INTRODUCTION
Liver is unique in its capacity to regenerate in response to injury or tissue loss. Following two-thirds partial hepatectomy, hepatocytes exit the G0 phase of the cell cycle and synchronously re-enter the cell cycle to regenerate the liver mass completely in 6-7 d in rodents, or 3-4 mo in humans[1-4]. Liver stem/progenitor cells do not seem to be required for this process. However, when this response is impaired, as in the case of a hepatocyte-selective proliferative defect or acute liver failure, the contribution of liver progenitors becomes much more relevant[5,6]. A population of small cells with a low cytoplasmic/nuclear ratio that arose from a small number of portal cells, not from hepatocytes, were first observed in rodents and became known as “oval cells”[7,8]. Although several lines of evidence suggest that oval cells derive from the biliary compartment, other origins have been also suggested[4].
Significant work in the past few years has focused on the signaling pathways, as well as cell-cell interactions, that control the initial proliferation/expansion and the terminal differentiation of liver stem/progenitor cells. Oval cells express MET, the receptor of the hepatocyte growth factor (HGF). The superabundance of HGF-producing cells in the immediate vicinity of oval cell proliferation and differentiation suggests that this growth factor is involved in all aspects of stem cell behavior, proliferation, migration, and differentiation, through a paracrine mechanism[9]. Oval cells also express receptors for epidermal growth factor (EGF)-like ligands, and in vivo infusion of a combination of HGF and EGF enhance the mitogenic response of oval cells after administration of 2-acetylaminofluorene[10], which reveals the relevance of both growth factors in liver stem/progenitor cell biology. Additionally, different studies have revealed that oval cells also respond to other growth factors in an autocrine/paracrine manner[11]. The transforming growth factor (TGF)-β family of cytokines play a relevant role in the maintenance of embryonic stem cell identity, and it has been shown that the specification of pancreas and liver progenitors is restricted by the TGF-β pathway[12]. All these growth factors and cytokines might modulate not only proliferation of liver stem/progenitor cells, but also cell death, as well as contributing to their terminal differentiation.
This review gives an update on recent relevant studies of the growth factors and cytokine-driven intracellular pathways that govern liver stem/progenitor cell expansion and differentiation, and the relevance of these signals in liver development, regeneration and carcinogenesis.
TYROSINE KINASE RECEPTOR-MEDIATED SIGNALING PATHWAYS
HGF
HGF was firstly identified in the 1980s as a potent mitogen for hepatocytes[13-15]. A factor secreted by fibroblasts and smooth muscle cells was discover separately, which promoted epithelial cell scattering[16]. Later studies unraveled that HGF and scatter factor were indistinguishable[17,18]. HGF is a growth factor that induces a wide range of biological activities, including stimulation of proliferation, migration, morphogenesis, and survival of a variety of cell types[19-24], which plays a major role in tissue formation and homeostasis. HGF acts through binding to its tyrosine kinase receptor, Met. Ligand-receptor binding results in autophosphorylation of the receptor in specific tyrosine residues located in the C-terminal domain, and subsequent phosphorylation/activation of multiple adapter and signal transducing proteins, such as growth factor receptor-bound protein 2 (Grb2)/Sos, Ras-mitogen-activated protein kinase, Grb2-associated binding protein 1 (Gab1), phosphoinositide 3-kinase (PI3K), phospholipase C-γ, p38, and signal transducer and activator of transcription (STAT)-3, among others, which mediate the biological activities of HGF/c-Met[25,26]. For decades, HGF has been recognized as a growth factor involved in the hepatocyte proliferative response during liver regeneration (a recent review on the role of HGF in liver regeneration can be found in[3]), but an unequivocal demonstration of an essential role of the HGF/c-Met signaling in liver regeneration has only been provided recently. Thus, liver specific c-Met and HGF conditional knock-out mice show an impairment of the regenerative response[27-29]. Hepatocytes that lack a functional c-Met display reduced basal survival and a higher sensitivity to Fas-induced liver damage, both in vivo and in vitro. Moreover, after toxic liver injury induced by exposure to CCl4, c-met-/- livers showed delayed healing from necrotic injury[28]. Complete abolition of the cell cycle has also been demonstrated when c-Met is deleted after partial hepatectomy, by using RNA interference techniques[30]. In addition to the effects on proliferation, these authors have described an alteration in expression of apoptosis-related genes, particularly, increased expression of pro-apoptotic genes, and decreased expression of anti-apoptotic genes, and enhanced activation of caspase 3. Altogether, these studies provide clear evidences of a role for this ligand/receptor system in promoting hepatocyte proliferation, survival, and tissue remodeling during liver regeneration.
HGF/c-Met signaling is also essential during fetal liver development. Both HGF- and c-Met-deficient embryos show abnormally small livers and liver-to-body weight ratios and massive hepatocyte apoptosis[31-33]. Recent studies from Dr. Maina’s laboratory have demonstrated HGF/c-Met survival properties in primary embryonic hepatocytes, as shown by the ability of HGF to impair Fas-induced apoptosis by acting through PI3K and AKT to prevent FLICE inhibitory protein degradation[34]. Additionally, in the context of the adult liver, numerous works have reported an important contribution of the pro-survival activity of HGF in protecting liver during fibrosis and other hepatic dysfunction[35-40], thus expanding the scenarios in which the anti-apoptotic activity of HGF plays an active role.
