Published online Oct 7, 2010. doi: 10.3748/wjg.v16.i37.4677
Revised: July 5, 2010
Accepted: July 12, 2010
Published online: October 7, 2010
AIM: To investigate the effect of oncolytic adenovirus SG600-IL24 and replication-incompetent adenovirus Ad.IL-24 on hepatocellular carcinoma (HCC) cell lines and normal liver cell line.
METHODS: HCC cell lines (HepG2, Hep3B and MHCC97L) and normal liver cell line (L02) with a different p53 status were infected with SG600-IL24 and Ad.IL-24, respectively. Melanoma differentiation-associated (MDA)-7/interleukin (IL)-24 mRNA and protein expressions in infected cells were detected by reverse transcription-polymerase chain reaction (RT-PCR), enzyme-linked immunosorbent assay (ELISA), and Western blotting, respectively. Apoptosis of HCC cells and normal liver cells was detected by cytometric assay with Hoechst33258 staining. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to investigate proliferation of HCC cells and normal liver cells, and cell cycle was assayed by flow cytometry.
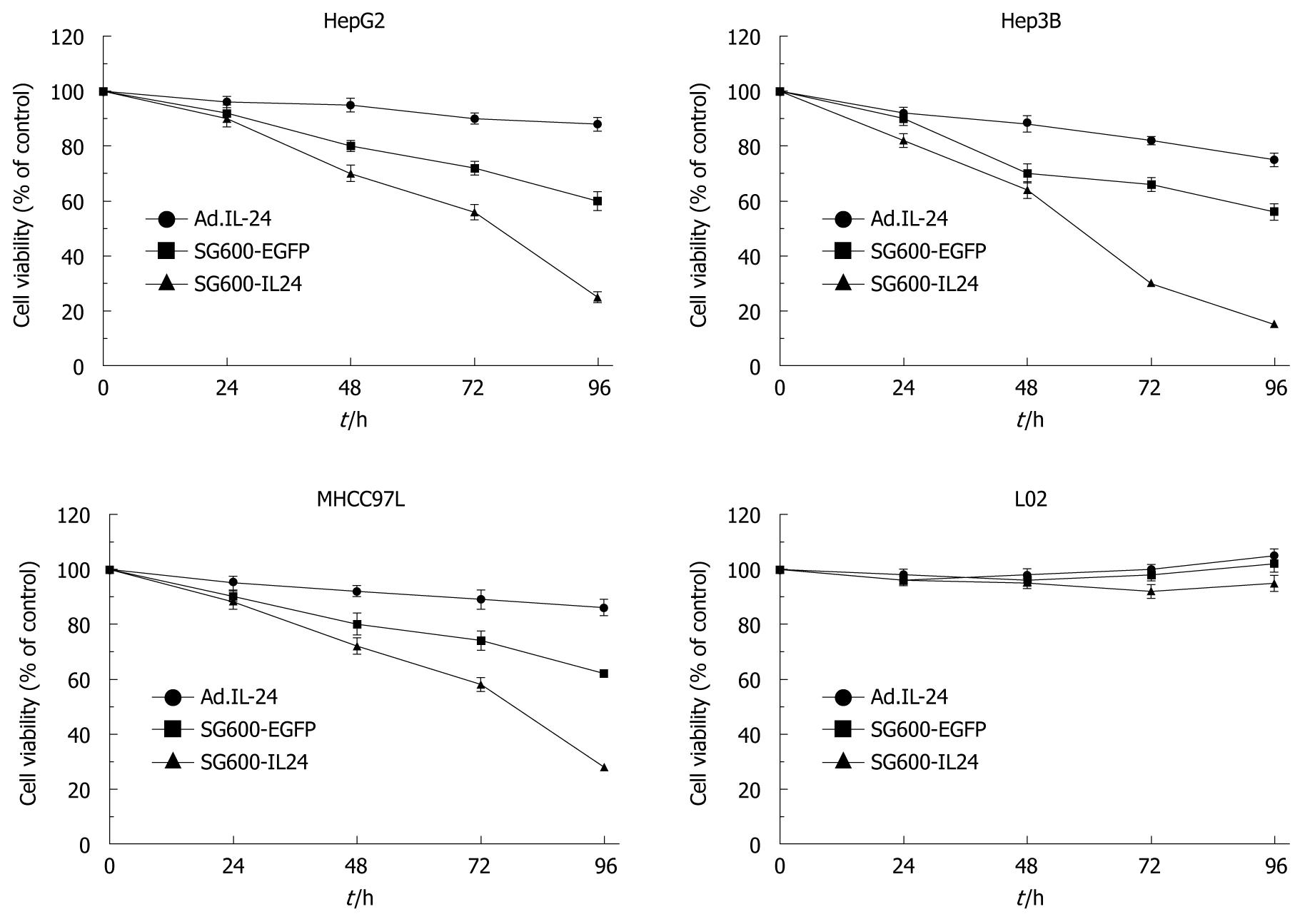
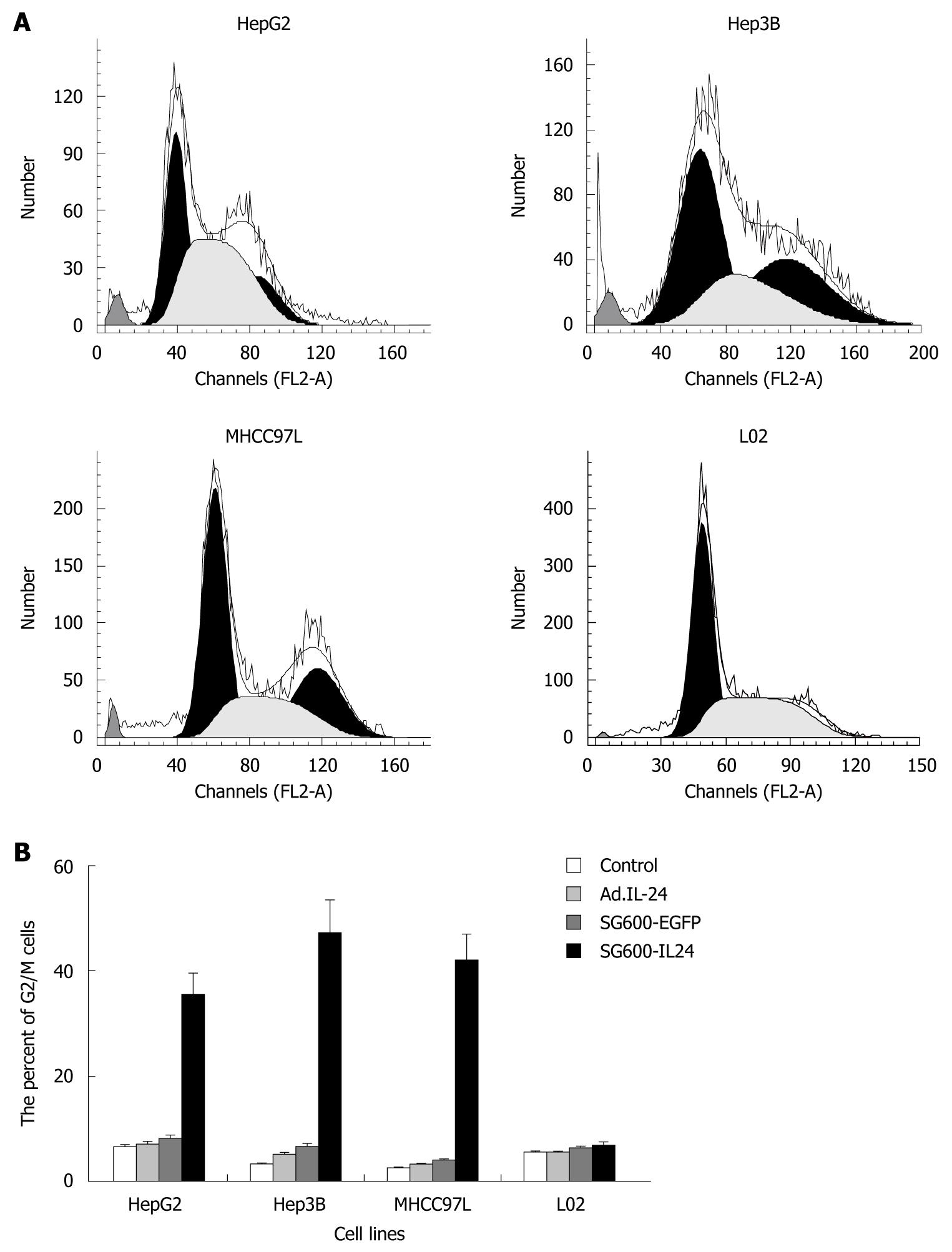
RESULTS: RT-PCR, ELISA and Western blotting showed that the exogenous MDA-7/IL-24 gene was highly expressed in cells infected with SG600-IL24. MTT indicated that SG600-IL24 could suppress the growth of HepG2, Hep3B, MHCC97L, with an inhibition rate of 75% ± 2.5%, 85% ± 2.0%, 72% ± 1.8%, respectively (P < 0.01), promote the apoptosis of HepG2, Hep3B, MHCC97L, with an apoptosis rate of 56.59% ± 4.0%, 78.36% ± 3.5%, 43.39% ± 