INTRODUCTION
Barrett’s esophagus (BE) develops as a consequence of long standing gastro-esophageal reflux diseases (GERD) and is characterized by replacing the distal stratified squamous epithelium by columnar lined mucosa containing specialized intestinal metaplasia (SIM). The diagnosis of Barrett’s is established with endoscopic presence of salmon colored mucosa proximal to the gastric folds and associated histopathological examination confirming intestinal metaplasia. BE is a pre-malignant condition which predisposes to the development of esophageal adenocarcinoma (EAC). These patients carry a cancer risk that is 30-125 times higher than that of an age-matched population[1]. The metaplastic epithelium acquires genetic changes over a period of time and malignant transformation occurs in a stepwise manner progressing through low grade dysplasia (LGD), high grade dysplasia (HGD), and finally cancer[2]. Medical and surgical therapy for GERD has not been shown to prevent development of EAC or dysplasia. However, some observational studies suggested that the use of proton pump inhibitors decreased the incidence of dysplasia[3]. In the absence of any preventive strategy, regular surveillance to identify early neoplasia is the most pragmatic approach and hence most of the international gastroenterological societies advise surveillance programmes in patients with BE[4]. Endoscopic surveillance can detect curable early neoplasia, and asymptomatic cancers discovered during surveillance are less advanced than those found in patients who present with cancer symptoms, such as dysphagia and weight loss[5,6]. Early neoplastic lesions can be treated by endotherapy, avoiding the morbidity and mortality associated with major surgery. In the absence of mucosal abnormalities, random four quadrant biopsies every 1-2 cm is the standard practice; however the yield of dysplasia with such a labor intensive endoscopic biopsy protocol is suboptimal[7,8].
HGD and early cancer are often difficult to identify, as many of them will be flat lesions with no obvious mucosal irregularity or nodules. The use of mucolytic agents, such as N-Acetyl Cysteine (NAC), has been shown to improve visibility during endoscopy[9] and should be considered in all cases undergoing a thorough mucosal examination. In this article we will address newer imaging modalities that have been developed with the hope of improving dysplasia detection.
ERA OF ENDOSCOPIC TECHNOLOGICAL ADVANCES
Conventional video endoscopes have a focal distance between 1 and 2 cm from the tip of the endoscopes and use less than 200 000 pixels to construct an image. A close examination of the area of interest would be compromised due to blurring of image if moved close to the mucosa. Technological advances over the past decade have allowed enhancement of the endoscopic image by increasing the resolution of the charge coupled devices (CCD) and have improved the clarity of the images by using high definition monitors. Currently, endoscopes with integrated zoom lenses and microscopes are available, and with these techniques, tissue can be imaged at the cellular and nuclear levels, which provides in-vivo optical histology. Image enhancement using dye (chromoendoscopy) or optical methods [Narrow Band Imaging (NBI), Fuji Intelligent Chromo Endoscopy (FICE) and I-Scan] could allow improved detection and characterization of dysplastic lesions in BE.
USE OF MAGNIFICATION AND HIGH RESOLUTION ENDOSCOPY IN BE SURVEILLANCE
Optical magnification is closely related to the concept of resolution, which is the ability to discriminate between two points, and in an electronic image, this is the function of the pixel density. Current magnifying, or “zoom”, endoscopes enlarge the image up to 150 fold by optical magnification using a mechanically or electronically movable lens controlled by a lever at the head of the endoscope (optical zoom). This is different to the electronic magnification, where the images are magnified by only up to 1.5 times. Availability of high resolution endoscopes equipped with high density CCD (600 000-1 000 000 pixels) make high magnification possible without loss of resolution. These endoscopes also have variable focal distances, which helps to move the endoscope very close to the mucosal surface, thus providing a magnified image.
Use of magnification with indigocarmine chromoscopy was found to correctly identify specialized intestinal metaplasia (SIM) and high grade dysplasia (HGD)[10]. However, low grade dysplasia (LGD) was shown to have similar patterns to SIM. Various mucosal pit patterns, such as ridged/villous, circular and irregular/distorted patterns were identified in this study[10]. The presence of irregular/distorted patterns were found to be specific for HGD in a later multicenter study by the same investigators[11]. Acetic acid and methylene blue are also used as contrast agents with magnification endoscopy, but the results are inconsistent[12-14]. There was also a high reported inter-observer variability in some studies, questioning the accuracy of these techniques[13]. The role of magnification high resolution white light endoscopy without contrast agents is not well studied in this context.
NARROW BAND IMAGING
NBI is a relatively new technology of image enhanced endoscopy that was first described in 2004 by a Gono et al[15]. In endoscopic systems with NBI, an additional filter is activated by pressing a button on the hand control of the endoscope. This filter narrows the band widths of the emitted blue (440-460 nm) and green light (540-560 nm) and the relative contribution of blue light is increased. By narrowing the bandwidths of blue and green light, the superficial mucosal details are better visualized. Also, the blue light is absorbed by hemoglobin, enabling visualization of superficial vasculature. NBI is user friendly and provides uniform visualization of the endoscopic field without the need for any additional dyes.