Although hepatocytes have long been considered the prime target of the actions of HGF, there is accumulating evidence of an important role for HGF/c-Met signaling on liver stem/progenitor cell function and behavior. Liver stem/progenitor cells express c-met[41]. Furthermore, during oval cell activation induced by N-acetyl-2-aminofluorene/partial hepatectomy (AAF/PH) in rats, HGF expression increases coincidentally with oval cell proliferation, mainly on the periportal regions where oval cells are located[9,41]. These data suggest that the HGF/c-Met system regulates some aspects of liver stem/progenitor cell biology. In support of this, in vivo infusion of HGF during AAF/PH-induced liver regeneration stimulates oval cell expansion into the liver lobules[10]. Similar results have been obtained by in vivo transfer of HGF cDNA into liver subjected to the Solt-Farber regime[42]. HGF-dependent mitogenic activity has also been shown in rat and mouse oval cell lines in vitro by either adenovirus-mediated transfer of the HGF gene or addition of exogenous HGF[43-46]. The molecular mechanisms that mediate the mitogenic effects of HGF in liver progenitors appear to be cell-type specific, because PI3K/AKT activation[43] and nuclear factor-κB activation, downstream of p38 and extracellular signal-regulated kinase (ERK) MAPKs[44], are involved. Additionally, bi-potential hepatoblast cell lines (precursors of both hepatocytes and cholangiocytes) have been established from transgenic animals expressing a constitutively active human Met[47]. However, HGF is much more than a mitogen for liver stem/progenitor cells. HGF effectively protects WB-F344 cell from apoptosis induced by tumor necrosis factor-α in a dose-dependent manner[44]. More recently, using a novel in vitro model of genetically modified oval cells that harbor an inactivated Met tyrosine kinase, we have demonstrated that loss of Met increases sensitivity to apoptosis caused by serum deprivation or treatment with TGF-β[46]. By virtue of these results, we hypothesize that Met-driven anti-apoptotic activity plays an important role supporting the expansion of liver progenitors following liver injury, by helping them to overcome the local tissue injuries and inhibitory signals. Therefore, the HGF/c-Met signaling pathway might be a major survival pathway in liver that operates during liver development, homeostasis, and regeneration (both hepatocyte and oval cell-mediated).
The role of HGF as a morphogen is already established[48]. Morphogenesis is a complex invasive growth program that plays a fundamental role in normal development[49,50]. Not surprisingly, morphogenic properties of HGF are exhibited in embryonic and postnatal mouse non-parenchymal epithelial cell-derived cell lines. In these cells, HGF induces a morphogenic response that includes cell scattering and ductal branching in collagen gels. These changes are not observed by treatment with other liver growth factors including EGF, TGF-β, acidic fibroblast growth factor (aFGF) and insulin, and are not inhibited by TGF-β[51]. A similar response has also been described in pancreatic oval cells in vitro; a cell population closely related to its hepatic counterpart[52]. Recently, HGF has been directly involved in promoting motility and invasiveness of human liver progenitor cells through Matrigel; an effect that is partially mediated by matrix-metalloproteinase-mediated extracellular matrix (ECM) proteolytic degradation and activation of the MAPK/ERK pathway[53].
Another important biological activity of HGF in liver progenitor cells that cannot be overlooked is its modulatory effect on the differentiation capacity of these cells. HGF has been reported to be required not only for an efficient proliferation and survival, but for hepatocytic differentiation of embryonic hepatic stem cells in vitro[54-57]. Furthermore, virtually all the strategies for in vitro differentiation of embryonic and adult stem/progenitor cells of different origin into hepatocytes include HGF as a hepatic-inducing factor (a thorough compilation of in vitro differentiation methods is found in[58]). Successful differentiation is generally achieved by step by step addition of growth factors, cytokines, and hormones, trying to emulate the sequence of events taking place during in vivo hepatogenesis. The requirement of HGF in this process relies on the specific role played by this growth factor at different liver developmental stages. During the commitment phase, HGF might antagonize differentiation of bi-potential hepatoblasts along the cholangiocytic lineage, which results in support of growth and differentiation of fetal hepatocytes[59]. In fact, results from Suzuki et al[57] have demonstrated that HGF can initiate differentiation of albumin-negative liver stem cells into albumin-positive hepatic precursors at the same time that allows their expansion and decreases expression of cholangiocyte-lineage markers, such as CK19 or γ-glutamyl transferase, but it cannot induce latter markers of hepatocyte differentiation, such as glucose-6-phosphatase or tryptophan-2,3-dioxygenase.
Later, after birth, the expression of both HGF and c-Met significantly increases in the liver, and activation of this pathway crucially assists during complete functional hepatic maturation[41,60]. We have also described that HGF combined with TGF-β helps to maintain the expression of hepatocyte differentiation markers in rat fetal hepatocytes in culture[61,62]. In rat primary neonatal hepatocytes, HGF promotes scattering, but this effect is not associated with a dedifferentiation process, as shown by an increase in the expression of hepatic differentiation markers using HGF treatment[63,64]. Although a role for HGF in hepatocyte differentiation seems to be beyond reasonable doubt, it should be pointed out that HGF has also been directly implicated in transdifferentiation of hepatocytes to biliary epithelial cells[65]. These data highlight the enormous complexity in terms of signaling networks and molecular mechanisms associated with regulation of phenotypic transitions in liver epithelial cells.
All these results provide sufficient evidence to support a crucial role for an HGF/c-Met-induced signaling pathway in liver stem/progenitor cell biology. What it is not totally clear, however, is its mode of action. HGF is mainly produced by mesenchymal cells, and c-Met is expressed in epithelial cells, therefore, this ligand-receptor system is generally considered to act in a paracrine fashion[66]. Consistent with this, the main producers of HGF in the liver are the stellate cells[67,68], although sinusoidal endothelial cells also express HGF[69]. c-Met is expressed in hepatocytes, biliary epithelial cells, and liver progenitor cells[41,54,70,71]. As mentioned above, in rat models of oval cell activation in vivo, HGF mRNA has been identified in the desmin-positive stellate cells that surround the ductal structures of oval cells, or interspersed with the oval cells, whereas c-Met expression is detected in oval cells[9,41]. These results have prompted the conclusion that the HGF/c-Met system operates in oval cells via a paracrine mechanism. However, in vitro studies carried out in our laboratory in oval cell lines have shown an autocrine regulatory mechanism for HGF/c-Met involved in protection against apoptosis[46]. Whether the autocrine regulation plays a role in vivo has not been explored, but these data suggest that oval cells might respond to both autocrine and paracrine Met signaling in a context-dependent fashion.
