Published online Nov 7, 2010. doi: 10.3748/wjg.v16.i41.5257
Revised: August 2, 2010
Accepted: August 9, 2010
Published online: November 7, 2010
AIM: To compare the staging systems for stratifying and predicting the prognosis of patients with hepatocellular carcinoma (HCC) after partial hepatectomy (PH).
METHODS: Clinical data about 438 HCC patients who underwent PH from January 1991 to December 2004 at our hospital were retrospectively analyzed. Tumor stage was evaluated following the Chinese tumor node metastasis (TNM) and barcelona clinic liver cancer (BCLC) staging systems, respectively. Survival curves for the HCC patients were plotted using the Kaplan-Meier method and differences were compared by the log-rank test. The accuracy of each system for predicting death of HCC patients was evaluated by calculating the area under the receiver operating characteristic curve.
RESULTS: The HCC patients were classified into stages I-III, stages I-IV and stages A-C, according to the 3 staging systems, respectively. Log-rank test showed that the cumulative survival rate was significantly different for the HCC patients at 3 Chinese system stages, TNM stages I and II, TNM stages III and IV, and 3 BCLC stages (P < 0.05). However, no significant difference was found in the HCC patients at TNM stages II and III. The accuracy of the Chinese and BCLC staging systems was higher than that of the TNM staging system for predicting the survival rate of HCC patients.
CONCLUSION: The Chinese and BCLC staging systems are better for stratifying and predicting the prognosis of HCC patients after PH than the TNM staging system.
- Citation: Xu LB, Wang J, Liu C, Pang HW, Chen YJ, Ou QJ, Chen JS. Staging systems for predicting survival of patients with hepatocellular carcinoma after surgery. World J Gastroenterol 2010; 16(41): 5257-5262
- URL: https://www.wjgnet.com/1007-9327/full/v16/i41/5257.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i41.5257
Hepatocellular carcinoma (HCC), one of the most common cancers in the world, especially in Eastern Asia, with a poor prognosis[1], is the first leading cause of cancer-related death in the southeast of China[2]. Treatment modalities for HCC are strongly dependent on tumor stage and the underlying liver diseases. Surgical intervention is the only potentially curative modality for it at present. However, the long-term prognosis of HCC patients remains dismal even after radical excision or liver transplantation[3].
Staging systems are used to define the prognosis of HCC and its treatment[4]. Generally, a better clinical staging system can stratify patients according to their survival time, and is reliable and useful for comparing the curative effects on HCC. Several HCC staging systems have been proposed, such as the Okuda staging system[5], tumor node metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC)[6], cancer of the liver Italian program (CLIP) scoring system[7], Barcelona clinic liver cancer (BCLC) staging classification[8], Japan integrated staging (JIS) score[9], and Tokyo score[10]. However, no worldwide consensus has been reached on the use of any given HCC staging systems. Therefore, more accurate classification of HCC patients with a homogeneous prognosis would at minimum improve the application of currently available treatment modalities.
The Chinese staging system, established by the Chinese Society of Liver Cancer (CSLC) in 1999[11,12], combines the tumor-related factors and liver function reserve. However, its application in stratifying and predicting the prognosis of HCC patients remains to be defined. This study was to evaluate and compare the Chinese staging system, the AJCC TNM staging system (7th edition) and the BCLC staging system for stratifying and predicting the prognosis of a large cohort of Chinese HCC patients after partial hepatectomy (PH).
Four hundred and thirty-eight patients with HCC who underwent PH from January 1991 to December 2004 at our hospital were included in this study. The diagnosis of HCC was pathologically confirmed. Clinicopathological features and survival rates of the patients were analyzed. The study was approved by the Ethical Committee of Sun Yat-Sen Memorial Hospital and in accordance with the Helsinki Declaration of 1975. Written informed consent was obtained from the patients or their guardians.
