Published online Oct 28, 2010. doi: 10.3748/wjg.v16.i40.5104
Revised: July 12, 2010
Accepted: July 19, 2010
Published online: October 28, 2010
AIM: To evaluate the feasibility, efficacy and safety of intraoperative radiofrequency ablation (RFA) combined with 125iodine seed implantation for unresectable pancreatic cancer.
METHODS: Thirty-two patients (21 males and 11 females) at the age of 68 years (range 48-90 years) with unresectable locally advanced pancreatic cancer admitted to our hospital from January 2006 to May 2008 were enrolled in this study. The tumor, 4-12 cm in diameter, located in pancreatic head of 23 patients and in pancreatic body and tail of 9 patients, was found to be unresectable during operation. Diagnosis of pancreatic cancer was made through intraoperative biopsy. Patients were treated with FRA combined with 125iodine seed implantation. In brief, a RFA needle was placed, which was confirmed by intraoperative ultrasound to decrease the potential injury of surrounding vital structures, a 125iodine seed was implanted near the blood vessels and around the tumor border followed by bypass palliative procedure (cholangio-jejunostomy and/or gastrojejunostomy) in 29 patients.
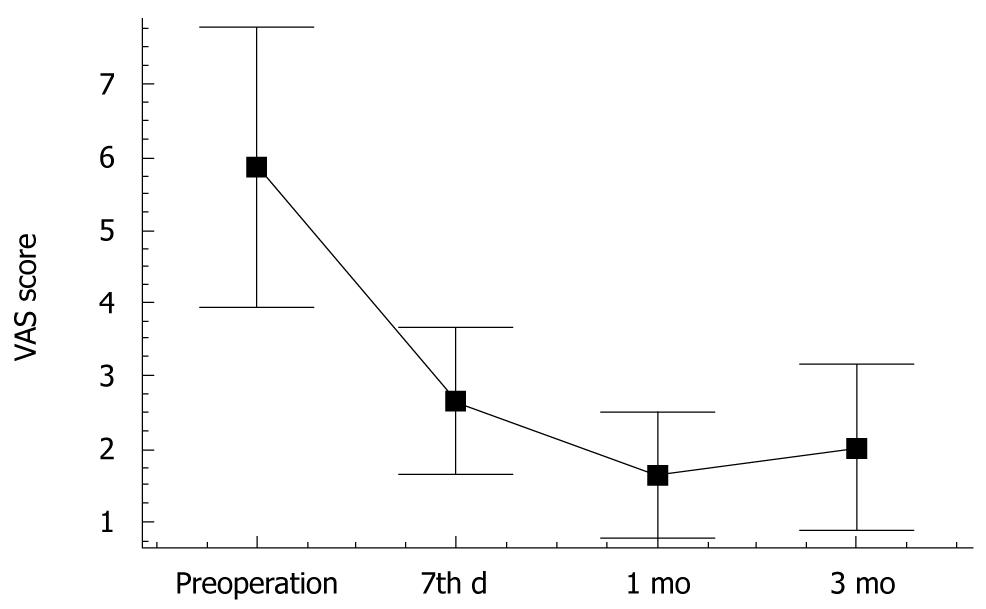
RESULTS: The serum CA 19-9 level was decreased from 512 ± 86 U/mL before operation to 176 ± 64 U/mL, 108 ± 42 U/mL and 114 ± 48 U/mL, respectively, 1, 3 and 6 mo after operation (P < 0.05). The pain score on day 7 after operation, 1 and 3 mo after combined therapy was decreased from 5.86 ± 1.92 before operation to 2.65 ± 1.04, 1.65 ± 0.88 and 2.03 ± 1.16, respectively, after operation (P < 0.05). The rate of complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD) in 32 patients was 21.8% (7/32), 56.3% (18/32), 15.6% (5/32) and 6.3% (2/32), respectively, 6 mo after operation, with a median overall survival time of 17. 5 mo. The median survival time of patients at stage III was longer than that of those at stage IV (19 mo vs 10 mo, P = 0.0026). The median survival time of patients who received and did not receive chemotherapy after operation was 20 mo and 16 mo, respectively (P = 0.0176). Of the 32 patients, 3 (10.6%) experienced postoperative complications including transient biliary leaks in 2 patients and acute pancreatitis in 1 patient. All the patients recovered well after conservative support treatment.
