Published online Oct 14, 2010. doi: 10.3748/wjg.v16.i38.4838
Revised: May 31, 2010
Accepted: June 7, 2010
Published online: October 14, 2010
AIM: To investigate the protective effect of erythropoietin (Epo) against ischemia-reperfusion injury (IR/I) following the Pringle maneuver (PM), in comparison with conventional steroid administration in a prospective randomized trial.
METHODS: Patients were randomized by age, sex, diagnosis, and surgical method, and assigned to three groups: (1) A steroid group (STRD, n = 9) who received 100 mg of hydrocortisone before PM, and on postoperative days 1, 2 and 3, followed by tapering until postoperative day 7; (2) An EPO1 group (n = 10) who received 30 000 U of Epo before the PM and at the end of surgery; and (3) An EPO2 group (n = 8) who received 60 000 U of Epo before the PM. Hemoglobin (Hb), hematocrit (Ht), aspartate aminotransferase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), lactate, interleukin-6 (IL-6), and tumor necrosis factor (TNF)-α were measured before and just after (Day 0) surgery, and on postoperative days 1, 3, 7 and 14.
RESULTS: There were no increases in Hb and Ht in the EPO1 and EPO2 groups. AST was significantly lower in EPO1 than in STRD on Day 0 (P = 0.041), and lower in EPO1 than in STRD and EPO2 on Day 1 (P = 0.018). ALT was significantly lower in EPO1 than in STRD and EPO2 on Day 0 (P = 0.020) and Day 1 (P = 0.004). There were no significant inter-group differences in the levels of LDH and lactate. IL-6 was significantly lower in EPO1 than in STRD and EPO2 on Day 0 (P = 0.0036) and Day 1 (P = 0.0451). TNF-α was significantly lower in EPO1 than in STRD and EPO2 on Day 0 (P = 0.0006) and Day 1 (P < 0.0001). Furthermore, hospitalization was significantly shorter in EPO1 and EPO2 than in STRD.
CONCLUSION: Epo has greater potential than steroids to ameliorate IR/I after the PM. Epo at a dose of 30 000 U, administered before PM and just after surgery, yields better results.
- Citation: Kato M, Sawada T, Kita J, Shimoda M, Kubota K. Erythropoietin ameliorates early ischemia-reperfusion injury following the Pringle maneuver. World J Gastroenterol 2010; 16(38): 4838-4845
- URL: https://www.wjgnet.com/1007-9327/full/v16/i38/4838.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i38.4838
The Pringle maneuver (PM) is a standard procedure used worldwide to reduce blood loss during hepatic resection. However, this procedure inevitably results in some degree of ischemia-reperfusion injury (IR/I). To ameliorate IR/I, several procedures have been used, such as steroid administration and ischemic preconditioning[1,2]. Although intravenous administration of steroids is widely used, its rationality has not been established, and major concerns have been raised regarding its possible side effects, such as infection, diabetes mellitus, and disturbance of wound healing[3].
Erythropoietin (Epo) is a hematopoietic peptide that has been used successfully for treatment of anemia in patients with end-stage renal disease. Recently, attention has been focused on the extra-hematopoietic effects of Epo, including an organ-protective effect against IR/I[4-6]. We have already reported the protective effect of Epo in a porcine liver IR/I model[7].
To elucidate and apply the protective effect of Epo against liver IR/I following the PM in a clinical setting, we performed a randomized prospective clinical trial.
The study was performed in accordance with the principles of the Declaration of Helsinki, and was approved by the ethics committee of Dokkyo Medical University Hospital. Because Epo has been used successfully in a clinical setting for more than 15 years, we did not perform a phase I study.
To determine the optimum dose, timing, and frequency of Epo administration, Epo was injected intravenously into groups of 3 patients who received each of the following doses: 6000 U (× 1), 12 000 U (× 1), 18 000 U (× 1), 24 000 U (× 1), 30 000 U (× 1), and 60 000 U (× 1) at 5 min before the PM, or at 30 000 U (× 2) at 5 min before the PM, and at the end of the operation. None of the patients in these groups showed any significant increase in red blood cell count or other side effects. We did not use doses of Epo exceeding 60 000 U (× 1) or 30 000 U (× 2), because experience with patients suffering from end-stage renal disease had shown that one or two injections of Epo did not increase hematopoiesis. Also, a study by Lipsic et al[8] had demonstrated the safety of a single injection of Epo at a dose of 60 000 U, and this dose was the maximum for a single injection available at the time of the study. We decided to use Epo at a dose of 30 000 U × 2 (at 5 min before the PM and at the end of the operation; EPO1 group), and 60 000 U × 1 (at 5 min before the PM, EPO2 group).
Patients treated with conventional steroids received 100 mg of hydrocortisone at 5 min before the PM, at the end of the operation, and on postoperative days 1, 2, and 3. Thus, the patients were allocated to 3 groups: a steroid group (STRD, n = 9), and EPO1 (n = 10) and EPO2 (n = 8) groups.
Eligibility criteria for participation were elective primary liver surgery with written informed consent. Patients who were expected to undergo non-curative hepatectomy were excluded.