HCC was divided into different stages following the criteria for the Chinese, TNM and BCLC staging systems, respectively. The detailed definition and criteria for the Chinese staging system are listed in Table 1.
| Stage | Tumor | Thrombi | CLN metastasis | Distant metastasis | Child-Pughscore |
| Ia | Solitary ≤ 3 cm | No | No | No | A |
| Ib | Solitary or two ≤ 5 cm in single lobe | No | No | No | A |
| IIa | Solitary or two ≤ 10 cm in single lobe, or two ≤ 5 cm in bilateral lobes | No | No | No | A |
| IIb | Solitary or two > 10 cm in single lobe, or two > 5 cm in bilateral lobes | No | No | No | A |
| Any | PV-branch, HV or BD | No | No | A | |
| Any | No | No | No | B | |
| IIIa | Any | PV-trunk or IVC | Yes/No | Yes/No | A/B |
| Any | Yes/No | Yes | Yes/No | A/B | |
| Any | Yes/No | Yes/No | Yes | A/B | |
| IIIb | Any | Yes/No | Yes/No | Yes/No | C |
All patients underwent non-anatomical resection. According to the Couinaud’s nomenclature for liver segmentation, minor hepatectomy (1-2 segments), major hepatectomy (≥ 3 segments), and wedge resection were performed in 179 (40.9%), 47 (10.9%), and 212 (48.2%) patients, respectively. Of the 438 patients, 24 (5.5%) with unresectable HCC underwent surgery after trans-catheter hepatic arterial chemoembolization (TACE, down-staged). Moreover, HCC patients with inoperable intrahepatic recurrence or extrahepatic metastases received combined therapies, including TACE, radiofrequency ablation (RFA), microwave coagulation therapy (MCT), percutaneous ethanol injection (PEI), and biotherapy or traditional Chinese therapy.
During the first 6 mo after operation, the patients were re-examined every 1-2 mo followed by every 3-6 mo. The clinical, laboratory and radiological (abdominal computed tomography scan and chest X-ray) data were collected at each follow-up. Three hundred and ninety-two (89.5%) HCC patients were followed-up until the end of January 2005 or death, while 46 (10.5%) HCC patients were lost during the follow-up. The median follow-up time was 21 mo (range 1-156 mo).
Statistical analysis was conducted with the SPSS software package (version 13.0, SPSS, Chicago, IL). Quantitative data were presented as mean ± SE. Survival curves for the HCC patients were plotted using the Kaplan-Meier method and examined by the log-rank test. The accuracy of each system for predicting the 1-, 3-, and 5-year rates of HCC patients was evaluated by calculating the area under the receiver operating characteristic curve. Patients censored before 1, 3 and 5 years were excluded from the analysis. P < 0.05 was considered statistically significant.
The general characteristics of the 438 HCC patients are summarized in Table 2. The mean and median ages of the patients were 50.0 ± 0.6 years and 49.0 years (range: 35-68 years), respectively. By the end of follow up, 223 HCC patients (50.9%) died. The 1-, 3- and 5-year postoperative overall survival rates were 72.2%, 53.5% and 43.3%, respectively, for the patients after PH.
