenal pathology; peptic ulcer, gastric cancer, gastric hyperplastic polyp, and chronic atrophic gastritis, which was possibly associated with H. pylori infection.
| Total | Odds ratio | 95% CI | P value |
| Type | 1.56 | 0.54-4.46 | 0.41 |
| Sessile | |||
| peduncle | |||
| Location | 3.55 | 1.32-9.54 | 0.01 |
| Bulb | |||
| 2nd portion | |||
| Size (cm) | 14.12 | 4.99-39.99 | < 0.01 |
| < 1.0 | |||
| ≥ 1.0 |
In our retrospective study, the prevalence of incidentally found duodenal polyps was 1.02% among cases undergoing routine diagnostic esophago-gastroduodenoscopy. The prevalence confirmed by histology was 0.44%. These results were consistent with the frequencies disclosed in previous reports. However, there were several limitations. First, the study population and the object of esophago-gastroduodenoscopy were not uniform-screening or diagnostic procedure for the gastrointestinal symptoms. Second, a tissue biopsy was not performed in all cases of duodenal polyp. Out of 510 polyps, only 221 were biopsied and the others were probably considered as non-neoplastic.
Several types of polyps can occur in the duodenum and most of them are non-neoplastic. Non-neoplastic duodenal polyps include Brunner’s gland hyperplasia, ectopic gastric mucosa, and hyperplastic polyps. Previous retrospective studies showed that ectopic gastric mucosa and Brunner’s gland hyperplasia are common in the duodenum, and that hyperplasic polyps were extremely rare[6,9,10]. However, in this study, most of the non-neoplastic polyps had normal duodenal mucosa or the lesions had distinguishable histopathology suggestive of an inflammatory polyp. In addition, hyperplastic polyps are a common type of duodenal polyp. Our results are similar to those of a previous prospectively designed study[3]. Inflammatory polyps are presumed to be part of a diffuse inflammatory process, with metaplastic or regenerative changes of the overlying mucosa on histology[4]. The duodenal inflammatory polyps were smaller than the other types of polyps.
Brunner’s gland hyperplasia and hamartomas were often encountered in the bulb of the duodenum[11,12], and our results showed that 76.2% (16/21 cases) were located in the bulb. The pathogenesis of these lesions is mostly unknown, and might be associated with duodenal injury observed in patients with acid hypersecretion. Macroscopically, they tend to be solitary sessile or pedunculated polyps with a central pore and are located in the posterior wall of the duodenal bulb. Their histological features include the proliferation of a mixture of mesenchymal tissues, such as adipose, smooth muscle, and Brunner’s gland, and cystic dilation of the Brunner’s gland. Rarely, adenoma or carcinoma can develop from a Brunner’s gland and in symptomatic cases with bleeding, endoscopic removal is necessary[13,14]. In our data, three cases were treated with a polypectomy for Brunner’s gland hyperplasia/hamartoma; in one case an adenoma developed from a Brunner’s gland and two cases were large polyps.
Although most duodenal polyps had a benign clinical course, some of them can show malignant transformation into adenomas or carcinoid tumors. Therefore, early diagnosis and treatment are important for these cases. In this study, adenomas were the most frequently detected neoplastic lesions, consistent with the findings of previous studies[6,9,10,15]. One case of an adenoma developed from a Brunner’s gland, and another case was associated with familial adenomatous polyposis (FAP). In addition, an adenoma of mixed hyperplastic and adenomatous morphology was observed as a serrated adenoma. A serrated adenoma of the duodenum is a very uncommon lesion and has been described in association with FAP[16]. A sporadic case of serrated adenoma has not been previously reported in the duodenum, and this appears to be the first report of one in the literature.