2.5%, respectively (P < 0.01), and block the HCC cell lines in the G2/M phase with a blocking rate of 35.4% ± 4.2%, 47.3% ± 6.2%, 42% ± 5.0%, respectively (P < 0.01) but not the normal liver cell line in a p53-independent manner.
CONCLUSION: SG600-IL24 can selectively suppress the proliferation and apoptosis of HCC cell lines in vitro but not normal liver cell line L02 in a p53-independent manner. Compared with Ad.IL-24, SG600-IL24 can significantly enhance the antitumor activity in HCC cell lines.
- Citation: Xue XB, Xiao CW, Zhang H, Lu AG, Gao W, Zhou ZQ, Guo XL, Zhong MA, Yang Y, Wang CJ. Oncolytic adenovirus SG600-IL24 selectively kills hepatocellular carcinoma cell lines. World J Gastroenterol 2010; 16(37): 4677-4684
- URL: https://www.wjgnet.com/1007-9327/full/v16/i37/4677.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i37.4677
Hepatocellular carcinoma (HCC), one of the most common lethal malignant diseases in the world, is responsible for a significant number of deaths annually[1]. The HCC-associated annual morbidity and mortality rank second among all tumors[2]. In addition, the prognosis of HCC patients is poor. Gene therapy has emerged as an attractive option in treatment of HCC, and its strategies include transgenic therapy with anti-oncogenes such as p53 and Rb, anti-sense nucleotide therapy, drug gene therapy (e.g. suicide gene like HSV-TK), and tumor vaccines[3]. However, its current strategies destroy both tumor and normal cells simultaneously, thus limiting their clinical application.