CONCLUSION: Intraoperative RFA combined with 125iodine seed implantation is a feasible and safe procedure for unresectable pancreatic cancer with acceptable minor complications, and can prolong the survival time of patients, especially those at stage III.
- Citation: Zou YP, Li WM, Zheng F, Li FC, Huang H, Du JD, Liu HR. Intraoperative radiofrequency ablation combined with 125iodine seed implantation for unresectable pancreatic cancer. World J Gastroenterol 2010; 16(40): 5104-5110
- URL: https://www.wjgnet.com/1007-9327/full/v16/i40/5104.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i40.5104
The prognosis of patients with pancreatic adenocarcinoma is dismal due to its delayed diagnosis and aggressiveness. Pancreatic adenocarcinoma, characterized by a late presentation, is one of the most lethal human cancers and currently the fifth and sixth most common causes of cancer-related death in men and women, respectively[1]. Only 10% of the tumors are confined to the pancreas at the time of presentation, 30%-40% are locally advanced and 50% have distant metastases. Surgical resection is the only potentially curative procedure for pancreatic cancer. However, only 5%-22% are resectable at the time of presentation[2]. The median survival time of such patients is 3-4 mo if they are untreated, and less than 5% of such patients can survive 5 years after treatment[3]. The 5-year survival rate for most patients who undergo resection and adjuvant therapy does not exceed 29%[4]. Some palliative therapeutic modalities have been applied in treatment of unresectable locally advanced pancreatic cancer, such as chemotherapy, chemoradiation therapy, external-beam radiation therapy, intraoperative radiation therapy (IORT), iodine-125 implantation and RFA[2,3,5-7]. However, these modalities have almost no effect on the overall survival rate of such patients, indicating that more effective treatment modalities should be developed for improving their prognosis.
The aim of this study was to evaluate the feasibility, efficacy and safety of intraoperative RFA combined with iodine-125 implantation for unresectable pancreatic cancer.
Thirty-two patients (21 males and 11 females) at the age of 68 years (range 48-90 years) with unresectable locally advanced pancreatic cancer admitted to our hospital from January 2006 to May 2008 were enrolled in this study. The patients presented with variable symptoms including anorexia, nausea/vomiting, fatigue, weight loss, pruritus. Of these patients, 19 had painless obstructive jaundice, and 12 had mild epigastric or back pain with 2 almost addicted to pethidine or morphine because of intolerable pain. Abdominal ultrasound, contrast-enhanced computed tomography, abdominal and pelvis MRI imaging showed pancreatic cancer in all patients. Their mean serum CA 19-9 level was 512 ± 86 U/mL (range 344-1028 U/mL). The tumor, 4-12 cm in diameter, located in pancreatic head of 23 patients and in pancreatic body and tail of 9 patients, was found at operation to be unresectable due to infiltration of adjacent vessels and metastases in peritoneum or distant organs. Diagnosis of pancreatic cancer was thus made through intraoperative biopsy with hematoxylin and eosin staining. The tumor was classified as stage III in 24 patients and stage IV in 8 patients according to the TNM staging system (UICC, 2002)[8]. The patients were treated with intraoperative FRA combined with 125iodine seed implantation followed by a bypass palliative procedure (cholangio-jejunostomy and/or gastrojejunostomy) for 25 patients. All patients and their relatives were informed about the treatment options. All possible and potential complications were explained in detail to the patients and their relatives before they gave their written consent. The study was approved by the Hospital Ethical Committee.
