Published online Nov 7, 2010. doi: 10.3748/wjg.v16.i41.5203
Revised: August 2, 2010
Accepted: August 9, 2010
Published online: November 7, 2010
AIM: To classify the histological severity of Helicobacter pylori (H. pylori) infection-associated gastritis by confocal laser endomicroscopy (CLE).
METHODS: Patients with upper gastrointestinal symptoms or individuals who were screened for gastric cancer were enrolled in this study. Histological severity of H. pylori infection-associated gastritis was graded according to the established CLE criteria. Diagnostic value of CLE for histological gastritis was investigated and compared with that of white light endoscopy (WLE). Targeted biopsies from the sites observed by CLE were performed.
RESULTS: A total of 118 consecutive patients with H. pylori infection-associated gastritis were enrolled in this study. Receiver operating characteristic curve analysis showed that the sensitivity and specificity of CLE were 82.9% and 90.9% for the diagnosis of H. pylori infection, 94.6% and 97.4% for predicting gastric normal mucosa, 98.5% and 94.6% for predicting histological active inflammation, 92.9% and 95.2% for predicting glandular atrophy, 98.6% and 100% for diagnosing intestinal metaplasia, respectively. Post-CLE image analysis showed that goblet cells and absorptive cells were the two most common parameters on the CLE-diagnosed intestinal metaplasia (IM) images (P < 0.001). More histological lesions of the stomach could be found by CLE than by WLE (P < 0.001).
CONCLUSION: CLE can accurately show the histological severity of H. pylori infection-associated gastritis. Mapping IM by CLE has a rather good diagnostic accuracy.
-
Citation: Wang P, Ji R, Yu T, Zuo XL, Zhou CJ, Li CQ, Li Z, Li YQ. Classification of histological severity of
Helicobacter pylori -associated gastritis by confocal laser endomicroscopy. World J Gastroenterol 2010; 16(41): 5203-5210 - URL: https://www.wjgnet.com/1007-9327/full/v16/i41/5203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i41.5203
Helicobacter pylori (H. pylori) is a well accepted major etiological factor for gastric diseases, such as chronic gastritis, peptic ulcer and gastric carcinoma[1-3]. Therefore, endoscopists must determine the presence of H. pylori infection in the stomach during or after endoscopy. The updated Sydney system for classification of gastritis has established the association between H. pylori infection and histological evidence of gastritis[4]. Gastric atrophy especially accompanying intestinal metaplasia (IM), which is the severest stage of gastritis and has a high risk for gastric cancer, is closely associated with H. pylori infection[5]. Although the Sydney system has been widely used, multiple biopsies are rarely performed for gastritis evaluation but only for suspected lesions of cancer in clinical practice, thus leading to omission of some precancerous lesions which are difficult to find by white light endoscopy (WLE)[6]. Therefore, in vivo detection of H. pylori infection and its related complications by endoscopy is a simple, noninvasive and inexpensive procedure. H. pylori-induced gastritis must be evaluated and graded especially in Eastern countries because of a high prevalence of H. pylori infection and a high incidence of gastric cancer[7]. Furthermore, it can facilitate the clinical assessment and timely treatment of gastritis. The association between H. pylori infection and endoscopic findings has been extensively studied using modern endoscopic techniques such as magnification endoscopy and narrow-band imaging[8-10].
Confocal laser endomicroscopy (CLE) can detect gastrointestinal diseases, such as Barrett esophagus, gastric carcinoma and colonic neoplasia[11,12]. CLE can observe real-time histological-like cellular and subcellular conditions of gastric mucosal layer at the magnification × 500-1000. It was reported that acriflavine-aided CLE can observe H. pylori in vivo[13]. In addition, gastric pit patterns of various gastropathies have been accurately classified and IM has been studied by CLE[14,15]. However, no systematic data are available on the CLE characteristics of chronic gastritis and the classification of chronic gastritis has not been investigated by CLE.
In this study, the CLE features of gastritis mainly caused by H. pylori infection were compared to evaluate the accuracy of CLE in diagnosing H. pylori infection and the severity of gastritis.
Consecutive patients with upper gastrointestinal symptoms or individuals who were screened for gastric carcinoma, admitted to our hospital from June to November 2009, were enrolled in this study. The following patients were excluded from the study, including those who received proton pump inhibitors, antibiotics, or bismuth subsalicylate in the previous 6 wk, those with a history of using nonsteroidal anti-inflammatory drugs and medication for H. pylori infection, those undergone stomach surgery, those with systematic diseases or known gastric carcinoma, pregnant or breast-feeding females, those who did not give their informed consent or had an allergy to fluorescein. All participants gave their written informed consent before endoscopy. The study was approved by the Ethics Committee of Qilu Hospital.
A confocal laser endomicroscope (Pentax ISC-1000, Pentax, Tokyo, Japan) was used in this study. It is novel digestive endoscope with a confocal laser microscope integrated into the distal tip, can realize a histological-like examination during routine endoscopy and accurately diagnose gastrointestinal diseases at the magnification × 1000. CLE was performed to scan the gastric mucosa from the top layer to 250 μm beneath the surface. The CLE and white-light endoscopy (WLE) images were captured and stored.
CLE was performed by an endoscopist experienced with the system. All patients received oral chymotrypsin (20 000 U) to eliminate the slime layer of the stomach for better visualization. One mL of 2% fluorescein (a contrast agent) was intravenously injected before endoscopy. After a first WLE of the stomach, 10 mL of 10% fluorescein was intravenously injected, and CLE images could be seen after a few seconds. Five standardized sites (2 from the lesser and greater curvatures of the antrum about 2-3 cm near the pylorus, 2 from the middle portion of the lesser and greater curvatures of the corpus about 8 cm from the cardia, and 1 from the angulus), as recommended by the updated Sydney system, were examined separately on CLE images. Shallow-deep CLE images were captured at each site and stored as digital files for further analysis. At the standardized locations, real-time image assessment and targeted biopsies were performed by CLE for histopathology. For H. pylori testing, 2 specimens were taken from the greater curvature of the antrum and corpus, respectively. If necessary, other scanning and biopsies were performed for lesions such as those with color changes or erosions, polyps, ulcers, or abnormal folds.