Melanoma differentiation-associated (MDA)-7/interleukin (IL)-24 was identified by a combination of recombinant fibroblast interferon-β and protein kinase C activator mezerein subtraction hybridization by Fisher in 1995[4]. It has been shown that MDA-7/IL-24 can suppress the growth of melanoma, inhibit the proliferation and apoptosis of other cancer cells[5-8], and has thus been hailed as a novel and interesting development in experimental tumor therapy in the early 21st century[9]. Our previous study showed that Ad.IL-24 can selectively destruct a variety of liver cancer cells without any toxic effects and inhibit the proliferation of normal liver cell line L02. Ad.IL-24 can facilitate release of CytC and Smac from mitochondria into cytoplasm, independent of the Fas pathway[10]. MDA-7/IL-24 can also inhibit the growth and metastasis of high-transfer (HCCM6) liver xenograft tumors, with a synergistic effect when combined with doxorubicin. However, replication-defective adenovirus is limited in cancer gene therapy due to its poor targets, low transfection efficiency, low gene expression effect, and severe immune reaction. Oncolytic adenovirus, a non-defective virus, has a higher transfection efficiency because the exogenous gene can selectively replicate in tumor cells and kill tumor cells but not normal cells[11]. Besides, it combines the antitumor effect of viral vector and therapeutic gene. Therefore, we used this strategy to construct replication-competent oncolytic adenovirus SG600-IL24 carrying human MDA-7/IL-24. However, its effects on HCC cells with a different p53 status remain largely unknown. To clarify its effects, we first determined the expression of SG600-IL24 in HCC cell lines HepG2, Hep3B, MHCC97L, and normal human liver cell line L02 in vitro.
HCC cell lines HepG2 and Hep3B, MHCC97L and normal human liver cell line L02 were purchased from the Institute of HCC, Fudan University (Shanghai, China). Human embryonic kidney cells (HEK 293) were a gift from Professor Qi-Jun Qian, Laboratory of Viral and Gene Therapy, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Shanghai, China). The cell lines were cultured in a high glucose DMEM (HyClone, USA) supplemented with 10% FBS (Gibco, USA) at 37°C in a humidified incubator containing 5% CO2 and 95% air. L02 was cultured in RPMI-1640 (HyClone, USA) supplemented with 10% FBS.
Oncolytic adenoviruses SG600-IL24 and SG600-EGFP were constructed and amplified. In brief, IL-24 expression cassette was released from pZD55-IL-24 and introduced into pClon9-INS to produce pClon9-INS-IL24. Using a plasmid transfection method, we co-transfected pSG600-IL24 and adenovirus skeletal plasmid pPE3 into HEK293 cells to construct the recombinant adenovirus vector SG600-IL24 carrying the MDA-7 gene. The genomes were analyzed to confirm the recombinant structure, and the virus was plaque purified and amplified in HEK293 cells. The recombinant replication-defective Ad.MDA-7 virus was constructed and amplified as previously described[11]. The titer of SG600-IL24, SG600-EGFP and Ad.IL-24 was 2.25 × 1010 PFU/mL, 2.79 × 1010 PFU/mL, and 7.1 × 109 PFU/mL, respectively.
Six-well plates for each cell line were divided into control group, Ad.IL-24 group, SG600-EGFP group and SG600-IL24 group. Control group was treated with a serum-free DMEM. The cells were infected with oncolytic adenovirus with its multiplicity of infection (MOI) = 10.
Cells were harvested at 24 h following SG600-IL24 infection for detection of MDA-7/IL-24 mRNA expression. Total RNA was extracted and reverse transcription-polymerase chain reaction (RT-PCR) was performed as previously described[10,12]. The sequences of primers of MDA-7 mRNA are sense: 5'-GGGCTGTGAAAGACACTAT-3', antisense: 5'-GCATCCAGGTCAGAAGAA-3'. The length of amplified fragments was 381 bp. The sequences of primers of β-actin are sense: 5'-CCTTCCTGGGCAATGGAGTCCT-3', antisense: 5'-GGAACAATGATCTTGATCTT-3'. The length of amplified fragments was 201 bp. The PCR conditions were as follows: denaturation at 94°C for 5 min, followed by 30 cycles at 94°C for 30 s, at 56°C for 30 s, at 72°C for 30 s, a final extension at 72°C for 10 min. The PCR products were subjected to 1% agarose gel electrophoresis.
Two-antibody sandwich ELISA was developed for detection of human MDA-7/IL-24. The antibodies used were monoclonal mouse anti-human IL-24 antibody (R&D Systems) and peroxidaseconjugated rabbit anti-goat IgG antibody (R&D Systems). Cell culture supernatant was collected after 24, 48 and 72 h, respectively and stored at -20°C. Concentrations of MDA-7/IL-24 in supernatant were measured by ELISA at different time points. Absorbance was read at a wavelength of 450-nm. Concentration of IL-24 was measured according to the standard curve.