| Characteristics | n (%) |
| Sex | |
| Male | 380 (86.8) |
| Female | 58 (13.2) |
| Age (yr) | |
| ≤ 50 | 235 (53.6) |
| > 50 | 203 (46.4) |
| Child-Pugh score | |
| Class A | 391 (89.3) |
| Class B | 47 (10.7) |
| Tumor number | |
| Single | 374 (85.4) |
| Two | 29 (6.6) |
| Multiple | 35 (8) |
| Tumor size (cm) | |
| ≤ 5 | 166 (37.9) |
| > 5 | 272 (62.1) |
| Tumor location | |
| Single lobe | 389 (88.8) |
| Bilateral lobes | 49 (11.2) |
| Capsular invasion | |
| With | 346 (79) |
| Without | 92 (21) |
| Vascular invasion | |
| With | 84 (19.2) |
| Without | 354 (80.8) |
| Lymph node metastasis | |
| With | 11 (2.5) |
| Without | 427 (97.5) |
| Extra-hepatic metastasis | |
| With | 7 (1.6) |
| Without | 431 (98.4) |
| Histological grade | |
| G1 | 129 (29.5) |
| G2 | 188 (42.9) |
| G3 | 121 (27.6) |
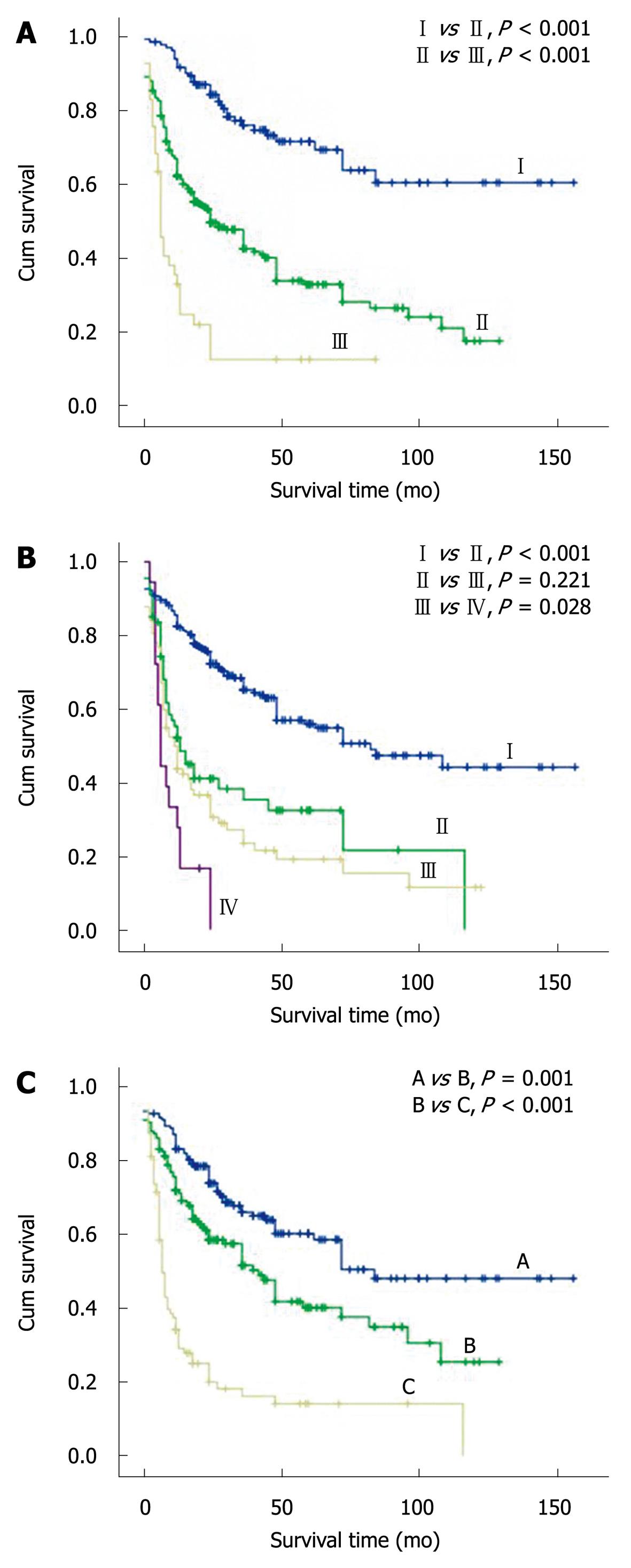
The 438 HCC patients were classified into stages I-III, stages I-IV and stages A-C according to the Chinese, TNM and BCLC staging systems, respectively. The log-rank test showed that the cumulative survival rate was significantly different for the HCC patients at 3 Chinese system stages, TNM stages I and II, TNM stages III and IV, and 3 BCLC stages (Table 3 and Figure 1, P < 0.05). However, no significant difference was found in the HCC patients at TNM stages II and III (P > 0.05).