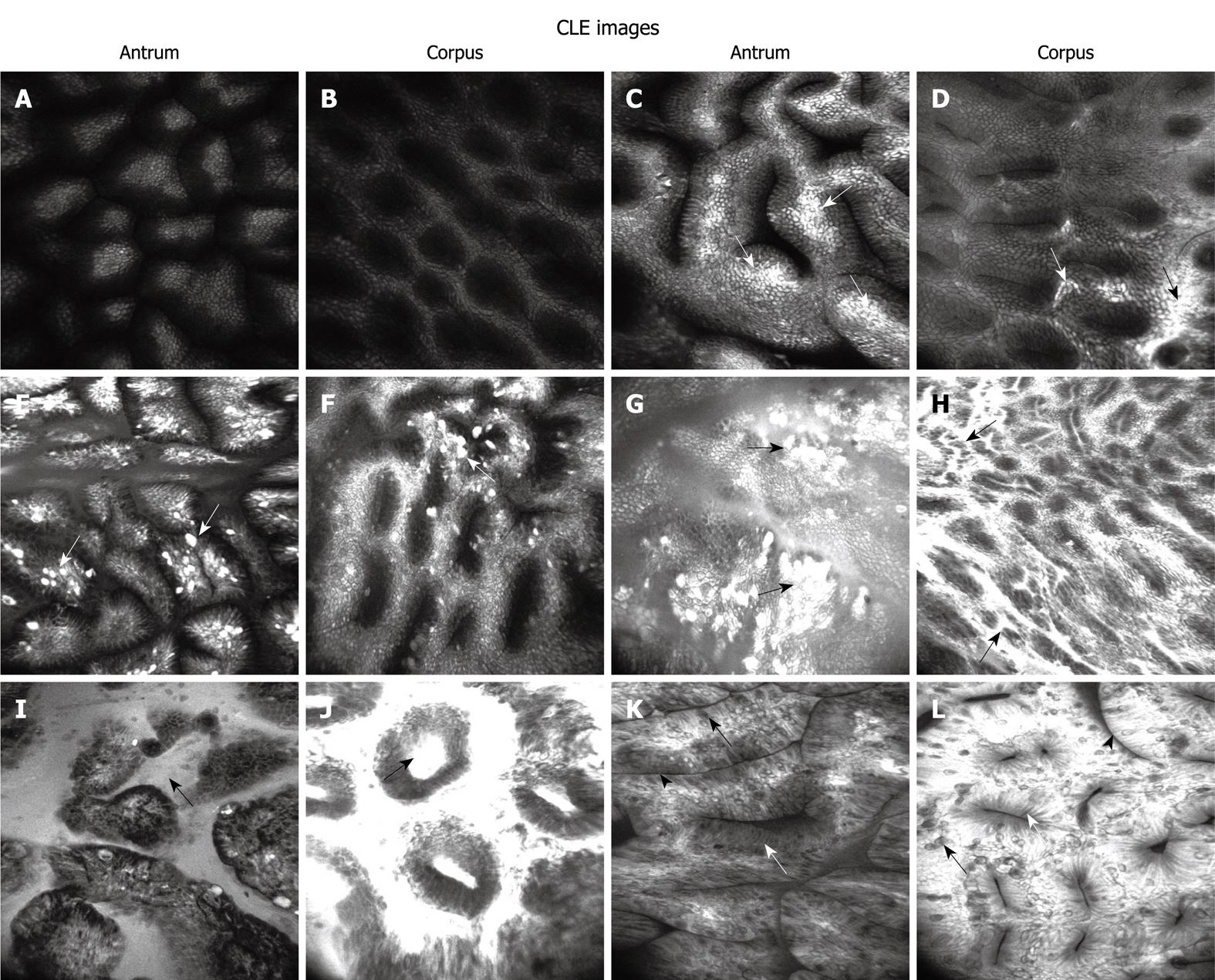
In our study, the severity of gastritis was divided into 6 levels by CLE: normal mucosa, H. pylori-associated active inflammation (3 levels), glandular atrophy and IM.
CLE criteria for normal gastric mucosa and H. pylori-associated active inflammation: The published CLE classification of gastric pit patterns was used for one part of our classification of H. pylori-associated active inflammation[14]. In addition, a new marker, fluorescein leakage, was introduced to define the active inflammation in stomach on CLE images (Figure 1). Each of the 5 standardized sites was assigned to a confocal gastritis score (CGS) and a corresponding histologic gastritis score (HGS).The CGS for normal mucosa, and mild, moderate and marked active inflammation was 0, 1, 2 and 3, respectively. The severity of histological activity and chronic inflammation was also defined as normal, mild, moderate and marked with a score of 0-3, respectively. Finally, the mean CGS and HGS (mCGS and mHGS) were calculated for specimens from each stomach.
CLE criteria for glandular atrophy and IM: Glandular atrophy and IM are the severest stage of chronic gastritis. The CLE classification of glandular atrophy and IM was defined as decreased gastric pits with a dilated opening and as villous-like gastric pits with goblet cells, absorptive cells and brush border, respectively (Figure 1). To assess the CLE images, the mean number of gastric pits on each grade image was calculated and compared, except for that on IM images. The clinical values for IM features on CLE images were assessed by calculating their mean ratio present in each IM site on CLE images.
