Published online Oct 14, 2010. doi: 10.3748/wjg.v16.i38.4865
Revised: June 18, 2010
Accepted: June 25, 2010
Published online: October 14, 2010
AIM: To determine the effect of pituitary adenylate cyclase-activating polypeptide (PACAP) on left gastric artery (LGA) flow and to unveil the structural or functional important sites that may be critical for discrimination of different receptor subtypes.
METHODS: Peptides, including PACAP-27, PACAP-38, amino acid substituted PACAP-27 and C-terminus truncated analogues PACAP (27-38), were synthesized by a simultaneous multiple solid-phase peptide synthesizer. Flow probes of an ultrasound transit-time blood flowmeter were placed around the LGA of beagle dogs. When peptides were infused intravenously, the blood flow was measured.
RESULTS: [Ala4, Val5]-PACAP-27 caused a concentration-dependent vasodepressor action which was similar to that caused by PACAP-27. The LGA blood flow response to [Ala4, Val5]-PACAP-27 was significantly higher than that to PACAP-27, which was similar to that to vasoactive intestinal polypeptide (VIP) at the same dose. [Ala6]-PACAP-27 did not increase the peak LGA flow. [Gly8]-PACAP-27 showed a similar activity to VIP. [Asn24, Ser25, Ile26]-PACAP-27 did not change the activity of peptides at all doses.
CONCLUSION: NH2 terminus is more important to biological activity of peptides and specific receptor recognition than COOH-terminus.
- Citation: Wei MX, Hu P, Wang P, Naruse S, Nokihara K, Wray V, Ozaki T. Possible key residues that determine left gastric artery blood flow response to PACAP in dogs. World J Gastroenterol 2010; 16(38): 4865-4870
- URL: https://www.wjgnet.com/1007-9327/full/v16/i38/4865.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i38.4865
Pituitary adenylate cyclase-activating polypeptide (PACAP) was originally isolated because of its similarity with vasoactive intestinal polypeptide (VIP). PACAP stimulates adenylyl cyclase activity in anterior pituitary cells of rats. So far, two forms of PACAP (PACAP-38 and PACAP-27) have been described. Considering that the 27-residue polypeptide (PACAP-27) corresponds to the N-terminus 27-amino acid sequence of PACAP-38 and shows a 68% identity with VIP[1] (Table 1), PACAP showing a 68% of identity with VIP has been considered a member of VIP-glucagon-growth hormone releasing factor-secretin superfamily.
| Peptides and their analogues | Amino acid sequence |
| PACAP-27 | HSDG I, FTD S Y, SRYRK, QMAVK, KYLAA, VL-NH2 |
| VIP | HSDAV, FTDNY, TRLRK, QMAVK, KYLN S, ILN-NH2 |
| [Ala4]-PACAP-27 | HSDA I, FTDSY, SRYRK, QMAVK, KYLAA, VL-NH2 |
| [Val5]-PACAP-27 | HSDGV, FTDSY, SRYRK, QMAVK, KYLAA, VL-NH2 |
| [Ala4, Val5]-PACAP-27 | HSDAV, FTDSY, SRYRK, QMAVK, KYLAA, VL-NH2 |
| [Ala6]-PACAP-27 | HSDG I, ATDSY, SRYRK, QMAVK, KYLAA, VL-NH2 |
| [Gly8]-PACAP-27 | HSDG I, FTGSY, SRYRK, QMAVK, KYLAA, VL-NH2 |
| [Asn24, Ser25, IIe26]-PACAP-27 | HSDG I, FTDSY, SRYRK, QMAVK, KYLN S, IL-NH2 |
| PACAP 1-33 | HSDGI, FTDSY, SRYRK, QMAVK, KYLAA, VLGKR YKQ |
| PACAP 1-34 | HSDGI, FTDSY, SRYRK, QMAVK, KYLAA, VLGKR YKQR |
| PACAP 1-35 | HSDGI, FTDSY, SRYRK, QMAVK, KYLAA, VLGKR YKQRV |
| PACAP 1-36 | HSDGI, FTDSY, SRYRK, QMAVK, KYLAA, VLGKR YKQRV, K |
| PACAP 1-37 | HSDGI, FTDSY, SRYRK, QMAVK, KYLAA, VLGKR YKQRV, KN |
| PACAP 1-38 | HSDGI, FTDSY, SRYRK, QMAVK, KYLAA, VLGKR YKQRV, KNK-NH2 |
Both PACAP and VIP exhibit an ability to stimulate adenylate cyclase in pituitary cells and in neural, pancreatic, and liver membrane[2]. However, PACAP is much more potent than VIP in pituitary cells and liver membrane. In the cardiovascular system, PACAP acts as a vasodepressor like VIP. Similarity would demand the validation of effective dose, the duration of response, and the latent period, etc.[3].