Cells were seeded into 96-well tissue culture plates (1 × 103 cells per well), and treated with PBS, 10 MOI of Ad.IL-24, SG600-EGFP, SG600-IL24, respectively, on the next day. After cultured for 24 h, the medium was replaced with a fresh medium containing 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The cells were incubated at 37°C for 4 h. After about 150 μL of a solution was added to each well, the cells were incubated for an additional 10 min at 37°C with gentle shaking. Absorbance was read on a Bio-Rad microplate reader at a wavelength of 595 nm.
Cell lines were cultured in 6-well plates, and cells after different treatments were collected at the indicated time points. The cells were collected and suspended in lysis BCA for protein quantitation after 48 h. A total of 25 μg of protein was applied to 15% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes which were probed with polyclonal antibodies to MDA-7/IL-24 and β-actin. The corresponding fluorescent secondary antibody was hybridized. The second anti-PVDF membrane fluorescence signal was detected with an infrared imaging system.
Forty-eight hours after infection, the cells were washed twice with PBS and fixed in 1mL of 4% paraformaldehyde for 10 min at 4°C. After washed twice with PBS, the cells were stained with 100 μL Hoechst33258 in PBS for 15 min at room temperature in dark. Then 1000 cells were counted with their nuclear fragmentation visualized under a fluorescence microscope (TE2000-U, Nikon, Japan). Apoptotic cells were identified by condensation of nuclear chromatin and its fragmentation.
Cells were trypsinized and washed twice with complete media 48 h later. Aliquots of cells (1 × 106) were resuspended in 500 μL binding buffer and stained with 5 μL FITC-labeled Annexin-V according to its manufacturer’s instructions. Five microliters of propidium iodide (PI) was added to the samples after stained with Annexin-V to distinguish late apoptotic and necrotic cells, and then put in a dark place for 30 min. Flow cytometry (BD, FACSCalibur, USA) was performed immediately after staining.
Cells were cultured when they grew about 30%, and then treated with DMEM without FBS for 24 h for synchronization. The cells were divided into DMEM group, Ad.IL-24 group, SG600-EGFP group, and SG600-IL24 group 48 h later, and harvested using trypsin to adjust the cell concentration to l × 106 per mL. After fixed in 70% cold ethanol overnight at -20°C, the cells were washed with PBS, and aliquots of 1 × 106 cells were resuspended in 1 mL of PBS containing 1 mg/mL of RNase A and 0.5 mg/mL of PI. After incubated for 30 min, the cells were analyzed by flow cytometry using a FACScan flow cytometer (BD, FACSCalibur, USA). The treated cells were evaluated by FACS analysis for identifying cells at different stages of cell cycle. The percentages of cells at G0/G1, S, and G2/M stages were calculated using multicycle software and the results were analyzed using variance analysis.
All experiments were performed at least three times. Data were expressed as mean ± SD. Statistical comparisons were made by analysis of variance. P < 0.05 was considered statistically significant. All analyses were performed with SPSS14.0 software.
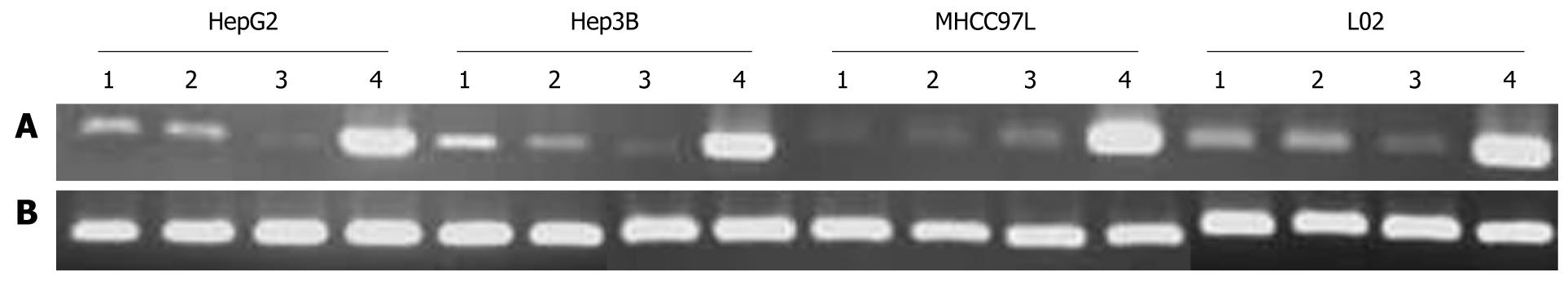
The expression of MDA-7/IL-24 mRNA was markedly increased both in normal liver cell line (L02) and in HCC cell lines (HepG2, Hep3B, MHCC97L) with a different p53 state that were infected with SG600-IL24. In contrast, the expression level of MDA-7/IL-24 was very low in cells infected with Ad.IL-24, SG600-EGFP, and DMEM (Figure 1).