| Tumor stage | n | Survival rate (%) | ||
| 1-yr | 3-yr | 5-yr | ||
| CS | ||||
| I | 132 | 0.916 | 0.760 | 0.693 |
| II | 265 | 0.622 | 0.426 | 0.281 |
| III | 41 | 0.330 | 0.126 | 0.126 |
| TNM | ||||
| I | 271 | 0.825 | 0.652 | 0.548 |
| II | 67 | 0.521 | 0.354 | 0.216 |
| III | 82 | 0.437 | 0.235 | 0.153 |
| IV | 18 | 0.278 | / | / |
| BCLC | ||||
| A | 178 | 0.870 | 0.678 | 0.602 |
| B | 165 | 0.756 | 0.574 | 0.402 |
| C | 95 | 0.375 | 0.182 | 0.142 |
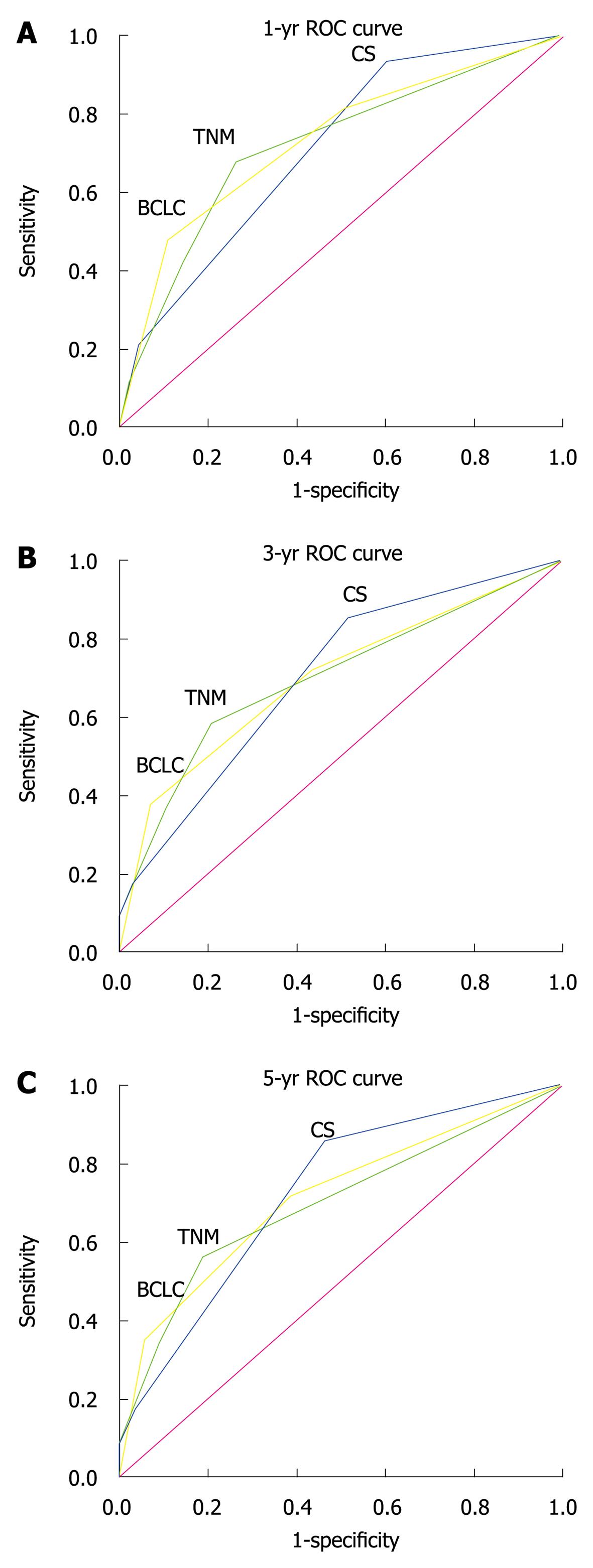
The accuracy of 3 staging systems for predicting the survival rate of HCC patients 1, 3 and 5 years after PH is summarized in Table 4. The accuracy of the Chinese and BCLC staging systems was higher than that of the TNM staging system for predicting the survival rates of HCC patients (Figure 2).