Preoperative random allocation to the 3 groups was made on the basis of 4 parameters: age (< 65 or ≥ 65 years), sex, indocyanine green excretion rate at 15 min (< 10% or ≥ 10%), and disease diagnosis (hepatocellular carcinoma or others). The operative procedure was standardized so that hepatectomy was performed by a single surgeon (co-author Kubota K). The patients’ clinical background factors are shown in Table 1.
| STRD (n = 9) | EPO1 (n = 10) | EPO2 (n = 8) | P-value | |
| Age (yr) | 62.7 ± 10.6 | 62.1 ± 15.8 | 69.3 ± 7.8 | 0.395 |
| Male/female | 6/3 | 7/3 | 6/2 | 0.976 |
| ICG R15 (%) | 14.0 ± 8.8 | 10.7 ± 4.8 | 11.3 ± 5.2 | 0.854 |
| Diagnosis (HCC/others) | 6/3 | 6/4 | 4/4 | 0.890 |
| Operation time (min) | 322 ± 122 | 245 ± 83 | 299 ± 57 | 0.105 |
| Pringle time (min) | 43.9 ± 26.8 | 39.2 ± 21.2 | 53.8 ± 15.1 | 0.153 |
| Operative blood loss (mL) | 674 (70-3673) | 469 (66-1900) | 356 (228-1362) | 0.386 |
| Operative method (anatomical/nonanatomical) | 6/3 | 4/6 | 6/2 | 0.321 |
Blood samples were taken on admission (Pre), just after surgery (Day 0), and on postoperative days 1 (Day 1), 3 (Day 3), 7 (Day 7), and 14 (Day 14). Aspartate aminotransferase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), lactate, hemoglobin (Hb), and hematocrit (Ht) were measured from each sample. AST, ALT, LDH, lactate, Hb, and Ht were measured at the central laboratory of our institution. Proinflammatory cytokines, interleukin (IL)-6 and tumor necrosis factor (TNF)-α, were measured on Day 0 and Day 1. IL-6 was determined using a Human IL-6 Quantikine ELISA kit (R&D System, Minneapolis, MN) and TNF-α was determined using a Human TNF-α Quantikine ELISA kit (R&D System). All samples were measured in triplicate, in accordance with the manufacturer’s recommendations.
The primary objective of the study was to compare the protective effects of Epo and steroid against IR/I following the PM. The secondary objective was to elucidate the frequency of Epo-associated side effects.
All statistical analyses were performed with GraphPad Prism 5.0 (Graphpad Software, La Jolla, CA). Comparisons between the two groups were made using Mann-Whitney U test. Kruskal-Wallis test with post-hoc test (Dunn’s multiple comparison test) was used for comparisons between the conventional, EPO1, and EPO2 groups. Differences at P < 0.05 were considered to be statistically significant.
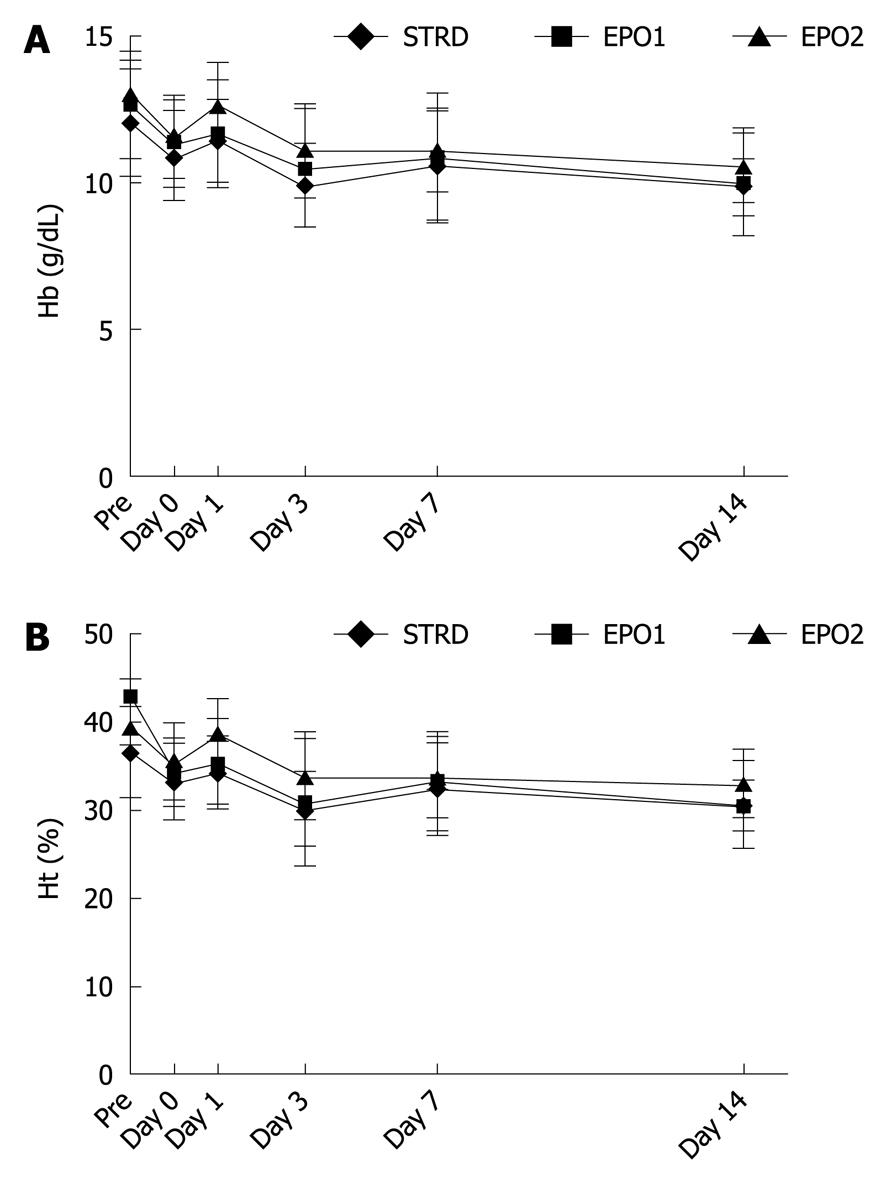
Figure 1 shows changes in Hb and Ht. During the perioperative period, there were no significant differences in Hb and Ht between the three groups, and no complications associated with the Epo treatments.