EGF receptor ligands
Another tyrosine kinase receptor family that regulates liver pathophysiology is the EGF receptor (EGFR) family. This receptor is part of a complex signaling system that includes multiple ligands, namely TGF-α, EGF, heparin-binding EGF, amphiregulin, betacellulin, epiregulin, epigen, and crypto; and four transmembrane receptors: EGFR (Her1/ErbB-1), ErbB-2 (Her2/neu), ErbB-3 (Her3), and ErbB-4 (Her4). Complexity of this pathway relies on differential ligand binding affinity to the receptors, as well as formation of receptor homo- and hetero-dimers, all of which leads to activation of distinct intracellular signaling cascade and diverse biological activities. Among all the ligands, TGF-α and EGF are the most widely studied. Both TGF-α and EGF bind and activate the same receptor, EGFR. Ligand binding results in dimerization and autophosphorylation of EGFR in tyrosine residues of the cytoplasmic domain. This leads to recruitment of adapter proteins, such as Grb2 and Shc, and subsequent activation of multiple downstream pathways, including PI3K, Ras-MAPK, c-Jun N-terminal kinase, p38, protein kinase C and STAT-3, which mediate cell proliferation, migration, differentiation and evasion from apoptosis[72-76]. TGF-α and EGF are well-known regulators of hepatocyte proliferation. Infusion of either one of the two factors initiates DNA synthesis in liver of adult rats[77,78], and when added exogenously, they stimulate growth of primary hepatocytes at all developmental stages: fetal, neonatal, and adult[79-82]. Together with HGF, EGFR ligands represent the only complete mitogens in adult hepatocytes in serum-free medium, and the most important growth factors involved in the proliferative response during liver regeneration[3]. Demonstration of a critical role for EGFR in hepatocyte proliferation during the initial phases of liver regeneration has recently been provided by generating mice with a liver-specific EGFR deficiency[83]. In addition to the regulatory role in proliferation, and similarly to Met, EGFR is a major survival pathway in the liver[84]. EGF-mediated EGFR activation is able to abolish completely the apoptotic response induced by TGF-β in fetal rat hepatocytes[62] or by Fas receptor stimulation in mouse hepatocytes[85]. Furthermore, EGFR ligands appear to be important modulators of hepatocyte differentiation as well. Morphological and gene expression studies from our laboratory and collaborators have shown that EGF, acting in cooperation with other cytokines and hormones, maintains primary fetal and neonatal hepatocyte differentiation[64,86,87].
Expression studies have suggested that ErbB1-triggered signaling also plays a role in stem/progenitor-cell-mediated liver regeneration. Indeed, both TGF-α and EGFR are transcriptionally upregulated during the period of active proliferation and differentiation of progenitor cells in the rat liver subjected to the 2-AAF/PH protocol[88], and they appear to drive the early proliferation of the progenitor cell compartment[89,10]. Although both HGF and EGF promote the expansion of oval cells in vivo, some differences are observed. HGF increased number of both ductal and Ito cells at a similar rate, whereas infusion of EGF mostly increases ductal cells. Our laboratory and others have also shown that EGF and TGF-α are mitogens in mouse and rat oval cells in vitro[11,46,90].
The effects of this signaling pathway on liver stem/progenitor cells are not restricted to mitogenesis. In vivo infusion with either EGF or HGF not only amplifies liver progenitor expansion following liver injury but also decreases apoptosis[10]. We also have evidence of an important role for EGFR-mediated signaling in regulating oval cell survival in vitro. Thus, inhibition of EGFR via treatment with a synthetic inhibitor increases basal apoptosis (in the absence of serum and exogenous stimuli) and strongly amplifies TGF-β-induced apoptosis (our unpublished results). EGFR-mediated motogenic, morphogenic, and differentiation activities have also been reported. EGF, combined with TGF-β, triggers a scattering response in mouse oval cell lines in vitro[11]. In addition to this, EGF is often included as a hepatogenic factor in strategies to induce in vitro differentiation of stem/progenitor cells into hepatocyte-like cells[58]. Consistently, TβT-FH cell lines - a cell population of fetal hepatocytes that has suffered an epithelial mesenchymal transition (EMT) and a dedifferentiation process after TGF-β treatment - recover the original epithelial phenotype and gain cytokeratin-19 expression by treatment with EGF and DMSO, which suggests conversion to hepatoblast-like cells[91].
It should be noted that we have seen autocrine regulation for EGFR-dependent signaling in oval cell lines (our unpublished results). Different to the HGF/c-Met system, an EGFR-ligand-mediated autocrine mechanism has been suggested, based on the detection of EGFR ligand transcripts in oval cells during liver regeneration[88]. In vivo and in vitro studies have shown that choline-deficient ethionine-supplemented diet-treated mouse livers express cytokines such as lymphotoxin-β, interferon-γ, and interleukin (IL)-6[92,93]. Collectively, these data strongly support that autocrine regulatory mechanisms are important in liver progenitor cells. The significance of the c-Met and EGFR autocrine loops in oval cells is not yet totally understood. Certainly, autocrine signaling has been mostly associated with malignancy. This seems to apply to stem/progenitor cells as well, because tumorigenic conversion of mouse oval cell has been associated with growth factor production and alteration in growth factor responsiveness[11]. More specifically, an HGF/c-Met autocrine loop has been identified in spontaneously transformed WB-F344 rat liver stem-like cells, which contributes to drive autonomous cell proliferation[94]. Spontaneously transformed oval cells express TGF-α. These cells form tumors when injected into nude mice, a capacity that is significantly reduced by transfection with TGF-α antisense gene[95]. A cross-talk between Met and EGFR has also been proposed. Thus, rat liver-epithelial-cell-derived tumor cell lines that constitutively express TGF-α display increased levels of both c-met gene and protein, as well as an amplified response to HGF[96]. However, 3,5-diethoxycarbonyl-1,4-dihydro-collidine (DDC)-treated mouse liver-derived oval cell lines, which show autocrine activation of Met and EGFR-dependent pathways, do not show any sign of transformation[46] (and our unpublished results). Whether this is due to a matter of time, dose, or lack of critical partners for the neoplastic transformation, it is not yet known. For now, awaiting further conclusive experiments, a direct link between the establishment of functional c-Met and EGFR-dependent autocrine loops and neoplastic transformation cannot be firmly established in liver progenitor cells.
All the data summarized above highlight not only a relevant role of Met and EGFR-triggered signaling pathways in regulating the liver progenitor cell compartment, but a striking parallelism between the two pathways, both of which mediate growth, survival, migration and hepatocytic differentiation (Figure 1). Further studies will clarify which of these biological activities are totally overlapping and which are not. In this sense, Met and EGFR mutant mice and cell models should provide very useful and invaluable tools in this regard.