A RITA 1500X RF generator (RITA Medical Systems Inc., Mountain View, CA, USA), which generates 100-150 W of power, and a UniBlate™ electrode (AngioDynamics Inc., Queensbury, NY, USA) were used. The UniBlate™ electrode, consisting of a 17-gauge insulated cannula, 15 cm in length with a 1-3 cm exposure length for tumor destruction, is a unique new concept in RFA electrode design that provides linearly scalable ablations from 1 to 3 cm in length and 1 to 2.5 cm in diameter. A built-in thermocouple provides full temperature feedback and RF power control as well as a cool down cycle and track ablation capabilities. Two-four ablations were performed for each pancreatic mass depending on its size. A RFA needle was placed, which was confirmed by direct vision and intraoperative ultrasound to decrease the potential injury of surrounding vital structures (duodenum and vessels). The target temperature controlled with a thermosensor at tip of the needle was 90-100°C. Each application of RFA energy lasted for 12 min to gain an about 3 cm × 2 cm ablation zone. Coagulation necrosis was confirmed by intraoperative ultrasound after the procedure. Each needle tract was packed with thrombin-coated Gelfoam to prevent possible pancreatic leakages. A drainage tube was left in the ablated tumor.
Type 6711 iodine-125 sealed seed sources were provided by HTA Co., Ltd (Beijing, China). The core source used is silver containing Na125I and the package used is a titanium alloy tube sealed with laser. Each seed source is 4.5 ± 0.3 mm in length and 0.8 ± 0.03 mm in diameter. The half-life of radioactivity of each seed is 59.43 d. The mean photon energy of each seed is 27.4-35.5 KeV, with a human tissue penetration distance of 1.6 cm, an initial dose rate of 7 cGy/h and a mean radioactivity of 0.694 ± 0.021 mCi (25.6 MBq). After RFA, iodine-125 was implantated. The seeds were implanted at the site of tumor near blood vessels and around the tumor border, with each seed at a distance of 1.0 cm. The number of implanted seeds depended on the tumor size. Thirty-two patients were treated with a median number of 18 seeds (range 16-26).
Of the 23 patients, 15 with lesions in pancreatic head underwent common bile duct-jejunostomy and 8 received both cholangio-jejunostomy and gastrojejunostomy. Of the 9 patients with lesions in pancreatic body and tail, 6 underwent gastrojejunostomy. The main criterion for cholangio-jejunostomy and/or gastrojejunostomy was anticipated tumor in pancreatic head with or without obstruction in bile duct and/or in duodenum or tumor invading in the retroperitoneal ligament of Treitz.
After operation, all patients were observed in wards for 10-14 d. The patients were instructed to stop eating for 4-6 d. All patients received prophylactic intravenous antibiotics for 5-7 d. An analogue of sandostatin LAR (Novartis) was intravenously infused for 5-7 d. Serum amylase and lipase levels were measured on days 1, 3, and 7, respectively, after operation. Drain output was also closely monitored for any significant amount of fluid and sent for fluid amylase estimation to rule out pancreatic leak or ascites formation.
Chemotherapy with gemcitabine (700 mg/m2)[9] was conducted for patients 2 wk after surgery. Gemcitabine was given intravenously for over 30 min on days 1, 8 and 15, respectively, after operation. This therapy was repeated every 4 wk for 6 cycles if tolerated.
All patients were followed up for 6-33 mo (mean 18.2 mo). Follow-up CT scanning was performed 4 wk and then every 3-6 mo after operation to assess the effectiveness of treatment and monitor disease progression in all patients. Laboratory tests, such as a complete blood cell count, liver function and serum CA 19-9 level, were also repeated. Tumor diameter and general condition of the patients were recorded during follow-up. The pain was scored using a 10-point visual analog scale (VAS)[10,11] as 0 (none), 1-3 (mild), 4-7 (moderate), and 8-10 (severe). The effect of treatment on pain control was assessed by collecting pain score 7 d, 1 and 3 mo, respectively, after operation. The response evaluation criteria for solid tumor (RECIST)[12] were adopted in assessment of change in tumor burden. Complete response (CR) was defined as complete disappearance of all lesions lasting for at least 4 wk. Partial response (PR) referred to the situation where the sum of maximum diameter of all lesions was decreased by more than 30%. Stable disease (SD) was defined as the sum of maximum diameter of lesions was decreased by less than 30%. Progressive disease (PD) defined as at least 20% increase in the sum of maximum diameter of lesions, or the appearance of one or more new lesions.
Statistical analysis was performed using SPSS version17 software (SPSS, Chicago, USA). Pain score of patients and serum CA19-9 level before and after treatment were compared using paired t-test or nonparametric methods. Survival time was calculated using the Kaplan-Meier method[13] and difference was assessed with the log-rank test. P < 0.05 was considered statistically significant.