PACAP and VIP are co-expressed in nerve fibers and neurons in ganglia of guinea pig gallbladder[4]. Our previous study showed that their actions on the gallbladder are opposite, namely VIP relaxes the gallbladder whereas PACAP induces its contraction[5].
It has been shown that PACAP-38 and PACAP-27 are potent VIP-like vasodilators of the femoral arterial bed of dogs, while PACAP-38 differs from PACAP-27 and VIP in its prolonged effects on femoral blood flow[6]. Small arteries and arterioles in the gastrointestinal tract and pancreas are innervated by VIP- or PACAP-positive fibers. Both peptides are very potent vasodilators of gastrointestinal blood vessels in conscious dogs. These findings suggest that PACAP may participate in regulation of the gastrointestinal circulation. However, its effect on gastric blood flow is unknown[7,8].
Three receptor subtypes that mediate PACAP and VIP have been identified[9], including PACAP-specific receptor (PAC1) with a high affinity for PACAP and a much lower affinity for VIP, and PACAP/VIP receptors (VPAC1 and VPAC2) with a similar affinity for PACAP and VIP. All of them belong to the group of 7 transmembrane G protein-coupled receptors. PACAP and VIP act primarily as an inhibitory transmitter on most gastrointestinal and vascular smooth muscle cells, suggesting that PACAP may participate in regulation of the gastrointestinal circulation.
In the present investigation, gastrointestinal blood flow response to PACAP38, PACAP27 and their analogues with amino acid substitutions of corresponding VIP residues as well as substituted analogues at putative functional/structural important sites and C-terminal truncated analogues were studied in conscious beagle dogs to unveil the dose-response and structure-response relationships of these peptides in the left gastric artery (LGA).
On the basis of sequence homology of PACAP and VIP as well as the structural results by NMR, positions 4, 5, 6, 8, 24, 25, and 26 of PACAP-27 and VIP were selected as substitution sites in the present study. Peptides, including PACAP-27, PACAP-38, amino acid substituted PACAP-27 and C-terminal truncated analogues PACAP 27-38 were synthesized by a simultaneous multiple solid-phase peptide synthesizer (PSSM-8; Shimadzu, Kyoto, Japan), using the 9-fluorenylmethoxycarbonyl strategy. After cleavage, all peptides were purified with the SynProPep System[10] and characterized by sequencing, amino acid analysis, and fast atom bombardment mass spectrometry to confirm the high homogeneity with the desired structure. VIP was purchased from Peptide Institute (Osaka, Japan).
The study was approved by the Ethical Committee of the National Institute for Physiological Sciences (Okazaki, Japan) on Animal Use for Experiment. Five beagle dogs of either sex weighing 8-14 kg were used. After fasted for 18 h, the animals were anesthetized with thiamylal (20 mg/kg) and atropine (0.5 mg) and maintained with N2O-O2-ether throughout the procedure. Flow probes of an ultrasound transit-time blood flowmeter (Transonic Systems, New York) were placed around the LGA. Connectors of the probes were pulled out of the abdominal cavity through a subcutaneous tunnel and fixed at the chest. After a 4-wk recovery period, the animals were restrained in Pavlov stands and experiments were conducted in the conscious state. PACAP27, PACAP38, VIP, and PACAP-27 analogues (2.5, 5, 10, 25, 50 and 100 pmol/kg in 1 min) were infused intravenously. The blood flow was measured. Blood flow response to oral ingestion of 300 mL milk served as a control.
All data were presented as mean ± SE. Statistical analysis was carried out by one-way analysis of variance using least-significant difference when equal variances or Tamhane’s T2 was assumed or when equal variances were not assumed for multiple comparisons. Independent sample t test was used for comparison between two independent data. P < 0.05 was considered statistically significant with n = the number of animals.