| System | AUC (95% CI) | ||
| 1-yr death | 3-yr death | 5-yr death | |
| CS | 0.709 (0.658-0.761) | 0.703 (0.645-0.761) | 0.720 (0.648-0.791) |
| TNM | 0.718 (0.662-0.774) | 0.702 (0.646-0.759) | 0.695 (0.629-0.760) |
| BCLC | 0.730 (0.675-0.785) | 0.701 (0.644-0.757) | 0.710 (0.645-0.776) |
HCC is the second most lethal cancer after pancreatic ductal adenocarcinoma, leading to about 600 000 deaths every year worldwide, with nearly 55% of the deaths occurred in China alone[3]. The long term prognosis of HCC patients is extremely dismal and the incidence of HCC is continuously growing globally[1,13,14]. In the southeast of China, HCC is the leading cause of cancer-related death despite aggressive conventional therapies[2]. Surgery remains the most effective treatment of HCC with a curative potential[15,16]. However, HCC patients even after curative resection of the tumor often have a high rate of relapse. Therefore, tools that can be used to stratify the prognosis of HCC patients after therapy are urgently needed. Gene-expression analysis has led to the successful molecular classification of HCC according to its prognosis, aetiology and intra-hepatic recurrence[17]. However, it has not been widely accepted due to its high cost.
Staging systems are often used to select primary and adjunctive therapies and to assess their outcome. Generally, a well defined clinical staging system not only can classify HCC patients and predict their survival time, but also can be generally applied globally. Increasing evidence shows that liver function reserve and tumor-related factors can also significantly influence the prognosis of HCC patients[4]. Chen et al[18] reported that a better staging system can provide more valid tumor-related factors and liver function parameters. Therefore, both tumor-related features and liver function reserve should be included in the clinical staging systems for HCC[19].
Several clinical staging systems are available for predicting the prognosis of HCC patients after PH at present. However, no worldwide consensus has been reached on which staging system is the best for predicting the prognosis of HCC patients after surgery[20]. Although the Okuda staging system[5], CLIP scoring system[7], JIS score[9], and Tokyo score[10] are widely used, they have been mostly applied to unresectable HCC, and none is universally accepted[21]. The TNM staging system is the most widely used system worldwide at present[21]. However, the AJCC TNM staging system does not include any measurement of liver functions and is thus not widely used[20]. Llovet et al[22] reported that the AJCC TNM staging system fails to adequately stratify HCC patients and predict their prognosis. Lu et al[23] also reported that the TNM staging system provides inadequate information for the prognosis of HCC patients.
In the present study, application of the CS system in stratifying and predicting prognosis of a large cohort of Chinese HCC patients (n = 438) after PH was investigated and compared to that of the AJCC TNM and BCLC staging systems. The log-rank test showed that the cumulative survival rate was significantly different for HCC patients at Chinese system stages, TNM stages I and II, TNM stages III and IV, and 3 BCLC stages, while no significant difference was found in the cumulative survival rate of HCC patients at TNM stages II and stage III. The accuracy of the Chinese and BCLC staging systems was higher than that of the TNM staging system for predicting the long-term survival (3- and 5-year) rates of HCC patients after PH, indicating that the Chinese and BCLC staging systems are better than the TNM staging system for stratifying and predicting the prognosis of HCC patients after PH. In our study, the AJCC TNM staging system could not accurately stratify the HCC patients with multiple nodules or vascular/peripheral invasion.
In conclusion, the Chinese and BCLC staging systems are better than the AJCC TNM staging system for stratifying and predicting the prognosis of HCC patients after PH. Further studies are needed to establish a single, worldwide staging system that can stratify and predict the prognosis of HCC patients after PH.
Staging systems are used to define the prognosis and treatment of hepatocellular carcinoma (HCC) patients. Several staging systems are available for predicting the prognosis of HCC patients, but no worldwide consensus has been reached on the use of any given HCC staging systems. Therefore, more accurate classification of HCC patients with homogeneous prognosis is urgently needed.