No major procedure-related death occurred. Of the 32 patients, 3 (10.6%) experienced postoperative complications, 2 had surgical anastomosis-associated transient biliary leak which was treated nonoperatively, 1 had RFA-related acute pancreatitis which was treated with somatostatin infusion for 2 wk. The patients recovered well. None required any form of surgical or radiological intervention for minor complications. All patients were advised to receive postoperative adjuvant chemotherapy. However, 6 patients refused any further treatment. Of the 32 patients, 15 completed the full course of chemotherapy and 11 did not because of severe toxicity after one cycle.
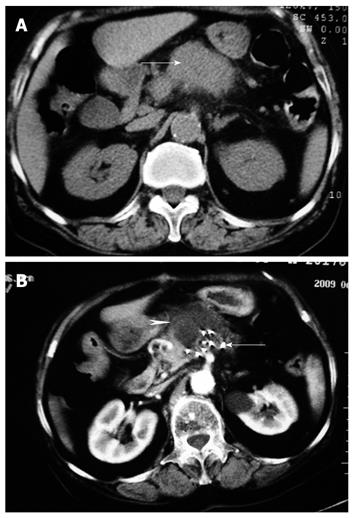
The serum CA 19-9 level was decreased from 512 ± 86 U/mL before operation to 176 ± 64 U/mL, 108 ±42 U/mL and 114 ± 48 U/mL, respectively, 1, 3 and 6 mo after operation (P < 0.05). The mean pain score for the 26 patients with mild epigastric or back pain before and after operation is shown in Figure 1. The pain score was decreased from 5.86 ± 1.92 before operation to 2.65 ± 1.04, 1.65 ± 0.88 and 2.03 ± 1.16, respectively, 7 d, 1 and 3 mo after combined therapy (P < 0.05). CT showed partial necrosis after therapy in all patients. Most patients showed varying degrees of tumor necrosis 3 mo after operation (Figure 2). The rate of CR, PR, SD and PD for 32 patients was 21.8% (7/32), 56.3% (18/32), 15.6% (5/32) and 6.3% (2/32), respectively, 6 mo after operation. No significant difference was observed in tumor diameter of patients with SD before and 3 mo after operation. Two or more new lesions were found in 2 patients with PD after operation.