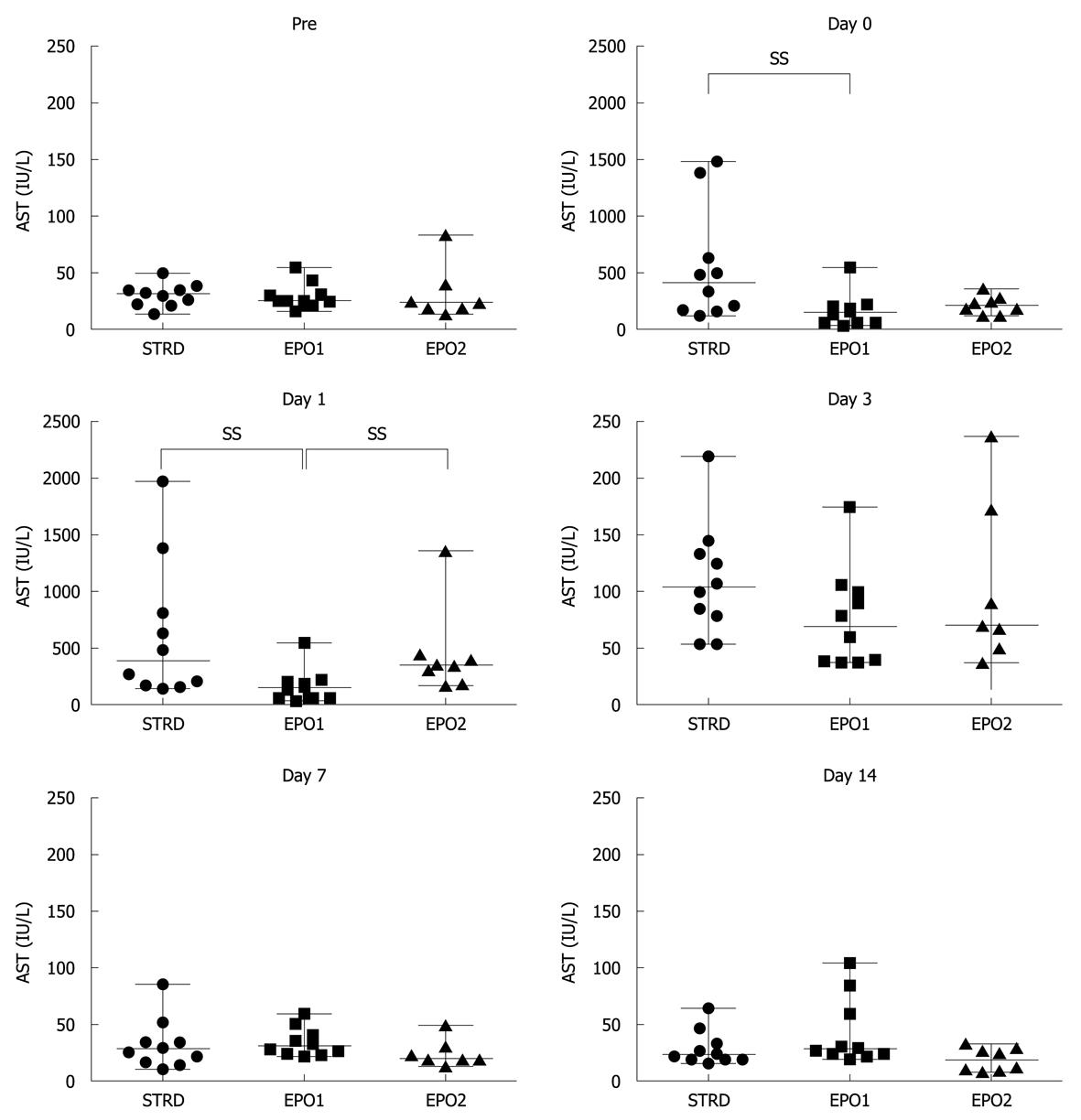
Figure 2 shows changes in the AST level at Pre, and on Days 0, 1, 3, 7 and 14. On Day 0, median values in the STRD, EPO1, and EPO2 groups were 409 (124-1481), 142 (34-552) and 205 (115-357) IU/L, respectively (P = 0.041, Kruskal-Wallis test). The AST level in the EPO1 group was significantly lower than that in the STRD group (P = 0.023). On Day 1, the corresponding median values were 275 (143.0-1389), 157 (55-552) and 342 (172-1361) IU/L, respectively (P = 0.018, Kruskal-Wallis test). The AST level in the EPO1 group was significantly lower than that in the STRD and EPO2 groups (P = 0.023 and 0.016, respectively). There were no significant inter-group differences in the AST level after Day 3.