Secreting MDA-7/IL-24 protein was detected by ELISA after SG600-IL24 infection. The concentrations of MDA-7/IL-24 protein in supernatants of cells infected with SG600-IL24 increased in a time-dependent manner. The expression of endogenous MDA-7/IL-24 was not detected in SG600-EGFP and control groups (Table 1).
| Concentration of MDA-7/IL-24 protein | ||||
| HepG2 | Hep3B | MHCC97L | L02 | |
| Control group | 8.0 ± 1.0 | 9.0 ± 0.5 | 4.0 ± 0.4 | 9.0 ± 0.8 |
| Ad.IL-24 group | 8.2 ± 0.5 | 9.0 ± 1.0 | 4.5 ± 0.5 | 10.0 ± 1.0 |
| SG600-EGFP group | 9.0 ± 0.8 | 10.0 ± 0.1 | 3.5 ± 0.5 | 9.0 ± 0.6 |
| SG600-IL24 group | ||||
| 24 h | 90 ± 10b | 60 ± 8a | 56 ± 10b | 110 ± 12b |
| 48 h | 160 ± 20b | 90 ± 15b | 180 ± 20b | 180 ± 15b |
| 72 h | 780 ± 80b | 800 ± 60b | 680 ± 50b | 920 ± 80b |
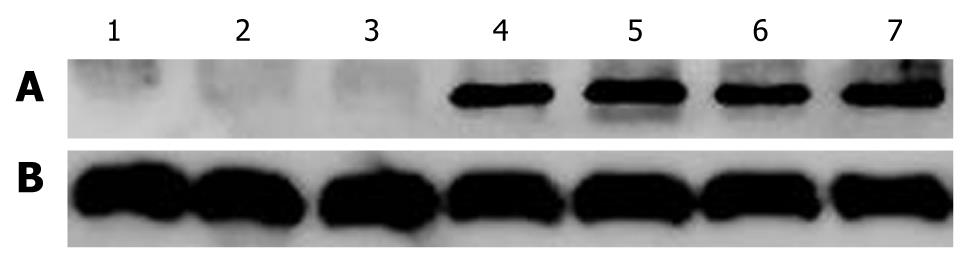
Mda-7/IL-24 protein was not expressed in control group, Ad.IL-24 and SG600-EGFP groups, while MDA-7/IL-24 was highly expressed in oncolytic adenovirus 48 h after SG600-IL24 infection (Figure 2).
To investigate whether SG600-IL24 can inhibit cell proliferation, HCC cell lines (HepG2, Hep3B and MHCC97L) and normal liver cell line L02 were infected with SG600-IL24. The cell proliferation and viability were determined by MTT. No proliferation arrest effect was observed on normal liver cell line L02 (Figure 3). However, the activity of SG600-IL24 in HCC cell lines (HepG2, Hep3B and MHCC97L) was significantly inhibited with an inhibition rate of 75%, 85% and 72%, respectively.
Hoechst staining showed that SG600-IL24 induced the apoptosis of human HCC cell lines of HepG2, Hep3B and MHCC97L (Table 2). The apoptosis level of HCC cells was higher in SG600-IL24 group than in other groups (HepG2: F = 156.6, Hep3B: F = 202.4, MHCC97L: F = 143.2, P < 0.05), indicating that SG600-IL24 can induce apoptosis of HCC cells. In contrast, no apparent change was observed in normal liver cell line L02, with an apoptosis rate of 1.0%, 1.4%, 1.2% and 2.0%, respectively (F = 1.78). Flow cytometry showed the effect of SG600-IL24 on the apoptosis of HCC cell lines of HepG2, Hep3B and MHCC97L and normal liver cell line L02 with Annexin-V and PI staining. The percentage of apoptotic HCC cells was significantly higher in SG600-IL24 group than in control group, SG600-EGFP and Ad.IL-24 groups (HepG2: F = 203.4, Hep3B: F = 313.2, MHCC97L: F = 160.6, P < 0.05, Table 2). In contrast, no significantly change was found in normal liver cell line L02 with an apoptosis rate of 0.75%, demonstrating that SG600-IL24 infection can kill HCC cells but not normal liver cells.
| Cell line | Apoptotic cells (%) | F-value | P-value | |||
| Control | Ad.IL-24 | SG600-EGFP | SG600-IL24 | |||
| Hoechst staining | ||||||
| HepG2 | 2.5 ± 0.1 | 4.0 ± 0.3 | 5.5 ± 0.3 | 42.0 ± 4.5 | 156.6 | 0.000007 |
| Hep3B | 3.0 ± 0.2 | 3.5 ± 0.3 | 4.5 ± 0.2 | 56.0 ± 3.8 | 202.4 | 0.000003 |
| MHCC97L | 1.6 ± 0.2 | 3.0 ± 0.4 | 2.6 ± 0.3 | 40.5 ± 1.9 | 143.2 | 0.000010 |
| L02 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.4 ± 0.1 | 2.0 ± 0.1 | 1.78 | 0.360 |
| Flow cytometry | ||||||
| HepG2 | 2.0 ± 0.1 | 4.2 ± 0.4 | 10.0 ± 2.0 | 56.5 ± 4.0 | 203.4 | 0.000003 |
| Hep3B | 2.5 ± 0.2 | 5.0 ± 0.2 | 13.5 ± 1.5 | 78.3 ± 3.5 | 313.2 | 0.000001 |
| MHCC97L | 1.4 ± 0.2 | 3.2 ± 0.4 | 10.4 ± 1.0 | 43.3 ± 2.5 | 160.6 | 0.000006 |
| L02 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.1 | 1.62 | 0.280 |
Cell cycle phase was assayed by flow cytometry after the fixed cells were stained with PI. The accumulation level of HCC cell lines at the G2/M phase was higher in SG600-IL24 group than in control group, Ad.IL-24 and SG600-EGFP groups (P < 0.05) with an accumulation rate of 35.4%, 47.3%, 42%, respectively (Figure 4). However, the accumulation rate of normal liver cell line L02 at the G2/M phase was 5.5%, 5.6%, 6.3%, and 6.8%, respectively, suggesting that SG600-IL24 infection can significantly increase the accumulation of HCC cell lines but not of normal liver cell line L02 at the G2/M phase.