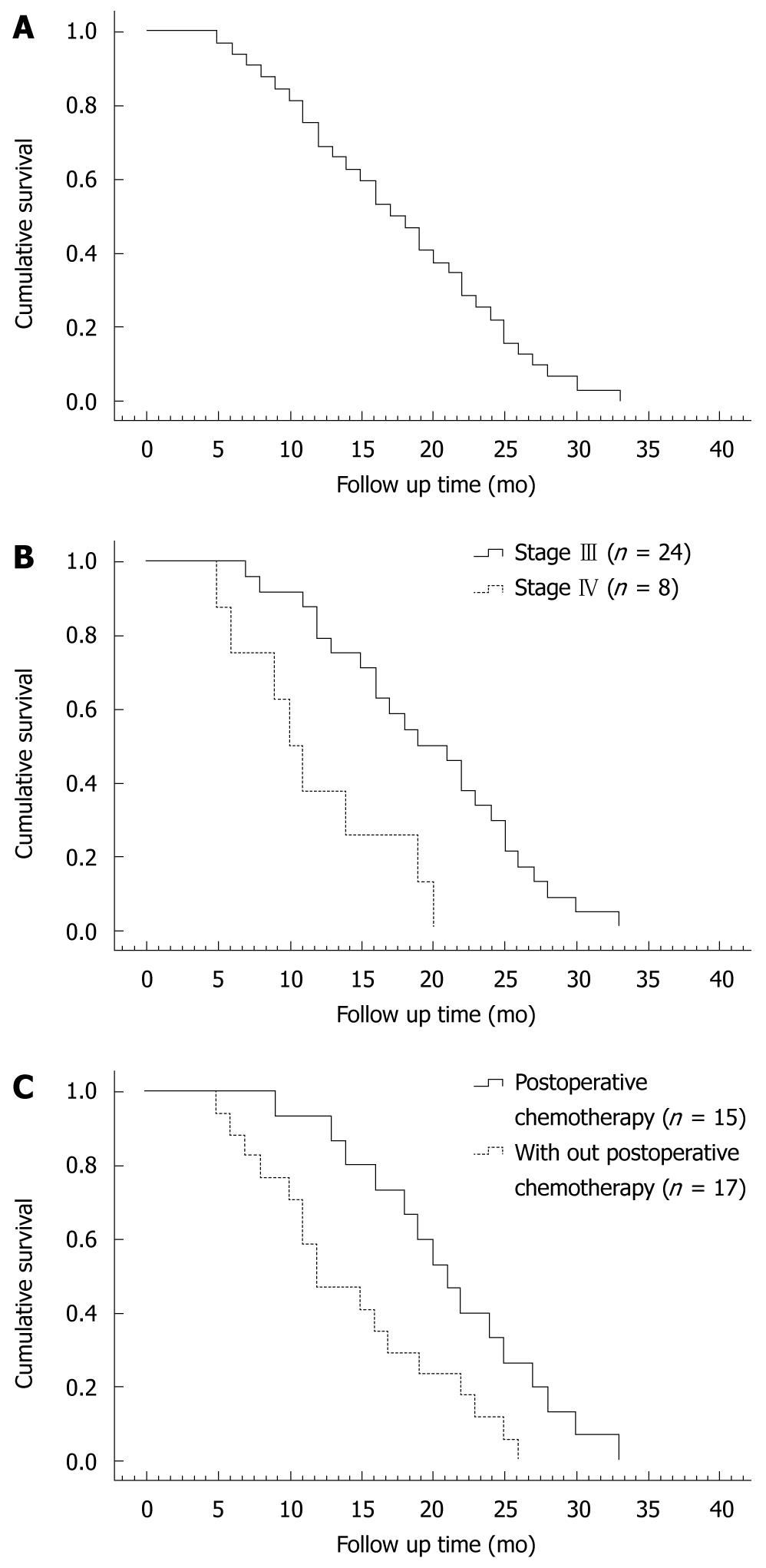
The survival time of all patients was calculated using the Kaplan-Meier method (Figure 3A). The mean and median survival time was 17.6 mo (95% CI: 15-20) and 17.5 mo (95% CI: 12-20), respectively. The overall 12 and 24 mo-survival rate was 65.6% and 21.9%, respectively. The maximun survival time was 33 mo in a patient who was still alive without evidence of disease progression at the time when we wrote this paper. The mean and median survival time of patients at stages III and IV was 19 mo (95% CI: 16-22) and 19 mo (95% CI: 14-23), and 11 mo (95% CI: 7-15) and 10 mo (95% CI: 7-12), respectively. The 12 mo and 24 mo-survival rate was 75% and 33.3% for patients at stage III, and 37.5% and 0% for patients at stage IV. Log-rank test showed that the survival rate for patients at stages III and IV was significantly different (P = 0.0026, Figure 3B). The mean and median survival time of patients who received and did not receive chemotherapy after operation (n = 15) was 19 mo (95% CI: 15-23) and 20 mo (95% CI: 13-26), and 16 mo (95% CI: 13-19) and 16 mo (95% CI: 9-22), respectively. The 12 and 24 mo-survival rate was 80% and 40% for patients who received chemotherapy after operation, and 47.1% and 11.8% for those who did not receive chemotherapy after operation. Log-rank test showed a significant difference in survival rates for patients who received or did not receive chemotherapy after operation (P = 0.0176, Figure 3C).