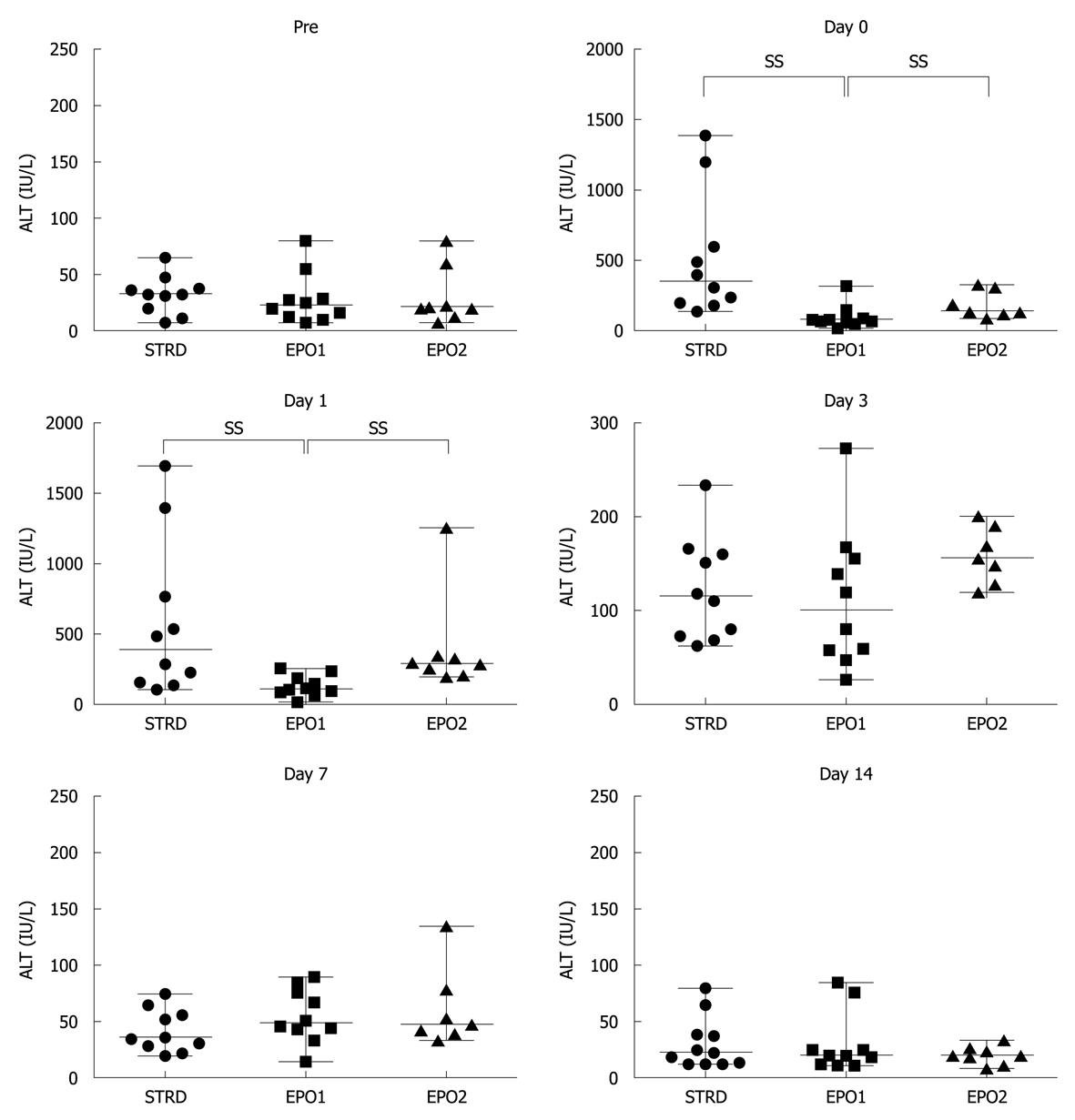
Figure 3 shows changes in the ALT level at Pre, and on Days 0, 1, 3, 7 and 14. On Day 0, the median levels in the STRD, EPO1, and EPO2 groups were 351 (140-1390), 76 (20-319) and 141 (85-323) IU/L, respectively (P = 0.020, Kruskal-Wallis test). The ALT level in the EPO1 group was significantly lower than that in the STRD and EPO2 groups (P = 0.023 and 0.017, respectively). On Day 1, the corresponding median levels were 300 (110-1700), 112 (20-256) and 289 (200-1253) IU/L, respectively (P = 0.004, Kruskal-Wallis test). The AST level in the EPO1 group was significantly lower than those in the STRD and EPO2 groups (P = 0.008 and 0.001, respectively). There were no significant inter-group differences in the ALT level after Day 3.
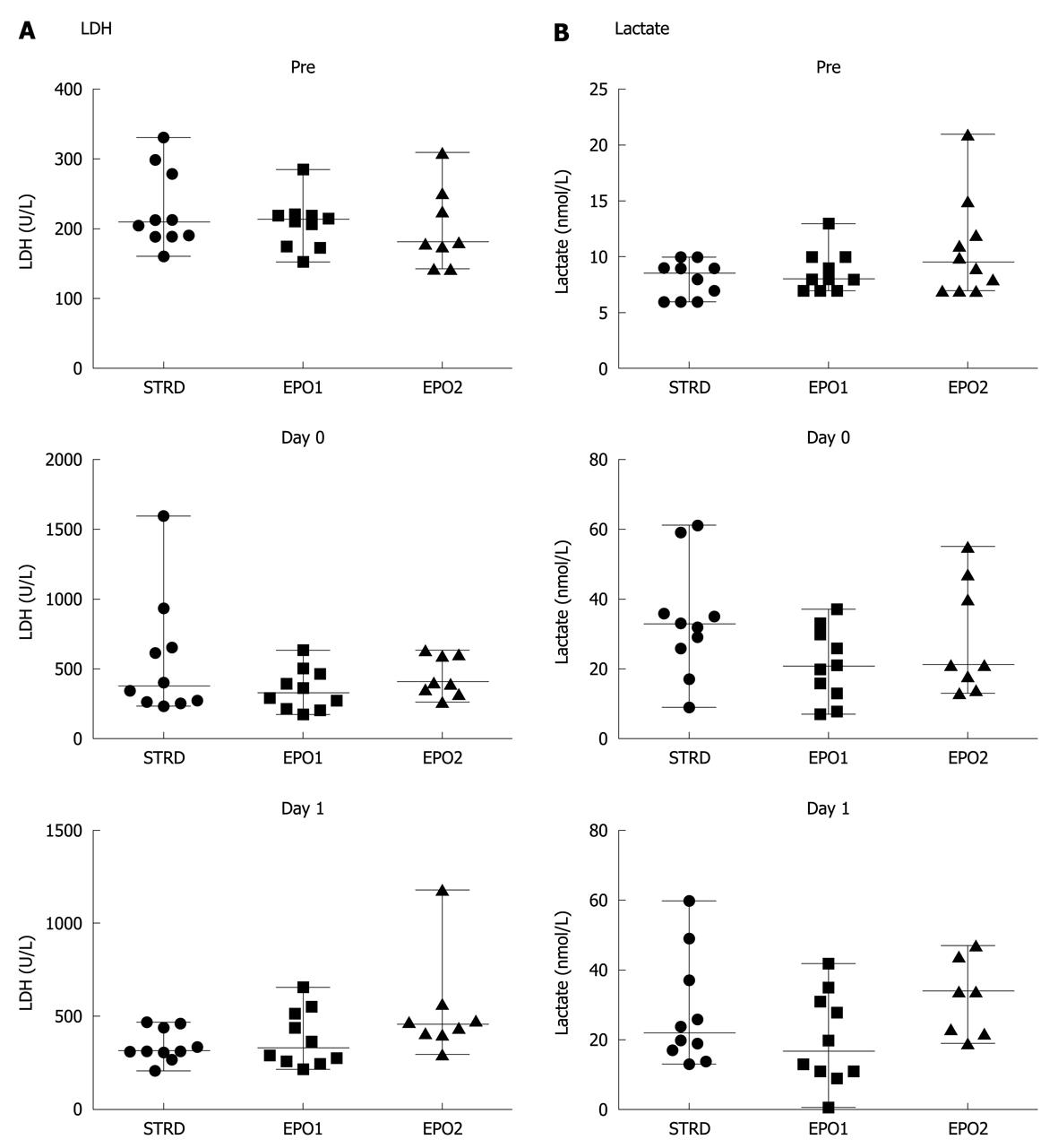
Figure 4 shows the changes in LDH and lactate levels at Pre, and on Days 0 and 1. There were no significant differences in LDH or lactate levels between the three groups.
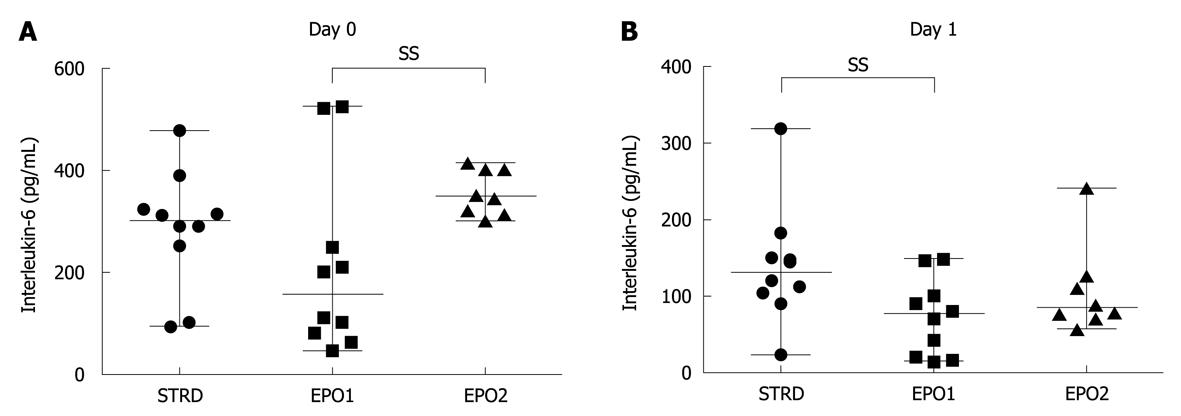
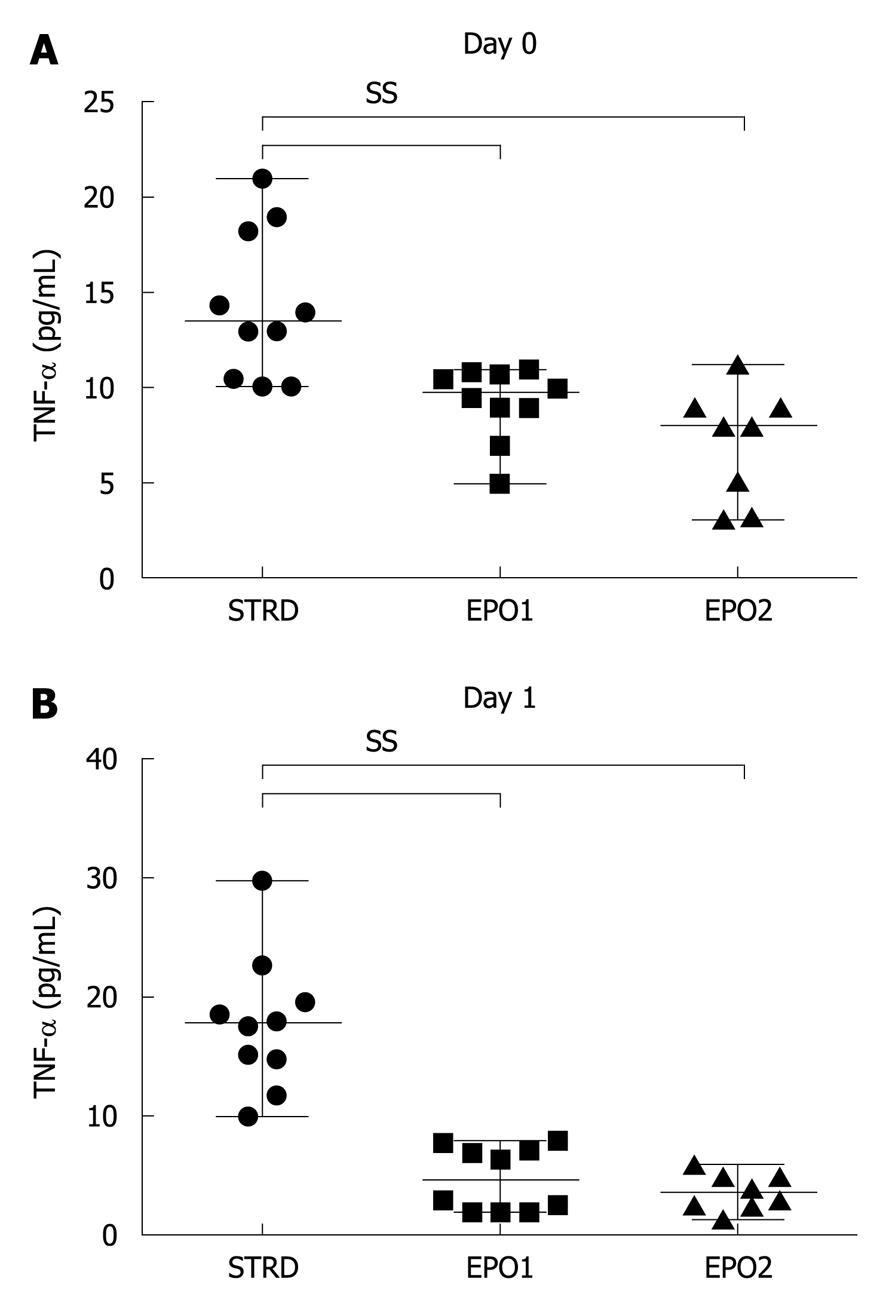