Rapid urease testing and Giemsa staining confirmed H. pylori infection. If only one test was positive for H. pylori infection, 13C-urea breath test was performed for further confirmation. The H. pylori infection was classified into Hp (-), Hp (+), Hp (++) and Hp (+++).
All specimens were fixed in 10% formalin. An experienced histopathologist analyzed the histological features of each sample with hematoxylin and eosin staining and made the diagnosis according to the updated Sydney classification. The histological parameters assessed in this study included histological activity, chronic inflammation, glandular atrophy and intestinal metaplasia, which were identified blinding to the results of CLE or WLE.
Data were collected from CLE images and histological examination. analysis of variance and box-plot analysis of mCGS were used in pairwise comparison of mCGS among the 4 H. pylori test groups [Hp (-), Hp (+), Hp (++) and Hp (+++)]. P < 0.05 was considered statistically significant. The efficacy of CGS for predicting H. pylori infection was evaluated by area under the receiver operating characteristic (ROC) curve analysis. On the other hand, the sensitivity, specificity, positive and negative predictive values (PPV, NPV) of the CLE criteria for diagnosing normal gastric mucosa, H. pylori-associated active inflammation, glandular atrophy and IM were calculated, respectively. Correlation between mCGS and mHGS was analyzed with the coefficient of determination. SPSS v16.0 (SPSS Inc., Chicago, IL) was used for all statistical analyses.
One hundred and eighteen patients including 74 males, at a mean age of 49.8 years (range 19-67 years) were enrolled in this study. A total of 12 243 CLE images (mean 104 images per patient) and 653 optical biopsy images (mean 5.5 biopsy images per patient) were obtained. The median endoscopy time was 23.2 min (range 15-33 min) (Table 1). The quality of over 90% of CLE images was good, while that of the remaining 10% was not satisfactory because it was difficult to fix the distal tip on the gastric angle and the artifacts were motile in gastrointestinal tract. No endoscopic complications or adverse reactions to fluorescein were observed.
| Patients | 118 |
| Gender | |
| Female | 44 |
| Male | 74 |
| Age (yr), median (range) | 49.8 (19-67) |
| H. pylori infection (mCGS, mean ± SD) | |
| Positive | 41 |
| + | 15 (1.11 ± 0.53) |
| ++ | 15 (1.68 ± 0.51) |
| +++ | 11 (2.42 ± 0.45) |
| Negative | 77 (0.61 ± 0.39) |
| Indication for CLE | |
| Upper GI symptoms | 87 |
| Screened for gastric cancer | 31 |
| Endoscopic diagnosis | |
| Normal stomach | 6 |
| Gastritis | 92 |
| Peptic ulcer | 5 |
| Polyps | 13 |
| Early gastric cancer | 2 |
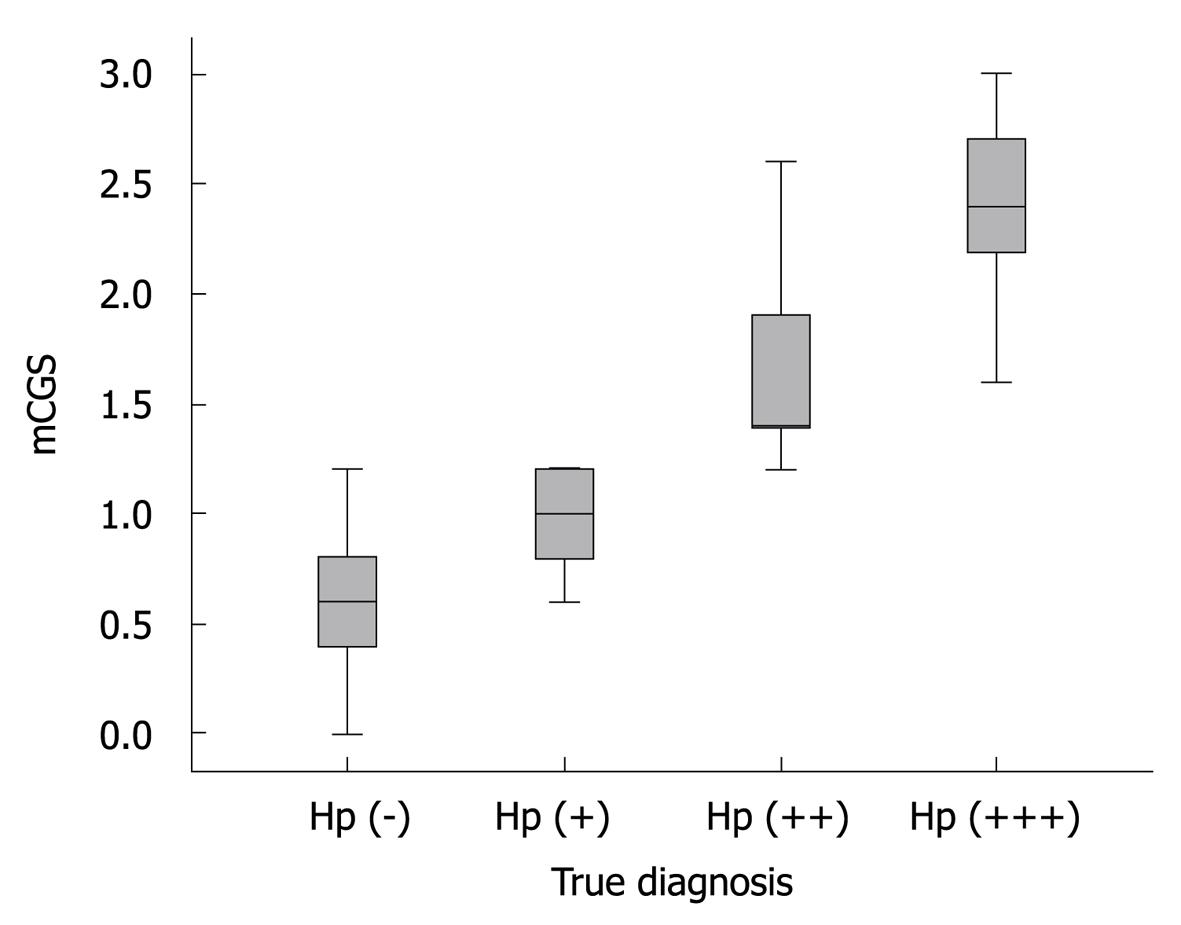
Association between mCGS and H. pylori infection: Of the 118 patients enrolled in this study, 41 were positive for H. pylori. Detailed data on H. pylori level are shown in Table 1. The mCGS was significantly differed among Hp (-), Hp (+),Hp (++), and Hp (+++) groups (P < 0.001). The median mCGS was significantly higher in Hp (+) and Hp (+++) groups than in Hp (-) and Hp (++) groups (P < 0.001), and higher in Hp (++) group than in Hp (+) and Hp (-) groups (P < 0.001) (Figure 2).