MDA-7/IL24 is a new member of the IL-10 class-II family of cytokines. It has been shown that MDA-7/IL24 not only inhibits the growth of melanoma but also the proliferation and apoptosis of other carcinoma cells, such as ovarian cancer[13,14], lung carcinoma[15,16], breast cancer[17], pancreatic cancer[18], glioma[18,19], prostate[20]and colon cancer[21]. It has been reported that increased expression of IL-24 gene suppresses cell growth and induces cell apoptosis in a variety of cancer cells with single or multiple genetic defects, including alterations in p53, p16/INK4a, and Rb[22,23]. It has been shown that some signal transduction pathways and molecules are regulated during IL-24-induced tumor suppression, including activation of caspase cascade, PKR, p38,STAT3, PI3K, GSK-3, ILK-1, BAX, BAK, Fas, DR4,TRAIL, inducible nitric oxide synthase (iNOS), IRF-1,IRF-2 and p53[24].
HCC gene therapy has become a current research focus. However, most methods used are not tumor specific and affect normal cells, thus limiting their clinical application. Gene therapy, a research hot-spot in cancer gene therapy, is able to selectively kill tumor cells without affecting normal cells[25]. In this study, oncolytic adenovirus, which is characterized by antitumor activity and can proliferate and replicate specifically in tumor cells, was used as a carrier. Oncolytic adenovirus can make the exogenous gene copy thousands of times and enhance the effect of anti-cancer gene in a similar manner, ultimately killing tumor cells[26]. Since tumor-specific oncolytic adenovirus can amplify many times in infected cancer cells and dissolve tumor cells, it can be used as a promising anti-tumor gene therapy vector[27]. Adenovirus SG600IL-24, which was constructed in this study, has the E1B 55 kDa defective oncolytic adenovirus (ZD55), replicates in tumor cells and causes significant cytotoxic effect, while normal cells show little or no toxicity[28]. SG600IL-24 was constructed with the oncolytic adenovirus SG600 vector using the telomerase reverse transcriptase promoter (TERTp) which is highly active in more than 85% of different human cancers, but inactive in most normal somatic cells, and can thus be applied to a wide range of cancers[29]. Hypoxia regulatory elements can control adenovirus proliferation genes E1a and E1b, and delete the E1a gene, thus promoting virus-specific replication in tumor cells and enhancing the effectiveness and safety of gene therapy[30].
In contrast to the replication-incompetent adenovirus Ad.IL-24, oncolytic adenovirus can replicate in tumor cells exclusively and kill tumor cells, inducing a high expression of MDA-7/IL-24 in these cells. MDA-7/IL-24 protein can also selectively kill cancer cells. Moreover, oncolytic adenovirus can selectively kill tumor cells and MDA-7/IL-24 protein can release into blood and kill distant micrometastatic tumor cells, resulting in a radical treatment of HCC because of the potent “bystander” antitumor activity[31].
Since MDA-7/IL-24 can selectively kill various cancer cells, it has been used in treatment of patients with HCC[32,33] with encouraging results. However, the treatment is restricted only to melanoma, but not to other tumors. HCC has much more cells, bulkier volume, and higher metastatic potential than melanoma. If replication-defective adenovirus is used as a vector, a large number of adenoviruses would be required and lead to mortal immune reaction to human bodies, thus limiting its further clinical application. Oncolytic adenovirus was constructed in this study as a vector carrying the MDA-7/IL-24 gene, which can proliferate in tumor cells exclusively and kill tumor cells, release a large number of adenoviruses which would infect other tumor cells, proliferate and kill tumor cells. Moreover, MDA-7/IL-24 protein releases and selectively kills tumor cells when tumor cells are dissolved. Therefore, few adenoviruses are required, thus greatly benefiting the clinical application of MDA-7/IL-24. Furthermore, SG600-IL24 has few or no toxic effects on normal cells because it cannot proliferate in normal cells[28].