On Day 0, the median levels of the proinflammatory cytokine, IL-6, in the STRD, EPO1, and EPO2 groups were 300 (93-477), 155 (44-523) and 347 (300-414) pg/mL, respectively (P = 0.0036, Kruskal-Wallis test) (Figure 5A). The IL-6 level in the EPO1 group was significantly lower than that in the EPO2 group (P = 0.0037). On Day 1, the corresponding median levels of IL-6 were 129 (22-317), 75 (13-146) and 83 (56-240) pg/mL, respectively (P = 0.0451, Kruskal-Wallis test) (Figure 5B). The IL-6 level in the EPO1 group was significantly lower than that in the STRD group (P = 0.0185). On the other hand, on Day 0, the median levels of TNF-α in the STRD, EPO1, and EPO2 groups were 13.5 (10.1-21.0), 9.7 (5.0-11.0) and 8.0 (3.1-11.3) pg/mL, respectively (P = 0.0006, Kruskal-Wallis test) (Figure 6A), the levels in the EPO1 and EPO2 groups being significantly lower than that in the STRD group. On Day 1, the corresponding median levels of TNF-α were 17.7 (10.0-29.7), 4.6 (2.0-8.0) and 3.5 (1.4-6.0) pg/mL, respectively (P < 0.0001, Kruskal-Wallis test) (Figure 6B), the levels of TNF-α in the EPO1 and EPO2 groups being significantly lower than that in the STRD group.
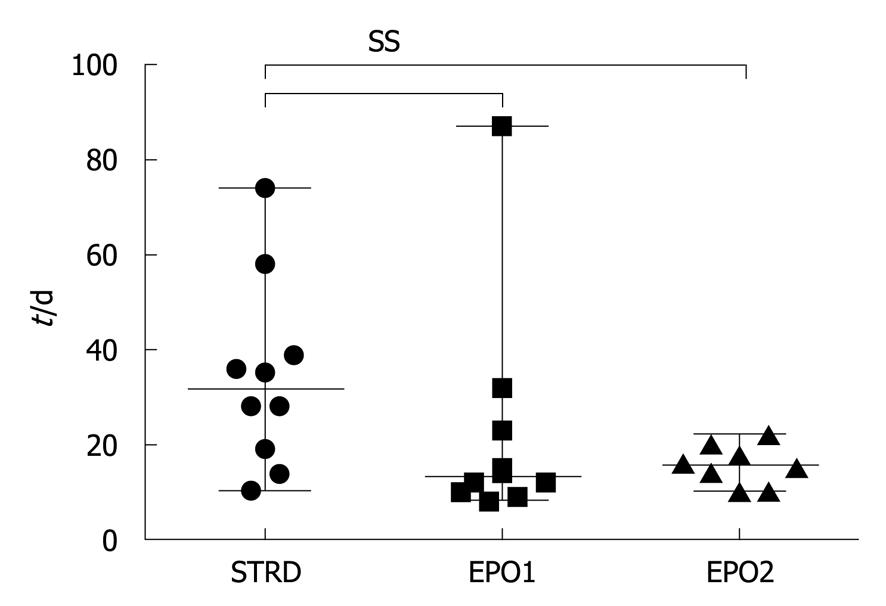
Postoperative complications were observed in two cases in the STRD group, but no such cases occurred in the EPO1 and EPO2 groups (Table 2). The median periods of hospitalization in the STRD, EPO1, and EPO2 groups were 32 (10-74), 13 (8-77) and 16 (10-22) d, respectively (P = 0.0463, Kruskal-Wallis test) (Figure 7), this period being significantly longer in the STRD group than in the EPO1 and EPO2 groups.
| Complication | Frequency (%) | |
| STRD (n = 10) | n = 2 | 20 |
| Prolonged ascites | ||
| Pleural effusion | ||
| EPO1 (n = 10) | 0 | 0 |
| EPO2 (n = 8) | 0 | 0 |
Most patients undergoing hepatic resection have associated chronic hepatic disease and deterioration of hepatic functional reserve. A dilemma therefore arises in deciding a balance between the curativeness of hepatic resection and the likelihood of postoperative hepatic failure. There is a tendency for surgeons to aim at removing as large a portion of liver as possible to ensure that any disease, such as carcinoma, is resected completely. On the other hand, excessive removal of liver parenchyma results in a higher risk of postoperative hepatic failure. Amelioration of IR/I is therefore a crucial factor to consider in the prevention of liver function deterioration.