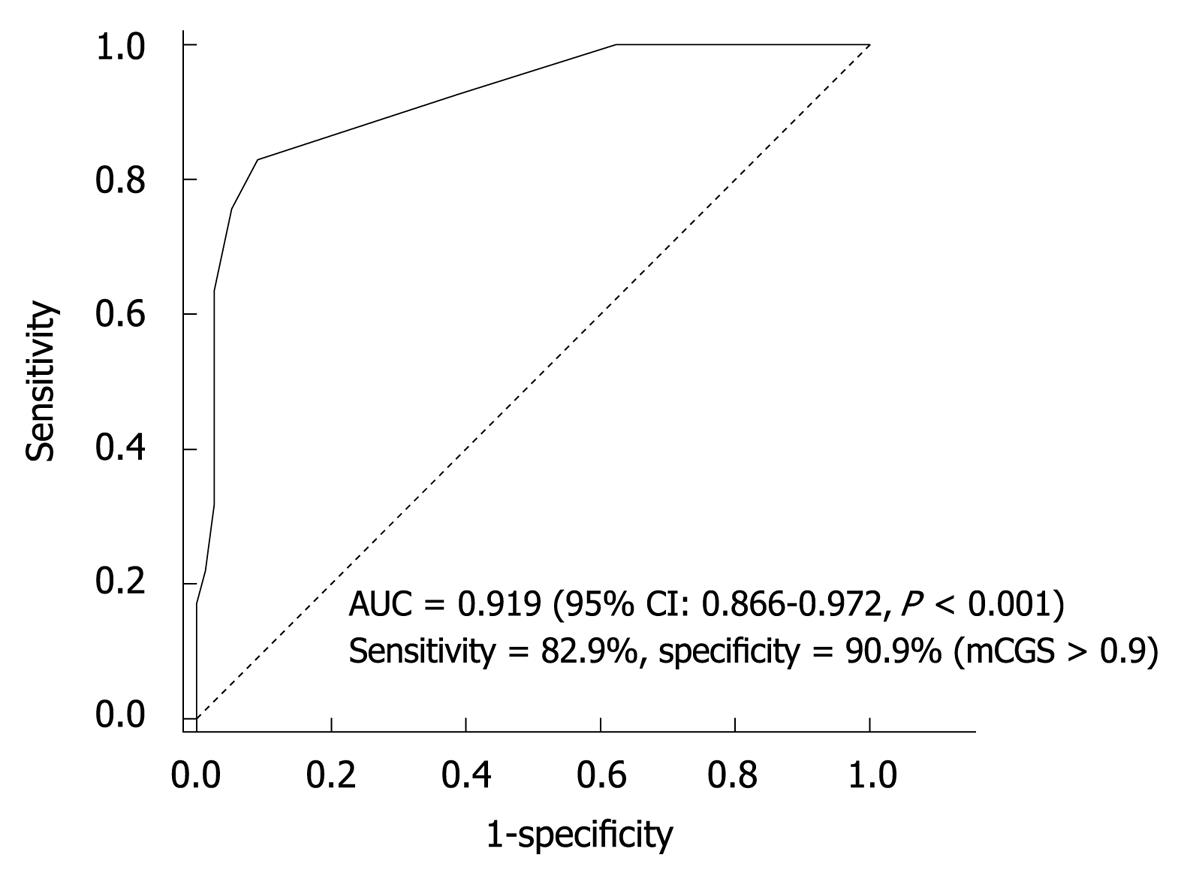
Sensitivity and specificity of CLE for H. pylori detection: The ROC curve for mCGS was plotted to determine a cut-off value of sensitivity relative to specificity for diagnosing H. pylori infection. The area under the ROC curve was 0.919 (95% CI: 0.866-0.972), showing an excellent accuracy (Figure 3). The sensitivity and specificity of CEL were 82.9% and 90.9%, respectively, for mCGS > 0.9. With this cut-off value, CLE could correctly diagnose H. pylori infection.