Pancreatic cancer is difficult to diagnose at its early stage and would be unresectable in most patients when they present with symptoms. Traditional therapeutic options for patients with advanced unresectable pancreatic cancer include chemotherapy, combined chemoradiation, external beam irradiation, IORT and radioactive seed implantation. Palliative chemotherapy with gemcitabine can improve the outcome of patients with advanced pancreatic cancer. It was reported that the median survival time of patients is only about 6 mo with a 12-mo survival rate of less than 20% after chemotherapy with gemcitabine although it is superior to bolus 5-Fu[14,15]. The effect of combined gemcitabine and bolus IV 5-FU on advanced pancreatic cancer is similar to that of gemcitabine monotherapy. However, it has been shown that the 1-year survival rate of patients with advanced pancreatic cancer is 23% after combined erlotinib and gemcitabine therapy and is 17% after gemcitabine monotherapy[15]. Chemotherapy with or without radiotherapy can improve the symptoms and quality of life of patients, but cannot prolong their survival time. Selection of patients, side effects and complications of chemotherapy and radiotherapy should also be considered[16]. IORT and conformal external-beam radiation therapy plus protracted 5-FU infusion for advanced pancreatic cancer have been evaluated in a phase II study[17], showing that combined therapy could not prolong the survival time of such patients, the median survival time of all enrolled patients, those without and with metastasis was 7.8, 12.9, and 5.8, respectively. It was reported that I-125 seed implantation controls growth of the tumor but increases perioperative morbidity[18,19].
RFA is a relatively new procedure for liver and lung tumors[20,21] and other solid tumors such as small breast cancer, renal tumor, cancers of adrenal gland, spleen, prostate, bone and brain[22-28]. However, RFA has not been widely performed as a treatment procedure for pancreatic tumors because of the fragile pancreatic parenchyma and the proximity to some important structures such as duodenum, common bile duct and vessels. The technical feasibility and effect of RFA have been studied in normal porcine pancreatic tissues with encouraging results because discrete zones of coagulation necrosis can be noticed with no major complications[29]. In 2000, Matsui et al[30] first reported 20 patients with stage IV pancreatic adenocarcinoma treated with RFA. Complications were observed only in 2 patients (a cyst and an abscess formation). No significant difference was found in the prognosis of these patients compared with those at the same stage who did not receive RFA, indicating that RFA is relatively safe and can be used in treatment of unresectable tumors without metastasis or benign pancreatic tumors. Since then, some reports on RFA for pancreatic cancer have been published[7,31-33]. However, the number of patients who were treated with RFA was small in most reports. It is difficult to comment on the improvement in survival. RFA for pancreatic tumors has also various complications such as acute pancreatitis, pancreatic fistula, and pancreatic ascites. It was more recently reported that the effect of RFA and palliative therapy on advanced pancreatic cancer is similar and RFA can prolong the survival time of patients with stage III unresectable pancreatic cancer[34].
In the present study, intraoperative FRA in combination with 125iodine seed implantation was performed in order to evaluate its feasibility, efficacy and safety in patients with unresectable pancreatic cancer.
In our series, no major complications occurred except for RFA-associated acute pancreatitis. In our experience, it is important to adequately expose the tumor before RFA by liberal kocherization and/or opening the mesocolon depending upon its location. Under intraoperative ultrasound guidance, the electrode can be accurately placed into the tumor. The ablation should be restricted within the tumor to avoid damage to normal pancreatic tissue, surrounding organs and nearby large blood vessels. To minimize the residual tumor near the blood vessels, 125iodine seed was implanted into duodenum and normal pancreatic tissue. Distal common bile duct injury caused by ablation was taken into consideration in patients with tumors in pancreatic head. A cholangio-jejunostomy was performed to circumvent this effect after RFA. Somatostatin analogues were used after operation to reduce possible complications such as acute pancreatitis, pancreatic fistula. Our results demonstrate that RFA combined with 125iodine seed implantation for unresectable pancreatic tumors is feasible and safe with acceptable minor complications.
Better tumor responses, significantly decreased tumor marker levels and pain score, were observed during follow-up. The pain was relieved in all patients, and 3 patients who were almost addicted to pethidine or morphine for intolerable pain stopped any related medication after combined treatment. Objective local cytoreduction was confirmed in tumor of all patients with contrast-enhanced CT during follow-up. The rate of CR, PR, SD and PD was 21.8%, 56.3%, 15.6% and 6.3%, respectively, 6 mo after operation. The median and maximun survival time of 32 patients was 17.5 and 33 mo in a patient who was still alive at the time when we wrote this paper. The 12 and 24 mo-survival rate was 75% and 33.3% for patients at stage III, and 37.5% and 0% for patients at stage IV, indicating that chemotherapy after operation can improve the survival rate of patients with pancreatic cancer[9]. In this study, the median survival time of patients who received and did not receive chemotherapy after operation was 20 and 16 mo, respectively, suggesting that systemic chemotherapy after operation can prolong their survival time. In this study, some patients did not receive chemotherapy due to their older age and organ dysfunction as well as toxicity of the therapy. Thus, further study is needed to verify the effect of postoperative chemotherapy on unresectable pancreatic cancer after intraoperative RFA combined with 125iodine seed implantation in.