In this study, RT-PCR, ELISA, and Western-blot demonstrated that MDA-7/IL-24 was successfully transfected into HCC cells and normal liver cells. Mda-7/IL-24 gene and protein were not expressed in control and SG600-EGFP groups. ELISA showed that the expression of secreted and intracellular MDA-7/IL-24 protein in SG600IL-24 group increased in a time-dependent manner. Flow cytometry showed SG600IL-24 significantly inhibited the proliferation of tumor cells, promoted the apoptosis of tumor cells, and blocked tumor cells in the G2/M phase. Normal liver cell line L02 was not affected although it increased by 1.3% in the G2/M phase. Flow cytometry showed that the early and late apoptosis rate of HepG2, Hep3B and MHCC97L was 56.59%, 78.36% and 43.39%, respectively, with Annexin-V and PI staining. MTT showed that SG600IL-24 could promote apoptosis and kill HCC cells but not normal liver cells with Hoechst33258 staining, indicating that the antitumor activity of SG600IL-24 is stronger than that of Ad.IL-24.
HCC cell line HepG2 is a wild type in gene p53 and a mutant type in gene Rb. Hep3B is a line with p53 gene deleted, and MHCC97L is a mutant type in gene p53[34-37]. Although these cell lines have different gene types, they induce apoptosis and growth arrest by infection with SG600IL-24, demonstrating that SG600IL-24 can kill different tumor cells independent of the p53 state.
In conclusion, SG600IL-24 selectively kills HCC cell lines in a p53-independent manner and enhances antitumor activity in HCC cell lines. SG600-IL24 can be used as a HCC gene therapy vector.
Gene therapy is able to selectively kill tumor cells without affecting normal cells and has thus become a research hot-spot in cancer gene therapy. However, in vitro experimental results of gene therapy for hepatocellular carcinoma (HCC) are imperfect. The aim of this study was to investigate the effect of replication-competent oncolytic adenovirus one HCC cell lines and normal liver cell line.
In this study, SG600-IL24 could selectively suppress the proliferation and apoptosis of HCC cells in vitro in a p53-independent manner. Compared with Ad.IL-24, SG600-IL24 could enhance antitumor activity in HCC cell lines.
Oncolytic adenovirus SG600 was constructed in this study, which has the telomerase reverse transcriptase promoter (TERTp) and hypoxia regulatory elements (HRE). The study showed that SG600 had an excellent antitumor activity in vitro on HCC cell lines with a different p53 status.
The oncolytic adenovirus SG600 vector which has TERTp, hypoxia and HRE, can replicate in tumor cells exclusively and kill HCC cells and other cancer cells. SG600-IL24 displays a better selective replication and antitumor effect than Ad.IL-24 and can thus be used as an efficient agent in anticancer therapies.
This is a good paper, although only in vitro data are provided.
Peer reviewers: Mark D Gorrell, PhD, Professor, Centenary Institute of Cancer Medicine and Cell Biology, Locked bag No. 6, Newtown, NSW 2042, Australia; Ezio Laconi, MD, PhD, Professor of General Pathology, Department of Sciences and Biomedical Technologies, Unit of Experimental Pathology, University of Cagliari, Via Porcell, 4, IV Piano, 09125 Cagliari, Italy
S- Editor Tian L L- Editor Wang XL E- Editor Lin YP
| 1. | Itamoto T, Nakahara H, Amano H, Kohashi T, Ohdan H, Tashiro H, Asahara T. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2007;141:589-597. |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 3. | Tanaka S, Arii S. Molecularly targeted therapy for hepatocellular carcinoma. Cancer Sci. 2009;100:1-8. |
| 4. | Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477-2486. |
| 5. | Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus WC. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010;70:1120-1129. |
| 6. | Park MA, Walker T, Martin AP, Allegood J, Vozhilla N, Emdad L, Sarkar D, Rahmani M, Graf M, Yacoub A. MDA-7/IL-24-induced cell killing in malignant renal carcinoma cells occurs by a ceramide/CD95/PERK-dependent mechanism. Mol Cancer Ther. 2009;Epub ahead of print. |
| 7. | Hamed HA, Yacoub A, Park MA, Eulitt P, Sarkar D, Dimitriev IP, Chen CS, Grant S, Curiel DT, Fisher PB. OSU-03012 enhances Ad.mda-7-induced GBM cell killing via ER stress and autophagy and by decreasing expression of mitochondrial protective proteins. Cancer Biol Ther. 2010;9:Epub ahead of print. |
| 8. | Maarof G, Bouchet-Delbos L, Gary-Gouy H, Durand-Gasselin I, Krzysiek R, Dalloul A. Interleukin-24 inhibits the plasma cell differentiation program in human germinal center B cells. Blood. 2010;115:1718-1726. |
| 9. | Los M. New, exciting developments in experimental therapies in the early 21st century. Eur J Pharmacol. 2009;625:1-5. |
| 10. | Wang C, Xue X, Yi J, Wu Z, Chen K, Zheng J, Ji W, Yu Y. Replication-incompetent adenovirus vector-mediated MDA-7/IL-24 selectively induces growth suppression and apoptosis of hepatoma cell Line SMMC-7721. J Huazhong Univ Sci Technolog Med Sci. 2008;28:80-83. |