Normal gastric mucosa: A good correlation was found between normal mucosa detected by CLE and histology. The sensitivity, specificity, PPV and NPV of CLE were 94.6% (95% CI: 91.9%-97.4%), 97.4% (95% CI: 95.8%-99.1%), 96.5% (95% CI: 94.2%-98.7%), and 96.1% (95% CI: 94.1%-98.1%), respectively, for predicting gastric normal mucosa lesions (Table 2).
| CLE classification | Sensitivity | Specificity | PPV | NPV |
| Normal mucosa | 94.6 (91.9-97.4) | 97.4 (95.8-99.1) | 96.5 (94.2-98.7) | 96.1 (94.1-98.1) |
| Active inflammation | 98.5 (97.2-99.8) | 94.6 (91.9-97.4) | 95.9 (93.7-97.9) | 98.0 (96.3-99.7) |
| Glandular atrophy | 92.9 (87.0-98.9) | 95.2 (93.3-97.0) | 72.5 (63.4-81.7) | 98.9 (98.1-99.9) |
| Intestinal metaplasia | 98.6 (95.7-100.0) | 100 (100.0-100.0) | 100 (100.0-100.0) | 99.8 (99.4-100.0) |
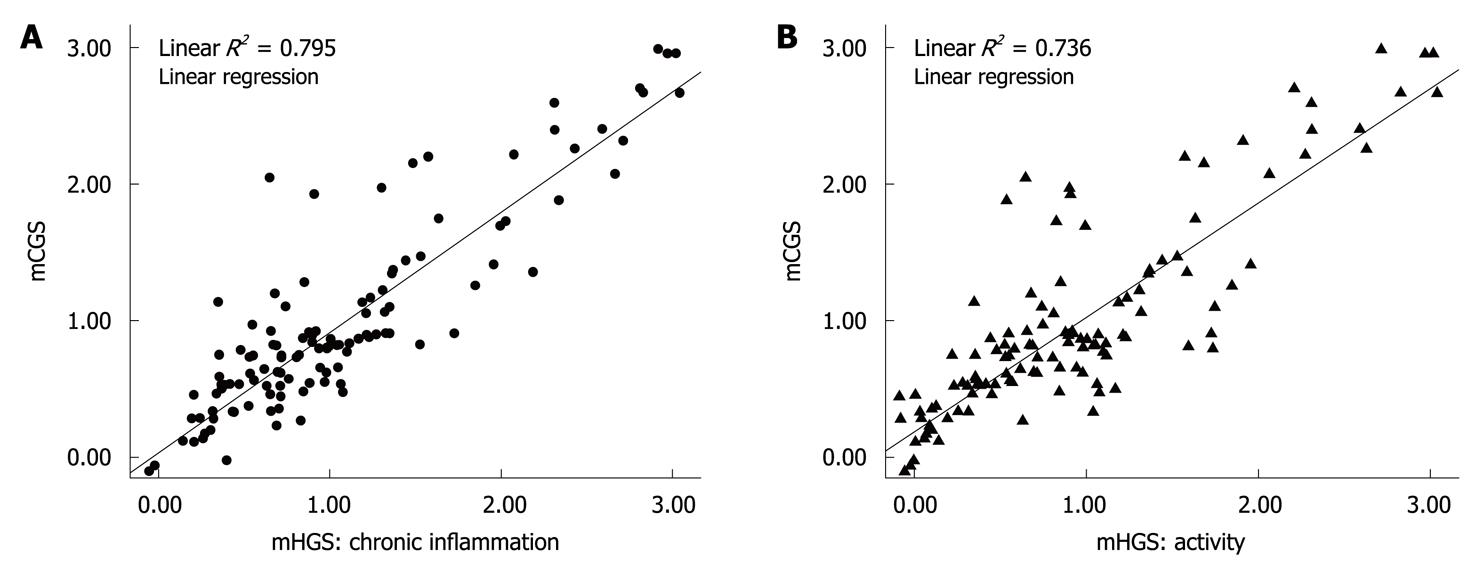
Chronic active inflammation: The CLE criteria correlated well with the active inflammation detected by histology. The sensitivity, specificity, PPV and NPV of CLE were 98.5% (95% CI: 97.2%-99.8%), 94.6% (95% CI: 91.9%-97.4%), 95.9% (95% CI: 93.7%-97.9%), and 98.0% (95% CI: 96.3%-99.7%), respectively, for predicting active inflammation detected by histology (Table 2). In particular, the histological activity and chronic inflammation were significantly differed among the 3 levels of active inflammation on CLE images, based on the evidence that histological activity and chronic inflammation were positively and linearly correlated with the mCGS (R2 = 0.795, R2 = 0.736, P < 0.001) (Figure 4).
Glandular atrophy and IM: The sensitivity, specificity, PPV and NPV of CLE were 92.9% (95% CI: 87.0%-98.9%), 95.2% (95% CI: 3.3%-97.0%), 72.5% (95% CI: 63.4%-81.7%) and 98.9% (95% CI: 98.1%-99.9%), respectively, for glandular atrophy (Table 2). Furthermore, a post-CLE analysis of CLE images showed that the mean number of gastric pits in glandular atrophy was significantly less than that in IM on CLE images (P < 0.001) (Figure 5).