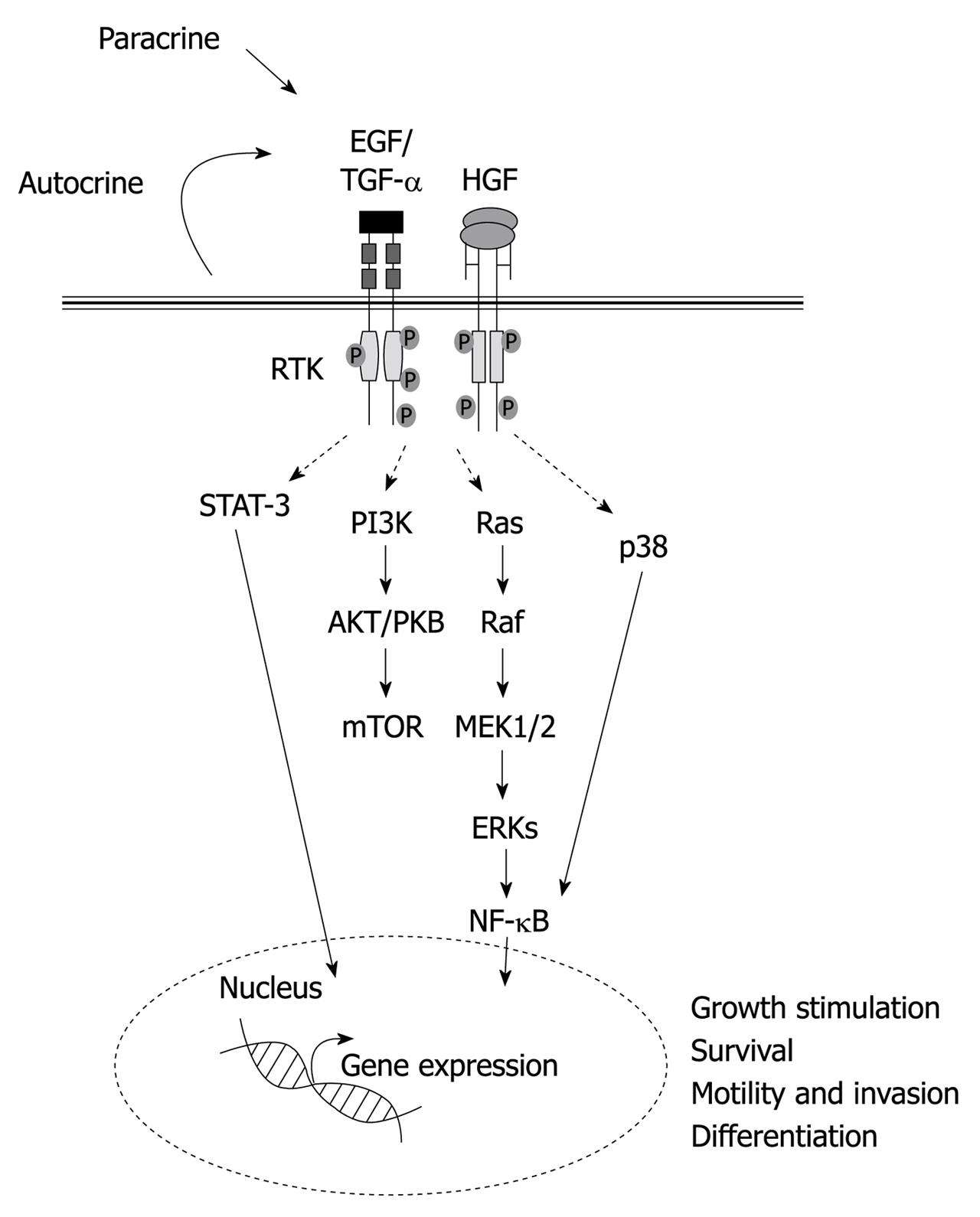
Figure 1 Schematic illustration of the major signaling pathways and biological activities induced by hepatocyte growth factor and epidermal growth factor receptor ligands in liver progenitor cells.
HGF: Hepatocyte growth factor; EGF: Epidermal growth factor; TGF: Transforming growth factor; STAT: Signal transducer and activator of transcription; PI3K: Phosphoinositide 3-kinase; NF-κB: Nuclear factor κB; mTOR: Mammalian target of rapamycin; MEK: Mitogen-activated protein kinase kinase; ERKs: Extracellular-signal-regulated kinases.
FGFs
The FGFs are a family of growth factors with high affinity for heparan sulfate proteoglycans, which bind to transmembrane tyrosine kinase FGF receptors (FGFRs). The role of these growth factors in hepatic fate specification of pre-hepatic endoderm cells during liver development is well known. There are a number of excellent reviews on this subject, some of which are cited here[97-99]. Activation of adult stem/progenitor cells is believed to use similar, if not identical, genetic programs as the embryonic progenitors, therefore, FGF is among the main targets to be analyzed in rodent models of oval cell expansion. Indeed, aFGF (also known as FGF-1) is upregulated at the stage of oval cell progression[89,100], being expressed by both oval cells and Ito cells. FGFR1 and FGFR2 are also expressed at high levels during the period of active proliferation and differentiation of oval cells, but exhibit a different pattern. Although FGFR1 is mainly expressed in oval cells, FGFR2 is expressed in both oval and Ito cells[101]. These results suggest a differential role for these two receptors during liver stem/progenitor-cell-mediated regeneration, as well as the establishment of both autocrine and paracrine signaling. In addition to these in vivo observations, in vitro studies have shown that aFGF is able to push Met murine hepatocytes-bi-potential precursors isolated from transgenic livers, which express a constitutively active human Met - to progress from a very early state of differentiation to a more mature state, associated with the expression of liver functions[47]. Moreover, FGF1-pretreated cells are resistant to the TGF-β dedifferentiation effect[102]. Essentially, FGFs have proved to be effective in mediating early hepatic differentiation, and therefore are included in most in vitro differentiation protocols[58].