The results of this study showed that the neoplastic lesions were larger than the non-neoplastic lesions, and had a significant predilection for the second portion of the duodenum compared with the non-neoplastic lesions. In addition, a previous report suggested that multiple, small polyps in the duodenal bulb are always benign and need neither biopsy nor treatment. Moreover, polyps in the second portion of the duodenum are rare. If polyps were present in the second portion of the duodenum, a substantial number would be adenomas[17]. Although duodenal carcinoid tumors were rare, their detection and treatment are important. On endoscopic examination, they were yellowish colored and had central dimpling or ulceration with erythematous changes[18]. It should be kept in mind that a polyp-like lesion might be a carcinoid tumor. For the subset of lesions measuring between 1 and 2 cm in diameter, endoscopic ultrasound (EUS) may ultimately offer the best diagnostic method to help guide the management of individual patients[19]. Nearly all neoplastic lesions were treated by local resection (e.g.: polypectomy or mucosal resection) or surgery, except for a metastasis from an esophageal cancer. Most of them could be managed with endoscopic therapy with minimal morbidity. If the lesions located in the second portion of the duodenum cannot be excised by endoscopic therapy, a pancreatico-duodenectomy may be required. However, this technique is associated with a high complication rate.
There have been several reports of hyperplastic gastric polyps associated with persistent H. pylori gastritis[20-22], but the association of H. pylori with duodenal hyperplastic polyps has been rarely described. Two case reports suggested that these lesions are rare and associated with H. pylori infection[6,7]. As a result, their clinical features are poorly understood. Based on our results, most of the patients with duodenal hyperplastic polyps had inflammatory mucosal pathology of gastric mucosa: chronic atrophic gastritis, peptic ulcer, or gastric cancer. A large number of patients were infected with H. pylori. Moreover, the association of H. pylori might have been underestimated because of the retrospective study design, and the fact that we could not exclude past exposure to H. pylori. Unfortunately, there was no control group and the interpretation of this result is limited.
In conclusion, although the frequency of clinically significant duodenal polyps was low, the polyps of more than 10 mm or polyps in the second portion of the duodenum should be carefully observed and evaluated by histological examination. Duodenal hyperplastic polyps might have an association with H. pylori infection and with various forms of gastro-duodenal disease.
Duodenal polyps are rare, although the diagnosis appears to be increasing, possibly due to the wide use of diagnostic endoscopy. Several types of polyps can occur in the duodenum. However, the clinical importance of non-ampullary duodenal polyps is not well understood and clear management guidelines do not exist.
The clinical and endoscopic characteristics of neoplastic non-ampullary duodenal polyps are not well known; therefore, this study evaluated their features, including age, sex, size, and endoscopic characteristics (location and gross appearance). In addition, the clinical characteristics of duodenal hyperplastic polyps were determined.
On univariate analysis, there were significant differences in shape, location,and size. Polyps of more than 10 mm in diameter or polyps in the second portion had independent risk factors for being neoplastic lesions identified on multivariate analysis. 79.3% of the hyperplastic polyps were accompanied by gastro-duodenal pathology that was possibly associated with H. pylori infection.
This study provides a consensus on guidelines for clinical management of non-ampullary duodenal polyps and helps to discriminate neoplastic polyps.
The study reports a retrospective single center evaluation of the prevalence and histology of duodenal polyps in over 50 000 subjects undertaking upper G-I endoscopy for several reasons. The main finding of the authors, on multivariate analysis, is that polyps of the 2° duodenal portion have a greater risk of being neoplastic. The study is interesting because of the large database analyzed; however the retrospective and hospital-based modality of screening should be better acknowledged as a limitation.