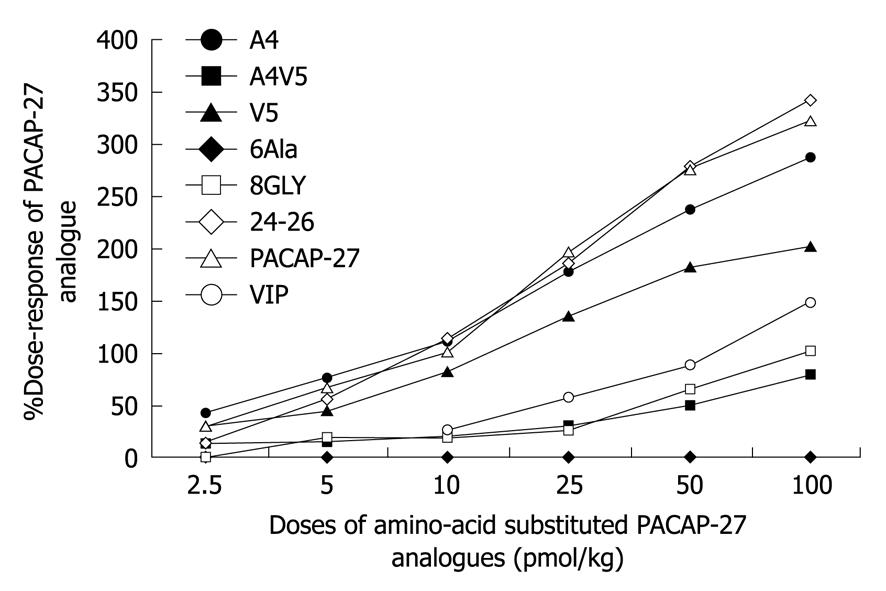
Different concentrations of PACAP-27, PACAP-38 and VIP were employed. The LGA blood flow responses to PACAP-27 at the doses of 2.5, 5, 10, 25, 50 and 100 pmol/kg were 30.07 ± 8.52, 66.62 ± 16.04, 100, 195.29 ± 35.07, 276.45 ± 47.33, 322.76 ± 60.36, respectively, while those to PACAP-38 at the same doses were 37.35 ± 5.11, 91.69 ± 11.15, 137.60 ± 13.81, 186.91 ± 25.66, 214.12 ± 31.42, 229.73 ± 42.11, respectively. The blood flow responses to VIP at the doses of 10, 25, 50 and 100 pmol/kg were 25.56 ± 8.32, 56.88 ± 9.56, 87.41 ± 1.72, 148.60 ± 17.17, respectively (Figure 1).
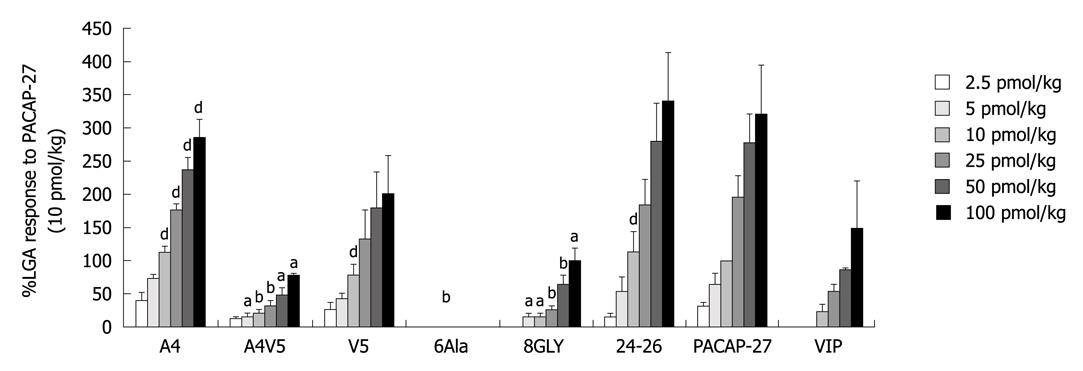
Effect of N-termini 4 and 5 substituted PACAP-27 analogues with corresponding VIP residues: Intravenous infusion of substituted PACAP27 analogues increased the peak LGA blood flow in a dose-dependent manner. [Ala4, Val5]-PACAP-27 caused a concentration- dependent vasodepressor action similar to that caused by PACAP-27. Both showed a comparable activity to PACAP-27 at the doses of 2.5-100 pmol/kg), demonstrating that a single amino-acid residue substitution at position 4 or 5 of PACAP-27 does not significantly change its biological function. Interestingly, analogues with a substitution at positions 4 and 5, [Ala4, Val5]-PACAP-27 (12.68 ± 4.88, 42.18% of PACAP27) showed a similar activity to PACAP-27 (30.07 ± 8.52) at the dose of 2.5 pmol/kg. However, the responses to [Ala4, Val5]-PACAP-27 (14.58 ± 6.73, 20.63 ± 6.08, 29.99 ± 9.77, 48.53 ± 10.79, 79.20 ± 4.66) at the doses of 5, 10, 25, 50 and 100 pmol/kg were significantly lower than those to PACAP27 (21.88%, 20.63%, 15.36%, 17.55% and 24.54%, P < 0.05) and those to PACAP27 at positions 4 and 5 (66.62 ± 16.04, 100, 195.28 ± 35.07, 276.45 ± 47.33, 322.76 ± 60.36), while exhibited a similar activity to VIP (25.56 ± 8.32, 56.88 ± 9.56, 87.41 ± 1.72, 148.60 ± 17.17) at the dose of 10-100 pmol/kg, suggesting that positions 4 and 5 of PACAP-27 are the key NH2-terminal residues of PACAP-27 that discriminate interactions of PACAP with specific receptor subtypes in the LGA (Figure 2).