TGF-β FAMILY
The TGF-β family of cytokines regulates hepatocyte proliferation and death, and plays relevant roles during liver regeneration[103]. However, TGF-β and other pro-inflammatory cytokines are important inducers of fibro-carcinogenesis, due to their ability to induce myofibroblast differentiation and ECM deposition[104,105]. Recent evidence has shown that TGF-β might also regulate liver stemness and phenotype[106].
TGF-β active form acts as a dimer, and signals by bringing together two receptors with serine-threonine kinase activity, which are known as type I and type II receptors (Figure 2). After TGF-β binding, the type II receptor phosphorylates and activates the type I receptor, which is responsible for phosphorylation of the receptor-regulated Smad family of transcription factors (R-Smads), i.e. Smad 2 and 3 in the case of TGF-β1, β2 and β3[107]. After phosphorylation, Smad 2 or 3 forms a complex with Smad 4 and shuttles to the nucleus, where it possesses DNA-binding activity, although it must associate with other DNA-binding co-factors to achieve high-affinity binding. Indeed, through combination with different transcription factors, the same TGF-β stimulus can induce or repress many different target genes. Activation of the Smad transcriptional activity can be counteracted by the presence of other members of the Smad family that play inhibitory effects (inhibitory Smads), such as Smad 6 and Smad 7, which compete with Smad 2 and 3 for binding either to the receptor complex or to Smad 4.
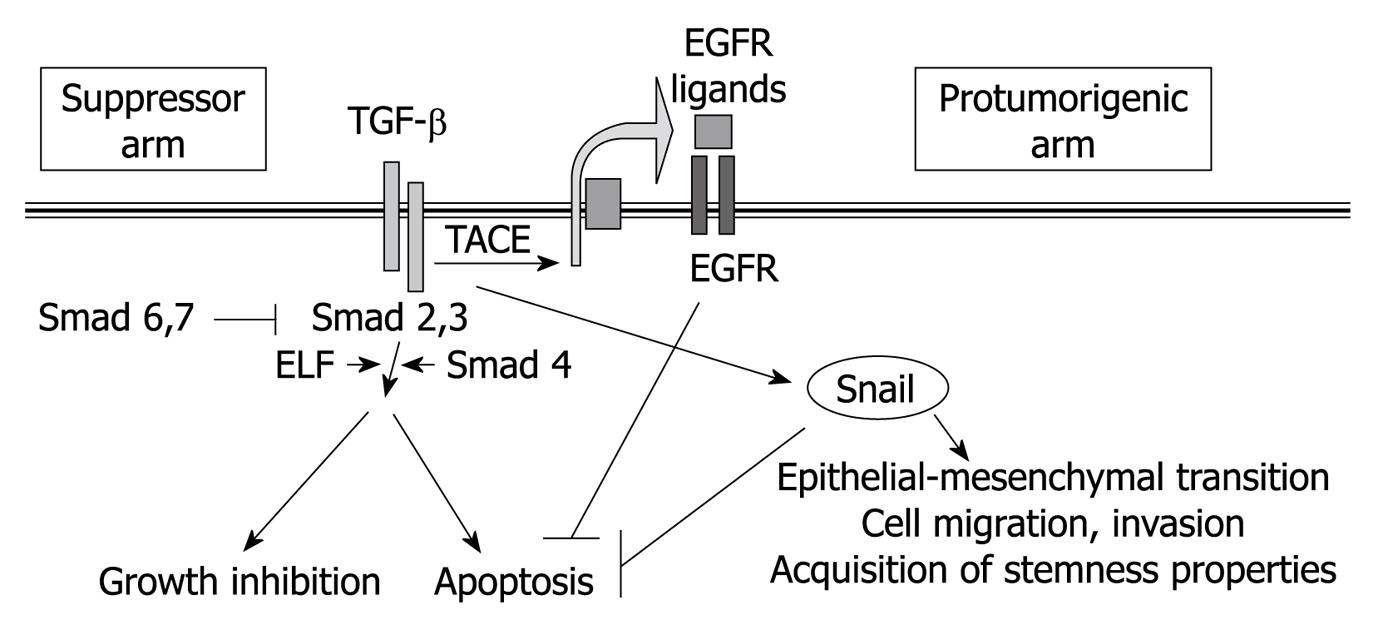
Figure 2 Overview of the major signaling pathways, and their cellular effects, induced by transforming growth factor-β in liver cells.
TGF: Transforming growth factor; EGFR: Epidermal growth factor receptor; ELF: Embryonic liver fodrin; TACE: Tumor necrosis factor-converting enzyme.
The most relevant Smad-mediated cell responses to TGF-β are growth inhibition and apoptosis. TGF-β inhibits proliferation in adult[108,109], as well as fetal or regenerating hepatocytes[82,109], and it is a well-known inducer of hepatocyte cell death[110,111]. The inhibitory effects of TGF-β on liver stem/progenitor cell growth and survival are somewhat unclear. TGF-β overexpression results in impairment of oval cell expansion induced by DDC[112]. Consistently, we have shown that treatment with TGF-β inhibits growth and induces apoptosis in DDC-derived oval cell lines in vitro[46]. However, when compared to adult hepatocytes, other authors have reported that the majority of the adult liver progenitor cells are more resistant to endogenously produced TGF-β anti-proliferative and pro-apoptotic effects in vitro[113], which indicates a differential sensitivity to TGF-β between liver progenitors and mature hepatocytes. Indeed, several mechanisms have been proposed that would explain this differential response. On the one hand, the ratio between R-Smads and inhibitory Smads might be different in oval cells. In this sense, Nguyen et al[114] have recently reported that oval cell lines show higher levels of Smad 6 than do adult hepatocytes. On the other hand, oval cells might show over-activation of survival signals, such as the MAPK/ERK pathway[115] that might be related to the autocrine production of growth/survival factors, such as HGF[46]. It is worth noting that, when combined with growth factors such as EGF or HGF, TGF-β might contribute to cellular scattering and morphological differentiation of hepatoblasts and liver stem cells[87,116,117]. In this same line of evidence, hepatocytes are undifferentiated and liver architecture is lost in Smad 2+/- and Smad 3+/- mice, as well as in ELF (a β-spectrin that is crucial for the propagation of the TGF-β signal) mutants[118-121]. Defects in Smad 2+/- and Smad 3+/- mutants are restored by HGF treatment. Although mechanisms by which TGF-β signals can be supplanted by HGF are unknown, data seem to support cooperation between two independent pathways, that converge on β1-integrin expression, rather than direct pathway crosstalk[119]. Emerging new data have reinforced the idea that TGF-β is important for stem cell transitioning to a progenitor cell phenotype, and ultimately, its conversion to a fully differentiated phenotype. Indeed, TGF-β signaling, and particularly ELF, appears to play a crucial role in hepatocyte proliferation and transitional phenotype during human liver regeneration, and its loss is associated with activation of liver progenitor cells[106]. It has also been shown recently that TGF-β might be a determinant in the appearance of progenitor/stem cells in hepatocarcinogenesis. The examination of human hepatocellular carcinoma (HCC) has revealed that cells that are labeled with stem-cell markers have unexpectedly lost the TGF-β receptor II and ELF, and show marked activation of the IL-6 pathway, a major stem-cell signaling pathway[121,122]. These data support that absence of TGF-β-driven epithelial differentiation favors carcinogenesis.