In conclusion, RFA combined with 125iodine seed implantation is a feasible and safe procedure for unresectable pancreatic cancer. However, a greater number of patients and long-term follow-up are needed to confirm its effect on the survival time and quality of life of such patients.
The prognosis of pancreatic cancer is dismal due to its late diagnosis and aggressiveness. Surgical resection is the only potentially curative procedure for pancreatic cancer. However, only 5%-22% are resectable and less than 5% of patients can survive for 5 years after operation. The 5-year survival rate for most patients does not exceed 29% after resection and adjuvant therapy. Some palliative therapeutic modalities can be used in treatment of unresectable locally advanced pancreatic cancer, such as chemotherapy, chemoradiation, external-beam radiation therapy, intraoperative radiation therapy, iodine-125 implantation and radiofrequency ablation (RFA). However, they cannot improve the overall survival rate of such patients, thus more effective treatment modalities should be developed.
RFA is commonly used in treatment of liver and lung tumors with encouraging results but not in treatment of pancreatic tumor because of the fragile pancreatic parenchyma and the proximity to some important structures. A few reports are available on RFA in treatment of pancreatic cancer but the number of patients is small in most reports. It is difficult to comment on its feasibility and safety. Moreover, no report is available on 125iodine seed implantation as a complementary therapy for pancreatic cancer after RFA.
This is the first study on RFA combined with 125iodine seed implantation for pancreatic cancer, which has a complementary effect on pancreatic cancer with different mechanisms of action.
Surgical resection is the only potentially curative procedure for pancreatic cancer, but only 5%-22% are resectable at the time of presentation, 30%-40% are locally advanced and 50% have distant metastases. RFA combined with 125iodine seed implantation is a feasible and safe procedure for unresectable pancreatic cancer.
Pancreatic adenocarcinoma accounts for 90% of all pancreatic cancers with 80% occurring in pancreatic head. Carcinoma in pancreatic body and tail is much less common. Microscopically, these tumors may vary from well-differentiated to undifferentiated tumors. Approximately two thirds of all pancreatic cancer patients will have metastasis at the time of diagnosis, and the tumor will become unresectable in the majority of the remaining patients. Ultrasound, computed tomography and magnetic resonance imaging are the currently used methods in diagnosis and staging of pancreatic cancer.
The authors described a series of pancreatic cancer patients with unresectable cancer who received intraoperative RFA along with i-125 seed implantation. The data are fairly compelling and the article is well written.