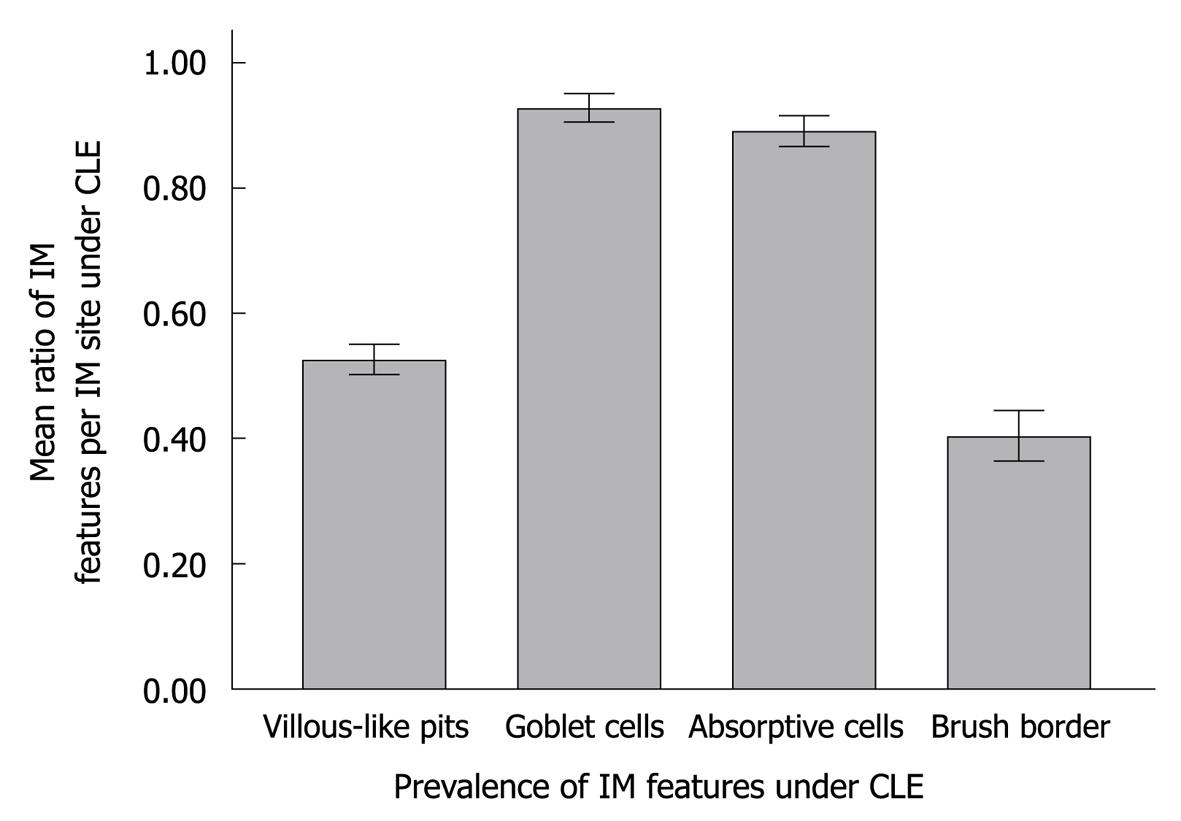
The sensitivity, specificity, PPV and NPV of CLE for IM were 98.6% (95% CI: 95.7%-100.0%), 100% (95% CI: 100.0%-100.0%), 100% (95% CI: 100.0%-100.0%), and 99.8% (95% CI: 99.4%-100.0%), respectively, for predicting IM (Table 2). Of the IM features on CLE images, goblet cells and absorptive cells were more common and easier to note than the other features (P < 0.001) (Figure 6).
CLE and WLE for predicting histological conditions: In our study, 54.5% (259/475) normal sites were found to be abnormal on WLE by CLE (173 inflamed sites, 41 atrophic sites and 45 IM sites). In contrast, of the 164 abnormal sites on WLE images, 18.9% (31/164) were found to be normal on CLE images. All the findings on CLE images were confirmed by histology, indicating that CLE can show more histological lesions than WLE (P <0.001) (Table 3).
Conventional endoscopy is not frequently correlated with histological alterations, WLE is not accurate for predicting histological gastritis with nonspecific erythema, prominent area gastrica, nodularity, or erosions, etc.[16], and gastroscopy without biopsy is incomplete in routine clinical practice. In recent years, novel endoscopies, such as magnification endoscopy and narrow band imaging, have been evaluated for gastric pathologic conditions with a good accuracy[17]. High resolution, magnification and advanced electronic dye techniques, can identify the mucosal and vascular details of H. pylori infection and its related complications, with a high diagnostic sensitivity and specificity[18].
More details of gastric pits can be clearly observed on CLE images at the magnification × 1000. Zhang et al[14] reported that CLE can reveal inflamed pits with marked morphological alterations. The sensitivity and specificity of type B’+D’ pits are 81.9% and 99.3%, respectively, for predicting moderate to severe active inflammation. In our study, H. pylori infection was positively correlated with progressively increasing changes in gastric pits due to direct injury caused by H. pylori and inflammatory infiltration. CLE could accurately distinguish normal mucosa from H. pylori-infected mucosa in the stomach. ROC curve analysis showed a high sensitivity (82.9%) and specificity (90.9%) of mCGS for diagnosing H. pylori infection. The mCGS cut-off value was 0.9, indicating an accurate and balanced result. Fluorescein leakage can classify active inflammation in ulcerative colitis[19] and fluorescein leakage into crypt lumen is positively correlated with histological active inflammation, because of increased colonic permeability. Fluorescein leakage is often present in the stomach, which contributes to the diagnosis of H. pylori-associated gastritis. When normal gastric mucosa is observed on CLE images, the fluorescence is confined to beneath the subepithelium and evenly distributed, epithelial cells are clearly visible and well-demarcated, showing a uniform gray cytoplasm and black border (Figure 1A and B). However, in H. pylori-infected mucosa, fluorescein easily leaks onto the surface through the damaged epithelium or normal epithelium with a high permeability. That is why this region is brighter than the surrounding normal epithelium. Sometimes, with a mass of the seepage, the entire field of vision can be white (Figure 1C-H). However, because fluorescein leakage is only an indirect response to mucosal damage and histological inflammation, the specificity of fluorescein is lower. In our study, fluorescein in combination with other CLE features was used to diagnose H. pylori infection following the active inflammation criteria defined by CLE[4]. The CLE scoring system used in our study could offer an objective evaluation of the entire stomach. High mCGS of the stomach indicated severe H. pylori infection, whereas low mCGS indicated mild or no H. pylori infection. Because the CGS agrees with the grading of confocal images, a high score represents severely injured gastric mucosa. It was reported that H. pylori organisms are usually observed singly or in groups along the surface epithelium but rarely in deeper mucosa[20]. Adherence of H. pylori to epithelial cells is the first step in H. pylori infection. When urease is secreted by H. pylori, and inflammatory and immune processes are activated by Vac A and Cag A toxin, the epithelium is depleted of mucin with irregular and missing cells (“drop-out cells”). In our study, similar alterations in moderate and severe active inflammation were found on CLE images, and clusters of destroyed epithelial cells (drop-out cells) on the two grade images were closely correlated with H. pylori infection, suggesting that CLE can display the drop-out cells because such microscopic lesions may progress to microerosions and precursors of gastric ulcer. However, when mCGS is less than 0.9, it can result in 7 false-positive and 7 false-negative diagnoses. False-positive cases are mainly diagnosed as mild active inflammation based on the CLE images of antrum, because normal mucosa on CLE images is sometimes misdiagnosed as mild inflammation when some fibrin or debris appears on the mucosal surface. The 7 false-negative cases are diagnosed as mild inflammation with a low H. pylori level and the corresponding mucosal changes are thus not obvious to be identified.