However, TGF-β is a pleiotropic cytokine inducing several, and sometimes contradictory, signals in epithelial cells. Indeed, in addition to the well-known Smad-mediated transcriptional responses that predominantly address tumor suppressor actions, TGF-β induces other Smad-dependent or independent effects that contribute to tumor progression[107]. Among these, related to the topic of this review, one of the more relevant is the capacity to induce EMT processes. EMT is a physiological process during embryogenesis, in which an epithelial cell loses expression of adhesion molecules, such as E-cadherin, and other components responsible for cell polarity. Instead, they express mesenchymal components of the cytoskeleton and acquire motility and scattering properties[123]. A closely related phenotypic conversion is also detected in fibrosis and neoplasia and is associated with disease progression[124]. Members of the TGF-β family can initiate and maintain EMT in a variety of biological systems and pathophysiological situations, through activation of major signaling pathways and transcriptional regulators integrated in extensive signaling networks[125,126]. In culture, hepatocytes and hepatoma cells undergo EMT in response to TGF-β[127-130], which support a potentially crucial role for TGF-β in the development and progression of hepatic fibrogenesis and cancer. The mechanisms that allow cells to escape from the apoptotic effects of TGF-β and undergo EMT are not completely understood. However, recent results have indicated that, in some epithelial cells, including fetal rat hepatocytes and hepatoma cells, TGF-β might induce both pro- and anti-apoptotic signals and their balance defines the cell fate[129,131-133]. In fact, certain evidence indicates a crosstalk between the genetic programs that control TGF-β-induced growth arrest/apoptosis and those that regulate EMT, because once the cell has adopted a mesenchymal phenotype, it does not respond to TGF-β suppressor effects[128,134,135]. Thus, TGF-β might regulate its own signaling to switch from tumor suppression to tumor progression. Indeed, it is interesting to point out that transcription factors of the Snail family, repressors of the E-cadherin gene, are required for cell survival during EMT[136,137].
In recent works from our group, we have demonstrated that transdifferentiation of liver cells from an epithelial to a mesenchymal phenotype, such as that mediated by TGF-β, induces dedifferentiation and allows enrichment in a population of cells with putative liver progenitor properties[91,128,138]. The phenotypic characteristics observed (elongated phenotype, strong expression of Thy-1, vimentin or α-smooth muscle actin) are also highly reminiscent of myofibroblasts[139]. These finding are in agreement with the recently proven fact that EMT not only endows cells with migratory and invasive properties, but also induces stem cell properties[123]. Furthermore, additional works have proved the capacity of TGF-β to induce/maintain a stemness phenotype in other tissues[140]. Preliminary results have indicated that human fetal hepatocytes respond to TGF-β downregulation of E-cadherin and upregulation of Snail, thus acquiring a fibroblastic-like morphology and a liver progenitor cell phenotype (unpublished observations from our group in collaboration with Dr. N. Fausto, University of Washington, Seattle, USA).
These findings might have implications for regenerative biology of the liver and open new perspectives for the in vitro isolation of putative liver stem/progenitor cells to be used in basic and translational research in the liver. Moreover, a recent study has also suggested that TGF-β treatment of HCC cells might induce the selection of cells expressing high levels of CD133, a putative stem-cell marker in diverse hematopoietic and non-hematopoietic tissues and cancers[141], through a mechanism partially dependent on the Smads pathway, and that involves epigenetic regulation of the CD133 promoter. These results indicate that TGF-β might also play a role in transdifferentiating liver tumor cells to liver cancer stem cells.
In the embryo, progenitors to pancreatic β cells and hepatocyte lineages arise from neighboring domains of ventral foregut endoderm. It has been recently found that the specification of pancreas and liver progenitors is restricted by the TGF-β pathway[12]. It has been speculated that TGF-β signaling restrains lateral endoderm specification until the cells move sufficiently far from the heart and into a bone morphogenetic protein signaling domain, with the latter then becoming the dominant Smad 4-dependent pathway, which leads to pancreatic induction. TGF-β signaling appears to be strongly inhibitory to the pancreatic lineage and modestly inhibitory to the liver lineage, which suggests that TGF-β also plays a relevant role in maintaining the stemness phenotype of endodermal precursors during liver development.
All these results suggest a dual role for the TGF-β signaling pathway in liver stemness and differentiation. On the one hand, TGF-β mediates progression of differentiation from a stem or progenitor stage, but on the other hand, it contributes to the expansion of liver stem cells. Further work is necessary to understand better the relevance of both effects in liver development, regeneration and carcinogenesis.