Peer reviewers: Rene Lambert, Professor, International Agency for Research on Cancer, 150 Cours Albert Thomas, Lyon 69372 cedex 8, France; Vito Annese, MD, Department of Internal Medicine, Unit of Gastroenterology, Hospital, Viale Cappuccini, 1, San Giovanni Rotondo 71013, Italy
S- Editor Tian L L- Editor Stewart GJ E- Editor Ma WH
| 1. | Höchter W, Weingart J, Seib HJ, Ottenjann R. [Duodenal polyps. Incidence, histologic substrate and significance]. Dtsch Med Wochenschr. 1984;109:1183-1186. |
| 2. | Reddy RR, Schuman BM, Priest RJ. Duodenal polyps: diagnosis and management. J Clin Gastroenterol. 1981;3:139-147. |
| 3. | Jepsen JM, Persson M, Jakobsen NO, Christiansen T, Skoubo-Kristensen E, Funch-Jensen P, Kruse A, Thommesen P. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483-487. |
| 4. | Remmele W, Hartmann W, von der Laden U, Schumann G, Schäfer E, Schmidt H, Linhart P, Bettendorf U, Schink U. Three other types of duodenal polyps: mucosal cysts, focal foveolar hyperplasia, and hyperplastic polyp originating from islands of gastric mucosa. Dig Dis Sci. 1989;34:1468-1472. |
| 5. | Franzin G, Novelli P, Fratton A. Hyperplastic and metaplastic polyps of the duodenum. Gastrointest Endosc. 1983;29:140-142. |
| 6. | Roche HJ, Carr NJ, Laing H, Bateman AC. Hyperplastic polyps of the duodenum: an unusual histological finding. J Clin Pathol. 2006;59:1305-1306. |
| 7. | Yousfi MM, el-Zimaity HM, Cole RA, Genta RM, Graham DY. Resolution of a metaplastic duodenal polyp after cure of Helicobacter pylori infection. J Clin Gastroenterol. 1996;23:53-54. |
| 8. | Gopalswamy N, Shenoy VN, Choudhry U, Markert RJ, Peace N, Bhutani MS, Barde CJ. Is in vivo measurement of size of polyps during colonoscopy accurate? Gastrointest Endosc. 1997;46:497-502. |
| 9. | Chong KC, Cheah WK, Lenzi JE, Goh PM. Benign duodenal tumors. Hepatogastroenterology. 2000;47:1298-1300. |
| 11. | Patel ND, Levy AD, Mehrotra AK, Sobin LH. Brunner’s gland hyperplasia and hamartoma: imaging features with clinicopathologic correlation. AJR Am J Roentgenol. 2006;187:715-722. |
| 12. | Schluger LK, Rotterdam H, Lebwohl O. Gastrointestinal hemorrhage from a Brunner’s gland hamartoma. Am J Gastroenterol. 1994;89:2088-2089. |
| 13. | Matsumoto T, Iida M, Matsui T, Yao T, Fujishima M. A large Brunner’s gland adenoma removed by endoscopic polypectomy. Endoscopy. 1990;22:192-193. |
| 14. | Gao YP, Zhu JS, Zheng WJ. Brunner's gland adenoma of duodenum: a case report and literature review. World J Gastroenterol. 2004;10:2616-2617. |
| 15. | Abbass R, Rigaux J, Al-Kawas FH. Nonampullary duodenal polyps: characteristics and endoscopic management. Gastrointest Endosc. 2010;71:754-759. |
| 16. | Ghazi A, Ferstenberg H, Shinya H. Endoscopic gastroduodenal polypectomy. Ann Surg. 1984;200:175-180. |
| 17. | Rubio CA. Serrated adenoma of the duodenum. J Clin Pathol. 2004;57:1219-1221. |
| 18. | Hirakawa K, Iida M, Matsui T, Matsumoto T, Nakao Y, Yao T, Fuchigami T, Fujishima M. Endoscopic findings in carcinoid tumor of the duodenum. Am J Gastroenterol. 1991;86:603-605. |
| 19. | Yoshikane H, Tsukamoto Y, Niwa Y, Goto H, Hase S, Arisawa T, Mizutani K, Naitoh Y, Mitake M, Segawa K. [Examination of endoscopical ultrasonography (EUS) on carcinoid tumors of gastrointestinal tract]. Nippon Shokakibyo Gakkai Zasshi. 1991;88:1297-1304. |
| 20. | Ji F, Wang ZW, Ning JW, Wang QY, Chen JY, Li YM. Effect of drug treatment on hyperplastic gastric polyps infected with Helicobacter pylori: a randomized, controlled trial. World J Gastroenterol. 2006;12:1770-1773. |
| 21. | Veereman Wauters G, Ferrell L, Ostroff JW, Heyman MB. Hyperplastic gastric polyps associated with persistent Helicobacter pylori infection and active gastritis. Am J Gastroenterol. 1990;85:1395-1397. |
| 22. | Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the stomach: associations with histologic patterns of gastritis and gastric atrophy. Am J Surg Pathol. 2001;25:500-507. |