Effect of N-terminus 6, 8 substituted PACAP-27 analogue: Intravenous infusion of [Ala6]-PACAP-27 did not increase the peak LGA flow, indicating that amino-acid residue replacement at position 6 of PACAP-27 results in loss of its biological function. Phenylalanine, an amino acid residue at position 6 of PACAP -27, was critical for PACAP-27 to exert its action on LGA flow.
The responses to [Gly8]-PACAP-27 were significantly lower at the doses of 5-100 pmol/kg (17.97 ± 4.21, 18.07 ± 4.13, 26.21 ± 7.22, 64.06 ± 15.82, 101.51 ± 17.40) than those to PACAP-27 (26.98%, 18.07%, 13.42%, 23.17%, 31.45%) (P < 0.05). [Gly8]-PACAP-27 at the dose of 10-100 pmol/kg showed a similar activity to VIP. Changes in amino-acid residue at position 8 made the biological function of PACAP-27 less potent, suggesting that position 8 of PACAP-27 plays a key role in conformation of PACAP-27 (Figure 2).
The replacement of C-terminal residues of PACAP-27, [Asn24, Ser25, Ile26]-PACAP-27 (14.75 ± 6.97, 55.43 ± 21.31, 112.66 ± 32.25, 185.23 ± 38.60, 279.30 ± 59.33, 341.83 ± 72.38) did not significantly change the responses at the doses of 2.5-100 pmol/kg, while the responses were 83.21%, 112.66% and 101.03% to PACAP-27 at 5, 10 and 50 pmol/kg, indicating that the three C-terminal residues are not critical for the difference between PACAP-27 and VIP (Figure 2).
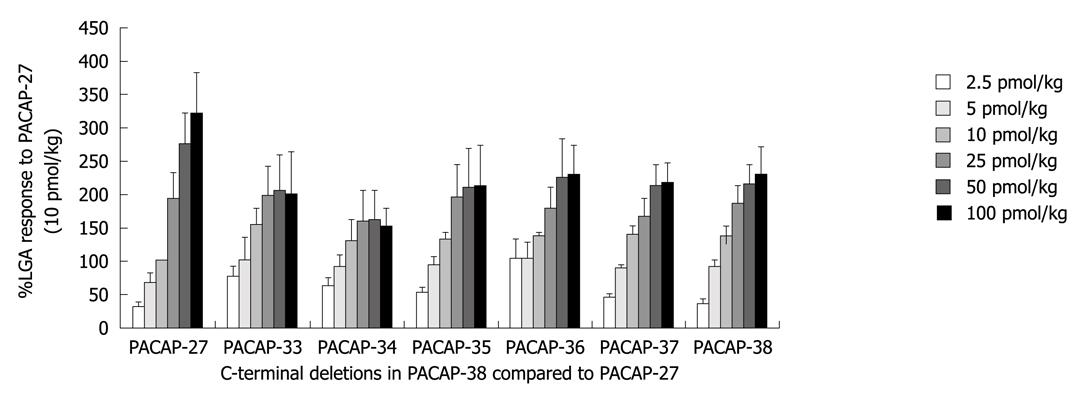
The effects of C-terminal deletion in PACAP-38 on the peak LGA flow were monitored and compared with the response to PACAP-27, showing that almost all C-terminal deletions in PACAP-38 had no significant effect on the peak LGA flow (Figure 3).