| 11. | Wei N, Fan JK, Gu JF, Liu XY. Double-regulated oncolytic adenovirus-mediated interleukin-24 overexpression exhibits potent antitumor activity on gastric adenocarcinoma. Hum Gene Ther. 2010;21:855-864. |
| 12. | Wang CJ, Xue XB, Yi JL, Chen K, Zheng JW, Wang J, Zeng JP, Xu RH. Melanoma differentiation-associated gene-7, MDA-7/IL-24, selectively induces growth suppression, apoptosis in human hepatocellular carcinoma cell line HepG2 by replication-incompetent adenovirus vector. World J Gastroenterol. 2006;12:1774-1779. |
| 13. | Mahasreshti PJ, Kataram M, Wu H, Yalavarthy LP, Carey D, Fisher PB, Chada S, Alvarez RD, Haisma HJ, Dent P. Ovarian cancer targeted adenoviral-mediated mda-7/IL-24 gene therapy. Gynecol Oncol. 2006;100:521-532. |
| 14. | Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R. Activation of the Fas-FasL signaling pathway by MDA-7/IL-24 kills human ovarian cancer cells. Cancer Res. 2005;65:3017-3024. |
| 15. | Kawabe S, Nishikawa T, Munshi A, Roth JA, Chada S, Meyn RE. Adenovirus-mediated mda-7 gene expression radiosensitizes non-small cell lung cancer cells via TP53-independent mechanisms. Mol Ther. 2002;6:637-644. |
| 16. | Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther. 2004;9:510-518. |
| 17. | McKenzie T, Liu Y, Fanale M, Swisher SG, Chada S, Hunt KK. Combination therapy of Ad-mda7 and trastuzumab increases cell death in Her-2/neu-overexpressing breast cancer cells. Surgery. 2004;136:437-442. |
| 18. | Lebedeva IV, Su ZZ, Sarkar D, Gopalkrishnan RV, Waxman S, Yacoub A, Dent P, Fisher PB. Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene. 2005;24:585-596. |
| 19. | Yacoub A, Mitchell C, Hong Y, Gopalkrishnan RV, Su ZZ, Gupta P, Sauane M, Lebedeva IV, Curiel DT, Mahasreshti PJ. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther. 2004;3:739-751. |
| 20. | Lebedeva IV, Su ZZ, Sarkar D, Kitada S, Dent P, Waxman S, Reed JC, Fisher PB. Melanoma differentiation associated gene-7, mda-7/interleukin-24, induces apoptosis in prostate cancer cells by promoting mitochondrial dysfunction and inducing reactive oxygen species. Cancer Res. 2003;63:8138-8144. |
| 21. | Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, Wang Y, Zhang J, Zhang Z, Huiwang J. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther. 2005;16:845-858. |
| 22. | Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708-718. |
| 23. | Huang EY, Madireddi MT, Gopalkrishnan RV, Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ, Alexandre D. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051-7063. |
| 24. | Gopalkrishnan RV, Sauane M, Fisher PB. Cytokine and tumor cell apoptosis inducing activity of mda-7/IL-24. Int Immunopharmacol. 2004;4:635-647. |
| 25. | Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, Yacoub A, Valerie K, Dent P, Fisher PB. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164-1180. |
| 26. | Zhang ZL, Zou WG, Luo CX, Li BH, Wang JH, Sun LY, Qian QJ, Liu XY. An armed oncolytic adenovirus system, ZD55-gene, demonstrating potent antitumoral efficacy. Cell Res. 2003;13:481-489. |
| 27. | Crompton AM, Kirn DH. From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets. 2007;7:133-139. |
| 29. | Su CQ, Wang XH, Chen J, Liu YJ, Wang WG, Li LF, Wu MC, Qian QJ. Antitumor activity of an hTERT promoter-regulated tumor-selective oncolytic adenovirus in human hepatocellular carcinoma. World J Gastroenterol. 2006;12:7613-7620. |
| 30. | Luo J, Xia Q, Zhang R, Lv C, Zhang W, Wang Y, Cui Q, Liu L, Cai R, Qian C. Treatment of cancer with a novel dual-targeted conditionally replicative adenovirus armed with mda-7/IL-24 gene. Clin Cancer Res. 2008;14:2450-2457. |
| 31. | Sarkar D, Su ZZ, Park ES, Vozhilla N, Dent P, Curiel DT, Fisher PB. A cancer terminator virus eradicates both primary and distant human melanomas. Cancer Gene Ther. 2008;15:293-302. |
| 32. | Eager R, Harle L, Nemunaitis J. Ad-MDA-7; INGN 241: a review of preclinical and clinical experience. Expert Opin Biol Ther. 2008;8:1633-1643. |
| 33. | Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience (Review). Int J Oncol. 2007;31:985-1007. |
| 34. | Yang J, Qin LX, Li Y, Ye SL, Liu YK, Gao DM, Chen J, Tang ZY. Molecular cytogenetic characteristics of the human hepatocellular carcinoma cell line HCCLM3 with high metastatic potential: comparative genomic hybridization and multiplex fluorescence in situ hybridization. Cancer Genet Cytogenet. 2005;158:180-183. |
| 35. | Tian B, Li Y, Ji XN, Chen J, Xue Q, Ye SL, Liu YK, Tang ZY. Basement membrane proteins play an active role in the invasive process of human hepatocellular carcinoma cells with high metastasis potential. J Cancer Res Clin Oncol. 2005;131:80-86. |
| 36. | Ding SJ, Li Y, Shao XX, Zhou H, Zeng R, Tang ZY, Xia QC. Proteome analysis of hepatocellular carcinoma cell strains, MHCC97-H and MHCC97-L, with different metastasis potentials. Proteomics. 2004;4:982-994. |