The second endpoint was to demonstrate the histological severity of gastritis by CLE, which showed that the 6 CLE grades were well correlated with the corresponding parameters of histological gastritis. Active inflammation was classified into 3 levels by CLE. On the other hand, obvious drop-out cells were observed at the moderate and marked levels with a high risk for gastric ulcer formation and management of these groups of patients. The severity of chronic inflammation was much higher at moderate and marked levels on CLE images (R2 = 0.736 and 0.795), probably because interstitial infiltration of inflammatory cells can easily squeeze the glands and damage the epithelium with an increasing severity of inflammation and H. pylori infection, leading to aggravated pit distortion and fluorescein leakage.
Gastric glands would be destroyed, and glandular atrophy and/or intestinal metaplasia would occur when they are infected with H. pylori and infiltrated with inflammatory cells. Glandular atrophy is manifested as thinner mucosa without gastric rugae but with visibly vascular pattern on WLE images[21]. However, gastric atrophy is poorly correlated with histological atrophy on WLE images[22]. It was reported that CLE shows a good sensitivity and specificity for gastric atrophy[14]. In our study, the diagnostic sensitivity and specificity of CLE were 92.9% and 95.2% for glandular atrophy. A further analysis of CLE-diagnosed glandular atrophy was performed with a more objective method. The mean number of gastric pits was calculated on different CLE images, and the mean number of pits in glandular atrophy was significantly less than that in gastritis on CLE images (P < 0.001). However, IM on CLE images was not analyzed because the gastric pits were replaced by intestinal mucosa, which became another form of atrophy or metaplasia. Although the sensitivity and specificity of CLE were high for glandular atrophy, the PPV was relatively low (72.5%) probably due to the low prevalence of atrophy in histology (71 sites/590 sites, 12.0%), indicating that there is a certain false positive rate when CLE is used to diagnose glandular atrophy, and identification of mild glandular atrophy is a big challenge for CLE.
Although the Sydney system has suggested a definition of endoscopic intestinal metaplasia for “grey-white patches with a slight opalescent tinge and/or a villous appearance on close inspection”, studies demonstrated that these features are quite difficult to be identified by conventional endoscopy[21,23]. The detection of IM needs multiple biopsy specimens. Tahara et al[18] reported that magnifying narrow-band imaging endoscopy can show IM with a sensitivity of 73.3% and a specificity of 95.6%. In this study, IM was further analyzed following the CLE criteria[15]. The sensitivity and specificity of CLE were 98.6% and 100%, respectively, for IM in our study, and were higher than those reported by Tahara et al[18]. In addition, goblet cells and absorptive cells were more frequently and easily observed than other features or parameters in IM on CLE images in our study (P < 0.001). Because diagnosis of IM is often disregarded by naked eyes or by chromoendoscopy in clinical practice, more powerful endoscopic techniques, such as CLE and magnifying endoscopy, should be used to map IM[24].
Microscopic examination is essential for endoscopists to make a final diagnosis of gastritis, because the term of gastritis has been used indiscriminately and whether histological inflammation is present or not. However, CLE plays a pathological role in improving the diagnostic rate of WLE. In this study, CLE could discover 54.5% of lesions missed by WLE and correct 18.9% of normal mucosal sites misdiagnosed as abnormal sites by WLE, because the micro-gastric inflammation may appear completely normal on WLE images, suggesting that the presence or absence of gastritis should not be determined only based on gross WLE images. However, CLE can detect these micro-changes.
The main limitations of our study are as follows. First, we did not establish a control group, or a follow-up group to validate our hypothesis from multiple aspects, leading some bias in determination of H. pylori infection and histological gastritis. Second, we did not evaluate inter- and intra-observer variability. The repeatability of our CLE criteria should be assessed in a future single study. Third, due to the scanning depth and resolution limitations of CLE, there is still some gap when compared with in vitro histology. Therefore, the CLE equipment needs to be constantly upgraded.
In conclusion, H. pylori infection is related to its related CLE image features. Histologically active inflammation can be classified by CLE. Two most accurate markers, goblet cells and absorptive cells for IM, are established. Although CLE itself still has some limitations as a novel technique, it is a promising new procedure for accurate histological assessment in vivo.
The accurate diagnosis of chronic gastritis still relies on histopathology. Confocal laser endomicroscopy (CLE) can show gastric pit patterns and epithelial cells in vivo, thus, contributing to an accurate diagnosis of chronic gastritis in vivo.
CLE has been extensively investigated in cancerous or precancerous mucosa of Barrett’s esophagus and its related neoplasia, early esophageal cancer, gastric intestinal metaplasia, neoplasia and early cancer, colonic neoplasia and cancer, and ulcerative colitis, etc.