HEDGEHOG FAMILY LIGANDS
Hedgehog (Hh) family ligands are widely acknowledged morphogens that regulate tissue remodeling during embryogenesis[142,143]. In particular, Indian hedgehog and Sonic hedgehog ligands, and their receptor, Patched, are expressed at different stages of liver organogenesis, and available data point to dynamic signal activation and temporarily restricted effects on hepatoblast regulation. Thus, Hh signaling is necessary to promote hepatoblast proliferation but it needs to be shut off to permit subsequent hepatoblast differentiation[143-148]. Hh signaling is also involved in the maintenance of a hepatic progenitor reservoir throughout life, both in humans and mice. Blockade of Hh activity in hepatic progenitors in vitro decreases survival, whereas stimulation of Hh activity inhibits endogenous apoptosis. Both autocrine and paracrine modes of action are suggested, based on simultaneous expression of Hh ligands and receptors in liver progenitor cells[149]. Additional observations suggest that Hh pathway activation is a common feature of various types of chronic liver injury associated with mobilization of hepatic progenitor populations. Participation of the Hh pathway has been demonstrated in the ductular reaction that is elicited by chronic alcohol-induced liver injury in mice and humans[150]; non-alcoholic steatohepatitis in humans[151]; methionine choline-deficient ethionine-supplemented diet[152] and fatty liver damage[153] in mice. Hh ligands are released by hepatocytes and myofibroblasts, and lead to enhanced viability and proliferation of bile ductular cells and hepatic progenitors, thus promoting the ductular response and fibrogenesis. Myofibroblasts, but not hepatocytes, also respond to Hh ligands with an increase in viability[152,154-157]. Either Hh paracrine signaling between myofibroblast and hepatic progenitors or autocrine signaling, or both, promote EMT in hepatic progenitors, which ultimately contributes to liver fibrosis[157-159].
The way in which Hh signaling interacts with other pathways to control the fate of hepatic progenitor populations during liver repair after damage or neoplasia is still not well understood. Nonetheless, some interesting pieces of information are emerging. Similar to that which has been described in embryogenesis[160], a functional crosstalk between the TGF-β and Hh signaling pathways has been proposed in the context of adult liver progenitor expansion during liver injury. In ethanol-fed mice, TGF-β induces production of Hh ligands in hepatocytes, which subsequently promotes survival and expansion of ductular/oval cell populations. This supports the concept that enhanced exposure to TGF-β contributes to the accumulation of cells with a ductular phenotype, which are protected from TGF-β-mediated apoptosis[155,161]. Reciprocally, Hh-induced EMT might depend, at least partially, on induction of TGF-β[159]. Further research is required to delineate the Hh-TGF-β interaction.
These findings suggest a role for the Hh pathway as a major mediator in the communication network established between myofibroblast and liver progenitors, which promotes progenitor accumulation and contributes to hepatic fibrosis pathogenesis through EMT induction.
WINGLESS/β-CATENIN PATHWAY
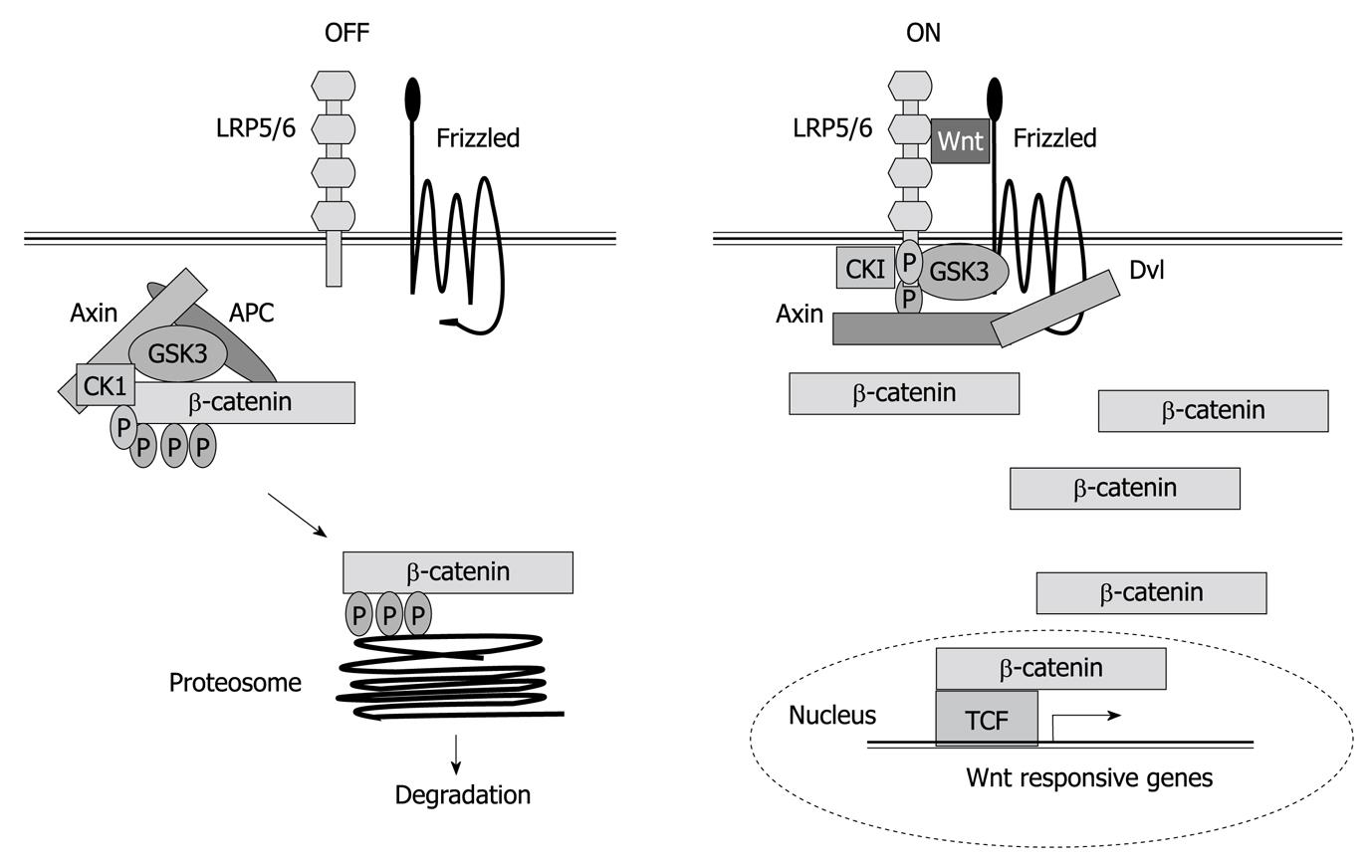
Proteins of the wingless (Wnt) family are secreted signaling molecules that regulate multiple processes in animal development and control tissue homeostasis. The Wnt signaling pathway is highly conserved throughout evolution and plays essential roles in controlling cell proliferation, cell to cell adhesion, and motility[162]. In the liver, it plays many crucial roles during hepatic development and regeneration, and its dysregulation is evident in aberrant growth during hepatocarcinogenesis[163]. Wnt signaling is itself inherently complex[164]. On the one hand, both the ligands and receptors involved in Wnt signal transduction belong to large multi-gene families, which allows for a large number of possible ligand-receptor interactions. On the other hand, Wnt-receptor interactions can elicit a variety of intracellular responses, the best-known of which results in the activation of β-catenin/T-cell factor (TCF) transcriptional complexes. In the absence of signals, β-catenin accumulates in adherens junctions and free β-catenin levels are very low because the tumor suppressor adenomatous polyposis coli forms a trimer complex with glycogen synthase kinase-3-β and axin/conductin, which interacts with and phosphorylates β-catenin, thus targeting it for degradation to the proteasome (Figure 3). Wnts bind a two-part receptor: a seven transmembrane Frizzled and low-density lipoprotein-related protein (LRP)5/6, both being required for canonical signaling. Ligand binding mediates phosphorylation of the cytoplasmic tail of LRP5/6, which creates an axin-binding site. Axin recruitment inactivates the destruction complex. This stabilizes β-catenin, which enters into the nuclei where it displaces Groucho from TCF, nucleating formation of a multiprotein activator complex, and activating Wnt