Peer reviewer: Oscar Joe Hines, MD, FACS, Professor, Director, Surgery Residency Program, Department of Surgery, UCLA School of Medicine, 10833 Le Conte Ave, Los Angeles, CA 90095-6904, United States
S-Editor Wang YR L-Editor Wang XL E-Editor Ma WH
| 1. | Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219-230. |
| 2. | Singh SM, Longmire WP Jr, Reber HA. Surgical palliation for pancreatic cancer. The UCLA experience. Ann Surg. 1990;212:132-139. |
| 4. | Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. |
| 5. | Russo S, Butler J, Ove R, Blackstock AW. Locally advanced pancreatic cancer: a review. Semin Oncol. 2007;34:327-334. |
| 6. | Lygidakis NJ, Jain S, Sacchi M, Vrachnos P. Adenocarcinoma of the pancreas--past, present and future. Hepatogastroenterology. 2005;52:1281-1292. |
| 7. | Siriwardena AK. Radiofrequency ablation for locally advanced cancer of the pancreas. JOP. 2006;7:1-4. |
| 8. | Sobin LH, Wittekind C, editors . UICC-TNM classification of malignant tumours. 6th ed. New York: Wiley-Liss 2002; . |
| 9. | Tani M, Kawai M, Terasawa H, Ina S, Hirono S, Uchiyama K, Yamaue H. Does postoperative chemotherapy have a survival benefit for patients with pancreatic cancer? J Surg Oncol. 2006;93:485-490. |
| 11. | Pietrabissa A, Vistoli F, Carobbi A, Boggi U, Bisà M, Mosca F. Thoracoscopic splanchnicectomy for pain relief in unresectable pancreatic cancer. Arch Surg. 2000;135:332-335. |
| 12. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 13. | Lee CI, Yan X, Shi NZ. Nonparametric estimation of bounded survival functions with censored observations. Lifetime Data Anal. 1999;5:81-90. |
| 14. | Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-5518. |
| 15. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. |
| 16. | Cohen SJ, Dobelbower R Jr, Lipsitz S, Catalano PJ, Sischy B, Smith TJ, Haller DG. A randomized phase III study of radiotherapy alone or with 5-fluorouracil and mitomycin-C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. Int J Radiat Oncol Biol Phys. 2005;62:1345-1350. |
| 17. | Furuse J, Kinoshita T, Kawashima M, Ishii H, Nagase M, Konishi M, Nakagohri T, Inoue K, Ogino T, Ikeda H. Intraoperative and conformal external-beam radiation therapy with protracted 5-fluorouracil infusion in patients with locally advanced pancreatic carcinoma. Cancer. 2003;97:1346-1352. |
| 18. | Peretz T, Nori D, Hilaris B, Manolatos S, Linares L, Harrison L, Anderson LL, Fuks Z, Brennan MF. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys. 1989;17:931-935. |
| 19. | Mohiuddin M, Rosato F, Barbot D, Schuricht A, Biermann W, Cantor R. Long-term results of combined modality treatment with I-125 implantation for carcinoma of the pancreas. Int J Radiat Oncol Biol Phys. 1992;23:305-311. |
| 20. | Lencioni R, Crocetti L. A critical appraisal of the literature on local ablative therapies for hepatocellular carcinoma. Clin Liver Dis. 2005;9:301-314, viii. |
| 21. | Simon CJ, Dupuy DE. Current role of image-guided ablative therapies in lung cancer. Expert Rev Anticancer Ther. 2005;5:657-666. |
| 22. | Singletary ES. Feasibility of radiofrequency ablation for primary breast cancer. Breast Cancer. 2003;10:4-9. |
| 23. | Boss A, Clasen S, Kuczyk M, Anastasiadis A, Schmidt D, Graf H, Schick F, Claussen CD, Pereira PL. Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol. 2005;40:583-590. |
| 24. | Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97:554-560. |
| 25. | Milićević M, Bulajić P, Zuvela M, Raznatović Z, Obradović V, Lekić N, Palibrk I, Basarić D. [Elective resection of the spleen--overview of resection technics and description of a new technic based on radiofrequency coagulation and dessication]. Acta Chir Iugosl. 2002;49:19-24. |
| 26. | Shariat SF, Raptidis G, Masatoschi M, Bergamaschi F, Slawin KM. Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-recurrent prostate cancer. Prostate. 2005;65:260-267. |
| 27. | Martel J, Bueno A, Ortiz E. Percutaneous radiofrequency treatment of osteoid osteoma using cool-tip electrodes. Eur J Radiol. 2005;56:403-408. |
| 28. | Gananadha S, Wulf S, Morris DL. Safety and efficacy of radiofrequency ablation of brain: a potentially minimally invasive treatment for brain tumours. Minim Invasive Neurosurg. 2004;47:325-328. |
| 29. | Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc. 1999;50:392-401. |
| 30. | Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14-20. |
| 31. | Elias D, Baton O, Sideris L, Lasser P, Pocard M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30:85-87. |
| 32. | Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F, Symeonides P. Radiofrequency ablation in pancreatic cancer. HPB (Oxford). 2006;8:61-64. |
| 33. | Varshney S, Sewkani A, Sharma S, Kapoor S, Naik S, Sharma A, Patel K. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74-78. |
| 34. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55-60. |