Endoscopic diagnosis of chronic gastritis and Helicobacter pylori (H. pylori) infection is still challenge to clinical endoscopists. This study first used CLE to classify H. pylori infection-associated chronic gastritis, which could accurately diagnose chronic gastritis in vivo without mutiple biopsy specimens.
By using the classification criteria for chronic gastritis established in this study, clinical endoscopits could use CLE to evaluate the conditions of the entire stomach, and calculate the abnormal mucosal sites in the entire stomach.
CLE is novel digestive endoscope. It is a conventional white-light endoscope with a confocal laser microscope integrated into the distal tip. CLE can realize a histological-like examination during routine endoscopy and accurately diagnose gastrointestinal diseases at the magnification × 1000.
The authors established the classification criteria for chronic gastritis, and compared CLE and white light endoscopy in diagnosis of gastritis and intestinal metaplasia, thus proving a novel method for the diagnosis of gastritis and intestinal metaplasia by CLE, which has a higher sensitivity and specificity than conventional endoscopy.
Peer reviewers: Jae J Kim, MD, PhD, Associate Professor, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50, Irwon-dong, Gangnam-gu, Seoul 135-710, South Korea; Dr. Katsunori lijima, Division of Gastroenterology, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aobaku., Sendai 980-8574, Japan
S- Editor Cheng JX L- Editor Wang XL E- Editor Lin YP
| 1. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. |
| 2. | Goldblum JR, Vicari JJ, Falk GW, Rice TW, Peek RM, Easley K, Richter JE. Inflammation and intestinal metaplasia of the gastric cardia: the role of gastroesophageal reflux and H. pylori infection. Gastroenterology. 1998;114:633-639. |
| 3. | Fox JG, Correa P, Taylor NS, Zavala D, Fontham E, Janney F, Rodriguez E, Hunter F, Diavolitsis S. Campylobacter pylori-associated gastritis and immune response in a population at increased risk of gastric carcinoma. Am J Gastroenterol. 1989;84:775-781. |
| 4. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 5. | Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, Lau JY, Sung JJ. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut. 2004;53:1244-1249. |
| 6. | Carpenter HA, Talley NJ. Gastroscopy is incomplete without biopsy: clinical relevance of distinguishing gastropathy from gastritis. Gastroenterology. 1995;108:917-924. |
| 7. | Wong BC, Lam SK, Ching CK, Hu WH, Kwok E, Ho J, Yuen ST, Gao Z, Chen JS, Lai KC. Differential Helicobacter pylori infection rates in two contrasting gastric cancer risk regions of South China. China Gastric Cancer Study Group. J Gastroenterol Hepatol. 1999;14:120-125. |
| 8. | Yagi K, Nakamura A, Sekine A. Characteristic endoscopic and magnified endoscopic findings in the normal stomach without Helicobacter pylori infection. J Gastroenterol Hepatol. 2002;17:39-45. |
| 9. | Bansal A, Ulusarac O, Mathur S, Sharma P. Correlation between narrow band imaging and nonneoplastic gastric pathology: a pilot feasibility trial. Gastrointest Endosc. 2008;67:210-216. |
| 10. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. |
| 11. | Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, Iguchi Y, Shimoyama Y, Nagasaka T, Maeda O, Ando T. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. 2006;38:1110-1114. |
| 12. | Dunbar KB, Okolo P 3rd, Montgomery E, Canto MI. Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. 2009;70:645-654. |
| 13. | Kiesslich R, Goetz M, Burg J, Stolte M, Siegel E, Maeurer MJ, Thomas S, Strand D, Galle PR, Neurath MF. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology. 2005;128:2119-2123. |
| 14. | Zhang JN, Li YQ, Zhao YA, Yu T, Zhang JP, Guo YT, Liu H. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc. 2008;67:843-853. |
| 15. | Guo YT, Li YQ, Yu T, Zhang TG, Zhang JN, Liu H, Liu FG, Xie XJ, Zhu Q, Zhao YA. Diagnosis of gastric intestinal metaplasia with confocal laser endomicroscopy in vivo: a prospective study. Endoscopy. 2008;40:547-553. |
| 16. | Elta GH, Appelman HD, Behler EM, Wilson JA, Nostrant TJ. A study of the correlation between endoscopic and histological diagnoses in gastroduodenitis. Am J Gastroenterol. 1987;82:749-753. |
| 17. | Yagi K, Honda H, Yang JM, Nakagawa S. Magnifying endoscopy in gastritis of the corpus. Endoscopy. 2005;37:660-666. |
| 18. | Tahara T, Shibata T, Nakamura M, Yoshioka D, Okubo M, Arisawa T, Hirata I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246-253. |
| 19. | Li CQ, Xie XJ, Yu T, Gu XM, Zuo XL, Zhou CJ, Huang WQ, Chen H, Li YQ. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol. 2010;105:1391-1396. |
| 20. | Smith VC, Genta RM. Role of Helicobacter pylori gastritis in gastric atrophy, intestinal metaplasia, and gastric neoplasia. Microsc Res Tech. 2000;48:313-320. |
| 21. | Tytgat GN. The Sydney System: endoscopic division. Endoscopic appearances in gastritis/duodenitis. J Gastroenterol Hepatol. 1991;6:223-234. |
| 22. | Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150-1158. |
| 23. | Guarner J, Herrera-Goepfert R, Mohar A, Sanchez L, Halperin D, Ley C, Parsonnet J. Interobserver variability in application of the revised Sydney classification for gastritis. Hum Pathol. 1999;30:1431-1434. |
| 24. | Wo JM, Ray MB, Mayfield-Stokes S, Al-Sabbagh G, Gebrail F, Slone SP, Wilson MA. Comparison of methylene blue-directed biopsies and conventional biopsies in the detection of intestinal metaplasia and dysplasia in Barrett's esophagus: a preliminary study. Gastrointest Endosc. 2001;54:294-301. |