It has been shown that PACAP has potent gastrointestinal effects[11]. The present study demonstrated that both PACAP and VIP were potent vasodilators of the left gastric arterial bed in dogs, and PACAP was more potent than VIP.
Similarly, it has been reported that although PACAP-27 and N-terminus 27 amino acids in PACAP-38 show a high homology with VIP[12], PACAP is more potent than VIP in stimulating adenylate cyclase in pituitary cells[13].
Autoradiography can clearly identify two PACAP binding sites: one is PACAP preferring, the other has an identical affinity to VIP and PACAP[14].
The physiological actions of these widely distributed peptides, including PACAP, VIP and their analogues, are produced by activating the three common G-protein coupled receptors (VPAC1, VPAC2 and PAC1) which preferentially stimulate adenylate cyclase and increase intracellular cAMP, although stimulation of other intracellular messengers, including calcium[15] and phospholipase D[16] has also been reported.
The three receptor subtypes have been classified into “VPAC receptors” for VIP and PACAP, and “PAC1 receptors” which are the PACAP-preferring subtype[17]. The VPAC receptors can be further divided into VPAC1R and VPAC2R subtypes, based upon helodermin binding/potency (VPAC2R is helodermin preferring).
The actions of PACAP and VIP on gallbladder are opposite, namely VIP relaxes the gallbladder, whereas PACAP induces its contraction both in vivo and in vitro. The three receptor subtypes that recognize PACAP and VIP as the gallbladder have been found in blood vessels of the gastrointestinal system. Intravenous infusion of PACAP analogues increases the intestinal system blood flow in a dose-dependent manner. The PACAP becomes more potent as the amino chain of PACAP extends.
The aim of this study was to determine the effect of PACAP on LGA flow and to unveil the structural or functional important sites that may be critical for discrimination of different receptor subtypes. PACAP-27 analogues with amino acid substitutions or deletions at selected sites were synthesized.
In the present study, deletion of amino-acid residues from C-terminus of PACAP-38 did not significantly change the effect of PACAP on LGA, which is not consistent with the reported findings in gallbladder[5], demonstrating that the COOH-terminus of PACAP-38 has no key residues for the activity of PACAP-27 in LGA, and that PACAP-27 and PACAP-38 may act on the same receptor subtype.
The C-terminal deletions in PACAP-38 had no significant influence on the peak LGA flow in this study, showing that no particular amino acid residue is responsible for the decreased potency of PACAP-27 and PACAP-38, which is consistent with the prolonged response of PACAP-38 to the femoral blood flow in dogs[18].
The change in single amino-acid residue at position 4 or 5 of the amino chain of PACAP-27 did not significantly change the biological function of PACAP-27. However, substituting the amino-acid residues at positions 4 and 5 with corresponding VIP residues significantly changed the biological function of PACAP-27. The responses of LGA flow to [Ala4, Val5]-PACAP-27 and VIP were similar, demonstrating that positions 4 and 5 are the key NH2-terminal residues of PACAP-27 that distinguish interactions with PAC1 receptors from those with VPAC1 and VPAC2 receptors in the LGA.
In our previous study on VIP and PACAP in guinea pig gallbladder[5], VIP induced relaxation while PACAP27 induced contraction of gallbladder. [Ala4, Val5]-PACAP27 were more potent than PACAP27 (P < 0.01) in stimulating the gallbladder. It has also been identified in a previous study[5] that [Ala6] PACAP-27 has no significant activity and [Gly8] PACAP-27 is significantly (P < 0.05) less potent (25%) than PACAP-27, which are consistent with the findings in the present study, demonstrating that position 4 and 5 are the key residues of PACAP-27 and substitutions at both sites with VIP residues may influence on specific receptor recognition. In this case, positions 4 and 5 substituted PACAP-27 may choose VPAC receptors instead of PAC receptors. Positions 6 and 8 are also important for the effect of PACAP27. It has been shown that a hydrophobic β-coil may form in the N-terminal region and that this structure may be important in receptor- binding affinity[19].