The present study demonstrated that Epo potently ameliorated IR/I following the PM. The AST level in the EPO1 group was significantly lower than that in the STRD group on Day 0, and significantly lower than in the STRD and EPO2 groups on Day 1. The ALT level in the EPO1 group was significantly lower than in the STRD and EPO2 groups on both Day 0 and Day 1. There were no significant differences in AST and ALT levels between the three groups after Day 3. Our results demonstrated that the protective effect of Epo against IR/I was most pronounced at a very early stage after surgery, and furthermore, was stronger than that of steroids. During the very early post-surgical phase, there were no differences in LDH level, although the lactate level tended to be lower in the EPO1 group than in the STRD and EPO2 groups. The levels of proinflammatory cytokines, IL-6 and TNF-α, showed similar tendencies. The level of IL-6 in the EPO1 group was significantly lower than in the EPO2 and STRD groups. The level of TNF-α in the EPO1 and EPO2 groups was significantly lower than that in the STRD group.
The mechanism responsible for the protective effect of Epo against IR/I has been studied extensively by various groups, including our own[9-11]. As shown in this study, Epo protects organs and tissues from IR/I by inhibiting IR/I-induced cell apoptosis. Furthermore, Epo induces neovascularization and inhibits the secretion of acute inflammatory cytokines[4-6]. Epo binds to its specific cell-surface receptor (EpoR) and transduces signals to the nucleus[11]. The most important signaling pathways are the Jak2/STAT, Jak2/PI3K/AKT, and Jak2/MAPK/p38 pathways[12,13]. These signals activate anti-apoptotic genes, such as XIAP and BclxL, and suppress proapoptotic genes, such as BAD and GSK3β, resulting in inhibition of apoptosis[10,14-17]. EpoR usually has a homodimeric structure, but Epo has been shown to bind to heterodimeric EpoR, consisting of EpoR and the β common receptor when exerting its extra-haematopoietic effect. This heterodimeric receptor is expressed in various organs, including the liver [18].
We employed two different dose regimes for Epo: EPO1 (30 000 U × 2) and EPO2 (60 000 U × 1). The results, in terms of AST, ALT, and IL-6 levels, indicated that EPO1 was more effective. In terms of the TNF-α level, the EPO2 regimen showed a stronger inhibitory effect than the EPO1 regimen, but not to a significant degree. Although the reason for this finding is unclear, previous studies have reported that Epo directly and indirectly inhibits the expression of IL-6 and TNF-α, and reduces their systemic levels[19-21]. The extra-hematopoietic effects of Epo have been studied in various animal models and clinical settings. These previous studies each employed different doses of Epo. The use of Epo in animal models has been reviewed by Sharples et al[13], Johnson et al[22], and Arcasoy[6]. In clinical studies, Ehrenreich et al[23] used Epo at a total dose of 100 000 U for the first 3 d after acute cerebral stroke, noting that this was safe and well tolerated, and also used a dose of 40 000 U for 3 mo to treat schizophrenia[9]. Lipsic et al[5] used a single bolus injection of Epo at a dose of 60 000 U for patients with acute myocardial infarction, and did not observe any significant increase in Hb. Mocini et al[24] used a single injection of Epo at a dose of 40 000 U before cardiac surgery, but did not observe any significant improvement or an increase in erythropoiesis. The results suggest that the optimal dose of Epo and the mode of administration may vary according to species and target organ. Our present findings indicate that 30 000 U of Epo × 2, rather than 60 000 U of Epo × 1, has a stronger inhibitory effect on IR/I following the PM, and that the doses and the mode of administration we employed did not affect haematopoiesis (Figure 1).
The period of hospitalization was significantly longer in the STRD group than in the EPO1 and EPO2 groups. This was a prospective study performed during a single period. Therefore, the patients who were treated with steroid stayed longer in hospital. The occurrence of complications in the STRD, EPO1, and EPO2 groups was 20%, 0% and 0%, respectively. In this study, we evaluated only grade II complications by Clavien’s classification[25]. Although the number of patients in each group was small and more studies are needed, the data suggest that Epo might reduce the rate of complications after hepatic resection.
We conclude that Epo has greater potential than steroids to ameliorate IR/I following the PM, and that Epo at a dose of 30 000 U, given just before the PM and just after surgery, yields better results. This protective effect of Epo may be applicable not only in patients undergoing hepatic resection, but also for much more severe IR/I, such as that occurring in liver transplantation.
Ischemia-reperfusion injury (IR/I) is an obstacle encountered in various medical fields. Especially in liver surgery, IR/I after the Pringle maneuver (PM) hinders curability. Up to now, steroids have been used for amelioration of IR/I, but stronger and more effective drugs to prevent IR/I are needed.
Erythropoietin (Epo) is a hematopoietic cytokine that has been used to treat anemia in patients with end-stage renal disease. Recently, extra-hematopoietic effects of Epo have been reported, including an organ-protective effect against IR/I. However, there have been no reports of the hepatoprotective effect of Epo in a clinical setting.
This study is the first prospective study to have confirmed the protective effect of Epo against IR/I after the PM, in comparison with steroid treatment. It was found that Epo strongly ameliorated IR/I after the PM, and that the effect was better than that of the steroid. Epo would therefore contribute to better preservation of liver function after liver surgery.