Although the Okuda and cancer of the liver Italian program scoring systems, Japan integrated staging score, and Tokyo score are widely used, they have been mostly applied to unresectable HCC patients. The tumor node metastasis (TNM) staging system for HCC does not include any measurement of liver functions and is thus not widely used. The Chinese staging system for stratifying and predicting the prognosis of HCC patients remains to be defined.
The Chinese staging system was studied for stratifying and predicting the prognosis of HCC patients. Furthermore, the applicability of the Chinese staging system was compared with other staging systems for stratifying and predicting the prognosis of HCC patients after partial hepatectomy.
The results of this study demonstrate that the Chinese staging system can be used for predicting the prognosis of HCC patients after partial hepatectomy.
The authors evaluated and compared the Chinese, American Joint Committee on Cancer (AJCC) TNM and Barcelona clinic liver cancer (BCLC) staging systems for stratifying and predicting the prognosis of a large cohort of Chinese HCC patients after partial hepatectomy. The results reveal that the Chinese and BCLC staging systems are better than AJCC TNM staging system for stratifying and predicting the prognosis of HCC patients after partial hepatectomy.
Peer reviewers: Tomoharu Yoshizumi, MD, PhD, Department of Surgery, Saiseikai Fukuoka General Hospital, 1-3-46, Tenjin, Chuou-ku, Fukuoka 810-0001, Japan; Takashi Kobayashi, MD, PhD, Department of Surgery, Showa General Hospital, 2-450 Tenjincho, Kodaira, Tokyo 187-8510, Japan
S- Editor Cheng JX L- Editor Wang XL E- Editor Ma WH
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. |
| 2. | Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346-353. |
| 3. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. |
| 4. | Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, Minami Y, Ueshima K, Fukunaga T. Review of current staging systems for hepatocellular carcinoma. Hepatol Res. 2007;37 Suppl 2:S210-S215. |
| 5. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. |
| 6. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors . AJCC cancer staging manual. 7th ed. New York, NY: Springer 2010; 237-246. |
| 7. | Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679-680. |
| 8. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. |
| 9. | Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38:207-215. |
| 10. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. |
| 11. | Yang BH, Ren ZG, Tang ZY. The consideration and proponent of the liver cancer staging classification. Zhonghua Gandan Waike Zazhi. 1999;5:67-68. |
| 12. | Yan P, Yan LN. Staging of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. New York, NY: Springer 2003; 491-495. |
| 14. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 15. | Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon’s perspective. Ann Surg. 2002;235:466-486. |
| 16. | Rougier P, Mitry E, Barbare JC, Taieb J. Hepatocellular carcinoma (HCC): an update. Semin Oncol. 2007;34:S12-S20. |
| 17. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. |
| 18. | Chen TW, Chu CM, Yu JC, Chen CJ, Chan DC, Liu YC, Hsieh CB. Comparison of clinical staging systems in predicting survival of hepatocellular carcinoma patients receiving major or minor hepatectomy. Eur J Surg Oncol. 2007;33:480-487. |
| 19. | Varotti G, Ramacciato G, Ercolani G, Grazi GL, Vetrone G, Cescon M, Del Gaudio M, Ravaioli M, Ziparo V, Lauro A. Comparison between the fifth and sixth editions of the AJCC/UICC TNM staging systems for hepatocellular carcinoma: multicentric study on 393 cirrhotic resected patients. Eur J Surg Oncol. 2005;31:760-767. |
| 20. | Yeh CN, Chen MF, Lee WC, Jeng LB. Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol. 2002;81:195-202. |
| 21. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. |
| 22. | Llovet JM, Bruix J, Fuster J, Castells A, Garcia-Valdecasas JC, Grande L, Franca A, Brú C, Navasa M, Ayuso MC. Liver transplantation for small hepatocellular carcinoma: the tumor-node-metastasis classification does not have prognostic power. Hepatology. 1998;27:1572-1577. |
| 23. | Lu W, Dong J, Huang Z, Guo D, Liu Y, Shi S. Comparison of four current staging systems for Chinese patients with hepatocellular carcinoma undergoing curative resection: Okuda, CLIP, TNM and CUPI. J Gastroenterol Hepatol. 2008;23:1874-1878. |