There is evidence that both N- and C-terminal regions are important for the biological activity of peptides and recognition of specific receptors[20,21]. It was reported that replacement of the COOH-terminal of PACAP-27 with VIP has no effect on the relaxation of LGA[22].
In conclusion, NH2 terminus plays an more important role in the recognition of specific receptors than the COOH-terminal. No particular amino acid residue is responsible for the decreased potency of PACAP-27 and PACAP-38. Further study is needed to determine the sites important to the structure and functions of PACAP.
Pituitary adenylate cyclase-activating polypeptide (PACAP) was originally isolated because of its similarity with vasoactive intestinal polypeptide (VIP). PACAP-27 corresponds to the N-terminal 27-amino acid sequence of PACAP-38 and shows a 68% identity with VIP. The effects of PACAP and VIP on the cardiovascular system and gallbladder have extensively studied. The effects of PACAP on excretion and motility of the gastrointestinal tract have also been investigated. The findings suggest that PACAP may participate in regulation of the gastrointestinal circulation. The influence of PACAP on left gastric blood flow was observed in the present study.
Small arteries and arterioles in the gastrointestinal tract and pancreas are innervated by VIP- or PACAP-positive fibers. Their actions on the gallbladder are opposite: VIP relaxes the gallbladder whereas PACAP induces its contraction. Both peptides were found to be very potent vasodilators of the gastrointestinal blood vessels in conscious dogs, suggesting that PACAP may participate in regulation of the gastrointestinal circulation.
Flow probes of an ultrasound transit-time blood flowmeter were placed around the left gastric artery (LGA). Connectors of the probes were pulled out of the abdominal cavity through a subcutaneous tunnel and fixed at the chest. After a recovery period, the animals were restrained in Pavlov stands and the experiments were conducted in the conscious state. This method can also be used in other experiments on gastrointestinal blood flow.
The motility and secretion function of gastrointestinal tract are closely related with blood flow. Based on the mechanisms of motility and secretion, the effects of brain-gut peptides on blood flow help understand the physiology of the digestive system and treatment of digestive diseases. The methods can also be used in other experiments on the effects of peptides on gastrointestinal blood.
In this study, peptides including PACAP-27, PACAP-38, amino acid substituted PACAP-27 and C-terminal truncated analogues PACAP (27-38) were synthesized and blood flow from the LGA of dogs was measured in response to these peptides infused at various concentrations. The results indicate that amino acid substituted PACAP can cause a concentration dependent vasodepressor action similar to that caused by PACAP-27. The study is interesting, but the data should be further clarified.
Peer reviewers: Dr. José Liberato Ferreira Caboclo, Professor, Rua Antônio de Godoy, 4120, São José do Rio Preto, Brazil; Parimal Chowdhury, Professor, Department of Physiology and Biophysics, College of Medicine University of Arkansas for Medical Sciences, 4301 W Markham Street, Little Rock, AR 72205, United States
S- Editor Tian L L- Editor Wang XL E- Editor Zheng XM
| 1. | Läuff JM, Modlin IM, Tang LH. Biological relevance of pituitary adenylate cyclase-activating polypeptide (PACAP) in the gastrointestinal tract. Regul Pept. 1999;84:1-12. |
| 2. | Gourlet P, Woussen-Colle MC, Robberecht P, de Neef P, Cauvin A, Vandermeers-Piret MC, Vandermeers A, Christophe J. Structural requirements for the binding of the pituitary adenylate-cyclase-activating peptide to receptors and adenylate-cyclase activation in pancreatic and neuronal membranes. Eur J Biochem. 1991;195:535-541. |