This study demonstrated that Epo shows promise for preventing IR/I after the PM. This finding extends its application not only to liver resection, but also liver transplantation, where amelioration of IR/I would improve survival.
IR/I is a tissue injury occurring after ischemia-reperfusion. Organs are continuously perfused with blood, but once the blood supply is interrupted, organs sustain damage due to an anaerobic environment (ischemia). Restoration of the blood supply then causes further organ damage (reperfusion injury).
This is an original study because it is the first prospective randomized study of its kind, although the number of patients in each group is small.
Peer reviewers: Tor C Savidge, PhD, Associate Professor, Department of Gastroenterology and Hepatology, University of Texas Medical Branch, Galveston, TX 77555, United States; Dr. Rene Schmidt, PhD, Department of Anesthesiology, Freiburg University Medical Center, Hugstetter Strasse 55, Freiburg 79106, Germany; Dr. Kemal Kismet, MD, 4th General Surgery Department, Ankara Training and Research Hospital, Ankara 06430, Turkey
S- Editor Wang JL L- Editor O’Neill M E- Editor Zheng XM
| 1. | van Gulik TM, de Graaf W, Dinant S, Busch OR, Gouma DJ. Vascular occlusion techniques during liver resection. Dig Surg. 2007;24:274-281. |
| 2. | Franchello A, Gilbo N, David E, Ricchiuti A, Romagnoli R, Cerutti E, Salizzoni M. Ischemic preconditioning (IP) of the liver as a safe and protective technique against ischemia/reperfusion injury (IRI). Am J Transplant. 2009;9:1629-1639. |
| 3. | Sileri P, Schena S, Fukada J, Rastellini C, Pirenne J, Benedetti E, Cicalese L. Corticosteroids enhance hepatic injury following ischemia-reperfusion. Transplant Proc. 2001;33:3712. |
| 4. | Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: biology and clinical promise. Kidney Int. 2006;70:246-250. |
| 5. | Lipsic E, Schoemaker RG, van der Meer P, Voors AA, van Veldhuisen DJ, van Gilst WH. Protective effects of erythropoietin in cardiac ischemia: from bench to bedside. J Am Coll Cardiol. 2006;48:2161-2167. |
| 6. | Arcasoy MO. The non-haematopoietic biological effects of erythropoietin. Br J Haematol. 2008;141:14-31. |
| 7. | Shimoda M, Sawada T, Iwasaki Y, Mori S, Kijima H, Okada T, Kubota K. Erythropoietin strongly protects the liver from ischemia-reperfusion injury in a pig model. Hepatogastroenterology. 2009;56:470-475. |
| 8. | Lipsic E, van der Meer P, Voors AA, Westenbrink BD, van den Heuvel AF, de Boer HC, van Zonneveld AJ, Schoemaker RG, van Gilst WH, Zijlstra F. A single bolus of a long-acting erythropoietin analogue darbepoetin alfa in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20:135-141. |
| 9. | Ehrenreich H, Hinze-Selch D, Stawicki S, Aust C, Knolle-Veentjer S, Wilms S, Heinz G, Erdag S, Jahn H, Degner D. Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol Psychiatry. 2007;12:206-220. |
| 10. | Patel NS, Sharples EJ, Cuzzocrea S, Chatterjee PK, Britti D, Yaqoob MM, Thiemermann C. Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo. Kidney Int. 2004;66:983-989. |
| 11. | Okada T, Sawada T, Kubota K. Asialoerythropoietin has strong renoprotective effects against ischemia-reperfusion injury in a murine model. Transplantation. 2007;84:504-510. |
| 12. | Westenfelder C, Biddle DL, Baranowski RL. Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int. 1999;55:808-820. |
| 13. | Sharples EJ, Thiemermann C, Yaqoob MM. Mechanisms of disease: Cell death in acute renal failure and emerging evidence for a protective role of erythropoietin. Nat Clin Pract Nephrol. 2005;1:87-97. |
| 14. | Parsa CJ, Kim J, Riel RU, Pascal LS, Thompson RB, Petrofski JA, Matsumoto A, Stamler JS, Koch WJ. Cardioprotective effects of erythropoietin in the reperfused ischemic heart: a potential role for cardiac fibroblasts. J Biol Chem. 2004;279:20655-20662. |
| 15. | Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641-647. |
| 16. | Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, Kieswich J, Allen D, Harwood S, Raftery M. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115-2124. |
| 17. | Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006;69:1806-1813. |
| 18. | Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907-14912. |
| 19. | Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971-975. |
| 20. | Mitsuma W, Ito M, Kodama M, Fuse K, Okamura K, Minagawa S, Kato K, Hanawa H, Toba K, Nakazawa M. Cardioprotective effects of recombinant human erythropoietin in rats with experimental autoimmune myocarditis. Biochem Biophys Res Commun. 2006;344:987-994. |
| 21. | Prutchi-Sagiv S, Golishevsky N, Oster HS, Katz O, Cohen A, Naparstek E, Neumann D, Mittelman M. Erythropoietin treatment in advanced multiple myeloma is associated with improved immunological functions: could it be beneficial in early disease? Br J Haematol. 2006;135:660-672. |
| 22. | Johnson DW, Forman C, Vesey DA. Novel renoprotective actions of erythropoietin: new uses for an old hormone. Nephrology (Carlton). 2006;11:306-312. |
| 23. | Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495-505. |
| 24. | Mocini D, Muso P, Guendouz E, De Marco L, Mele L, Cini R, Sordini P, Alois A, Costantino A, Arima S. Endogenous erythropoietin and a single bolus of 40,000 IU of epoetin alpha do not protect the heart from ischaemia-reperfusion injury during extracorporeal circulation for cardiac surgery. Perfusion. 2008;23:187-192. |