target genes[165] (Figure 3). Furthermore, β-catenin is at the crossroads of growth factor and cytokine signaling. Indeed, certain evidence indicates that TGF-β might induce nuclear β-catenin accumulation, through induction of platelet-derived growth factor signaling[166]. β-catenin expression leads to elevated EGFR levels in hepatocytes and immunohistological analysis shows high correlation between the expression of nuclear/cytoplasmic β-catenin and EGFR in most hepatoblastomas[167]. β-catenin also participates in homotypic cell-cell interactions through its association with E-cadherin. Thus, nuclear β-catenin accumulation in HCC cells might contribute to impaired E-cadherin expression, which mediates the EMT process, migration and survival. It is also known that β-catenin and Met form a complex on hepatocyte membranes. Upon HGF stimulation, the complex is dissociated, in a β-catenin tyrosine phosphorylation-dependent but Wnt-independent manner, which results in β-catenin nuclear translocation[168]. In addition, a role for β-catenin as a downstream effector of HGF in HGF-induced hepatomegaly has been demonstrated[169].
Figure 3 Wnt/β-catenin signaling.
Left figure shows how β-catenin is pushed to degradation in the absence of Wnt ligand. Right figure shows how in the presence of Wnt, β-catenin regulates gene expression. TCF: T-cell factor; LRP: Low density lipoprotein receptor-related protein; APC: Adenomatous polyposis coli; CK1: Casein kinase 1; GSK: Glycogen synthase kinase.
It has been recently suggested that oval cells respond to Wnt ligands in vitro with an increase in amino-terminus dephosphorylated β-catenin and cell cycle entry[170], and that canonical Wnt/β-catenin/TCF signaling plays a key role in the normal activation and proliferation of adult hepatic stem cells[171,172]. In this same line of evidence, repression of Wnt/β-catenin signaling in the anterior endoderm is essential for liver and pancreas specification[173], which indicates that turning Wnt signaling off is essential for liver differentiation. An opposite situation might be found during hepatocarcinogenesis, where reactivation of the Wnt/β-catenin pathway, or accumulation of nuclear β-catenin due to other alterations might contribute to the expansion of liver tumor stem cells. Indeed, recent reports have highlighted the relevance of Wnt/β-catenin signaling in the activation of tumorigenic liver progenitor cells[174] and the acquisition of hepatic stem-cell markers[175] in HCC. Accumulation of nuclear β-catenin has been shown to induce an early liver progenitor phenotype in HCC, which correlates with tumor recurrence[176].
In summary, Wnt family and β-catenin/TCF pathways are clearly involved in the maintenance of the liver stemness phenotype, and its repression is necessary for liver differentiation during development. Its overactivation during liver tumorigenesis might contribute to the acquisition of a liver cancer stem cell phenotype.
CONCLUSION
For the past 10-15 years, significant progress has been made in elucidating the cellular and molecular mechanisms that contribute to control of liver stem/progenitor cell behavior. Its complexity and multifaceted nature is evident. Among the diverse signaling pathways at play, the major growth factors, cytokines, and other ligand/receptor systems known to play an important role in liver development, homeostasis and regeneration, certainly occupy a central position. Many of the classical responses elicited by these factors are conserved. However, differences in the downstream mechanisms following ligand-receptor engagement, or signal interaction and crosstalk, as well as specific alterations in intracellular mediators, are being identified as contributing factors to the differential response of liver stem/progenitor cells compared with their mature counterparts. In addition, besides delivery of signals by the surrounding microenvironment, accumulating observations are pointing to co-existence of paracrine and autocrine modes of response as general regulatory mechanisms on this cell population. Collectively, these data indicate that liver stem/progenitor cells follow their own rules and regulations. It is also clear that the same signals that are essential for their activation, expansion, and differentiation, are good candidates to contribute, under adequate conditions, to the paradigm of transformation from a pro-regenerative to a pro-tumorigenic role. Unquestionably, from a clinical perspective, this is a fundamental issue for liver/stem progenitor cell-based therapies. Despite the fact that additional efforts are required to delineate perfectly how the functional switch takes place, the studies summarized here are steps in the right direction.
Peer reviewers: Dr. Thomas Kietzmann, Professor, Department of Biochemistry, University of Oulu, FI-90014 Oulu, Finland; Toshihiro Mitaka, MD, PhD, Professor, Department of Pathophysiology, Cancer Research Institute, Sapporo Medical University School of Medicine, South-1, West-17, Chuo-ku, Sapporo 060-8556, Japan
S- Editor Wang JL L- Editor Kerr C E- Editor Zheng XM