| 3. | Absood A, Chen D, Wang ZY, Håkanson R. Vascular effects of pituitary adenylate cyclase activating peptide: a comparison with vasoactive intestinal peptide. Regul Pept. 1992;40:323-329. |
| 4. | Mawe GM, Ellis LM. Chemical coding of intrinsic and extrinsic nerves in the guinea pig gallbladder: distributions of PACAP and orphanin FQ. Anat Rec. 2001;262:101-109. |
| 5. | Wei MX, Naruse S, Ozaki T, Hu P, Wray V, Nokihara K. Differences in Action of PACAP-27 and PACAP-38 on Guinea Pig Gallbladder Smooth Muscle Using Synthetic C-terminally Modified PACAP Peptides. Int J Pept Res Ther. 2009;15:227-232. |
| 6. | Naruse S, Suzuki T, Ozaki T, Nokihara K. Vasodilator effect of pituitary adenylate cyclase activating polypeptide (PACAP) on femoral blood flow in dogs. Peptides. 1993;14:505-510. |
| 7. | Sundler F, Ekblad E, Absood A, Håkanson R, Köves K, Arimura A. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46:439-454. |
| 8. | Naruse S, Nakamura T, Wei M, Ando E, Nokihara K, Wray V, Ozaki T, Kitagawa M, Hayakawa T. Effects of PACAP-VIP hybrid peptides on gastric blood flow in conscious dogs. Ann N Y Acad Sci. 1996;805:511-515. |
| 9. | Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269-324. |
| 10. | Nokihara K, Watanabe T, Yamaguchi M, Beck R, Herbst F. Development and applications of a novel ion-exchange HPLC columns for the preparative and analytical separation for proteins and peptides. Peptide chemistry 1991. Osaka: Protein Research Foundation 1992; 309-314. |
| 11. | Nokihara K, Ando E, Naruse S, Wei M, Wray V. Synthesis and structure-activity relationship of VIP-PACAP hybrid peptides. Peptide chemistry 1994. Osaka: Protein Research Foundation 1994; 53-56. |
| 12. | Sherwood NM, Krueckl SL, McRory JE. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev. 2000;21:619-670. |
| 13. | Tatsuno I, Uchida D, Tanaka T, Saeki N, Hirai A, Saito Y, Moro O, Tajima M. Maxadilan specifically interacts with PAC1 receptor, which is a dominant form of PACAP/VIP family receptors in cultured rat cortical neurons. Brain Res. 2001;889:138-148. |
| 14. | Shivers BD, Görcs TJ, Gottschall PE, Arimura A. Two high affinity binding sites for pituitary adenylate cyclase-activating polypeptide have different tissue distributions. Endocrinology. 1991;128:3055-3065. |
| 15. | Dickson L, Aramori I, McCulloch J, Sharkey J, Finlayson K. A systematic comparison of intracellular cyclic AMP and calcium signalling highlights complexities in human VPAC/PAC receptor pharmacology. Neuropharmacology. 2006;51:1086-1098. |
| 16. | McCulloch DA, Lutz EM, Johnson MS, MacKenzie CJ, Mitchell R. Differential activation of phospholipase D by VPAC and PAC1 receptors. Ann N Y Acad Sci. 2000;921:175-185. |
| 17. | Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265-270. |
| 18. | Laburthe M, Couvineau A. Molecular pharmacology and structure of VPAC Receptors for VIP and PACAP. Regul Pept. 2002;108:165-173. |
| 19. | Inooka H, Ohtaki T, Kitahara O, Ikegami T, Endo S, Kitada C, Ogi K, Onda H, Fujino M, Shirakawa M. Conformation of a peptide ligand bound to its G-protein coupled receptor. Nat Struct Biol. 2001;8:161-165. |
| 20. | Onoue S, Waki Y, Nagano Y, Satoh S, Kashimoto K. The neuromodulatory effects of VIP/PACAP on PC-12 cells are associated with their N-terminal structures. Peptides. 2001;22:867-872. |
| 21. | Onoue S, Matsumoto A, Nagano Y, Ohshima K, Ohmori Y, Yamada S, Kimura R, Yajima T, Kashimoto K. Alpha-helical structure in the C-terminus of vasoactive intestinal peptide: functional and structural consequences. Eur J Pharmacol. 2004;485:307-316. |
| 22. | Wei M, Fujiki K, Ando E, Zhang S, Ozaki T, Ishiguro H, Kondo T, Nokihara K, Wray V, Naruse S. Identification of key residues that cause differential gallbladder response to PACAP and VIP in the guinea pig. Am J Physiol Gastrointest Liver Physiol. 2007;292:G76-G83. |