NBI could be useful in detecting Barrett’s dysplasia compared to standard resolution white light endoscopy (WLE). In a prospective tandem endoscopy study of 65 patients, higher grades of dysplasia were detected by NBI compared to WLE. NBI directed target biopsies yielded more dysplasia than WLE directed biopsies and the number of biopsies taken by WLE were significantly more than that of NBI[16]. An earlier study by Kara et al[17] compared the dysplasia detection rates of high resolution endoscopy (HRE), indigocarmine chromoscopy (ICC), and NBI with magnification. Targeted biopsies with HRE alone had a sensitivity of 79% in detecting HGD. The addition of chromoscopy and NBI did not improve the yield significantly. The difference in observations could be related to the use of high resolution endoscopy in the latter study compared to standard WLE in the former study. A recent randomized cross-over trial presented as an abstract showed that NBI did not improve dysplasia detection rates on a per-patient analysis, but more neoplastic lesions were detected by NBI[18]. More well-designed studies are necessary to comment on the ability of NBI in detecting dysplastic lesions.
NBI with magnification, however, could help in assessing the micro-structural (pit) and vascular patterns of any suspicious areas detected in the Barrett’s segment. Various studies have identified different pit patterns and capillary patterns in BE[19-21]. Regular pit patterns include round, linear, tubular/ridged, and villous types. Irregular patterns and absent pit patterns are also reported. Micro-vascular patterns are classified as either regular or irregular. The sensitivity and specificity of the irregular micro vascular and pit patterns for prediction of HGD was as high as 90% and 100% in an observational study[19]. Similarly, the villous/ridged/absent pit patterns were thought be highly suggestive of specialized intestinal metaplasia (SIM) and the round patterns associated with columnar lined epithelium[21].
NBI is widely available for clinical use and magnification endoscopes are commercially available. The role of NBI in detecting dysplastic lesions remains controversial, but there are a number of studies that have used NBI to characterize the suspicious lesions[19-23]. These studies have shown good overall accuracy in diagnosing the lesions, especially so in cases of HGD and early cancer depicted by irregular pit patterns and/or vascular patterns. A recent meta-analysis confirmed a high diagnostic accuracy in characterizing HGD using NBI with magnification[24]. This would help in reducing the number of random biopsies and help in targeting lesions. We believe that, by adopting a standardized pit pattern and vascular classification, it is possible to improve the diagnostic accuracy of NBI with magnification in diagnosing dysplasia and SIM.
AUTOFLUORESCENCE ENDOSCOPY
The phenomenon of autofluorescence occurs when a light of shorter wavelength interacts with a tissue containing endogenous fluorophores, which in turn emits light of longer wavelength. A number of biological substances in the gastrointestinal tract, such as collagen, elastin, nicotinamide, and flavins, can act as endogenous fluorophores. Earlier autofluorescence imaging (AFI) systems used fiber optic endoscopes that failed to produce sufficient image quality for clinical utility. However the emergence of high resolution video endoscopy with a second CCD for autofluorescence imaging has made it possible to obtain pseudo-color images with a significant improvement in quality. AFI offers an easy way to distinguish between normal and dysplastic tissue, by combining an autofluorescence image on irradiating with a blue light of wavelength of 390-470 nm. The image of green reflected light depicts the absorbed light of hemoglobin, so that normal tissue appears pale green and dysplastic tissue appears magenta.
The role of AFI in Barrett’s esophagus has been widely studied. One of the earliest studies used a fiber based laser induced fluorescence system, which could be passed through the accessory channel of the endoscopes. Panjehpour et al[25] studied this system in 36 patients with BE. They found that 96% of non-dysplastic Barrett’s was classified as benign and 90% of HGD as pre-malignant. 5-aminolevulinic acid induced protoporphyrin IX fluorescence was found to identify areas of HGD with a modest sensitivity of 70% in a later study[26]. In vitro studies on surgical specimens showed that the highest fluorescence ratio was obtained in areas of adenocarcinoma, compared to dysplastic Barrett’s and non-dysplastic Barrett’s[27]. The next generation of light induced autofluorescence endoscopes (LIFE) was investigated at the turn of this century. In a randomized crossover trial, Kara et al[28] investigated the role of AFI in the detection of dysplasia in BE compared to WLE. The sensitivity of WLE targeted biopsies was better than that of AFI in this study (85% vs 69%)[28]. Thus, AFI did not improve dysplasia detection rates using this system. This resulted in the introduction of a video autofluorescence endoscope, which was studied in 2005 by the same group. Twenty-two patients with HGD were examined with AFI and WLE. AFI detected additional lesions in three patients compared to WLE. The use of AFI was found to be feasible and promising in detecting dysplasia[29].
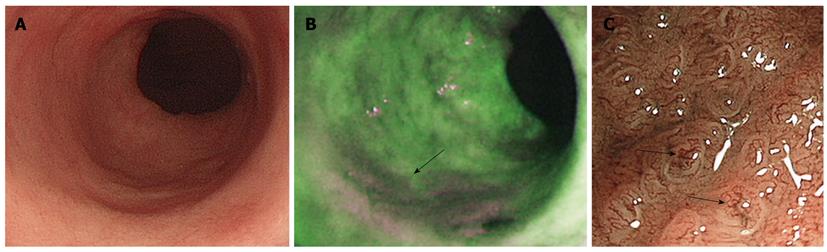
These earlier studies prompted the use AFI as a “red flag” technique to highlight suspicious areas in Barrett’s that could be closely examined with WLE or NBI with and without magnification. One of the disadvantages observed was the high false positive rates for AFI, and it was hypothesized that this could be improved by additional NBI use. Twenty patients with suspected HGD were observed with AFI and suspected areas were examined closely with NBI. All 28 lesions in this cohort were picked up by AFI; however, there was a false positive rate of 40%. This was reduced to 10% by the use of NBI, thus making a combined approach more specific[30]. In a randomized trial comparing AFI and WLE in Barrett’s surveillance patients, AFI was found to improve dysplasia detection rates. However, this did not suggest replacing the standard four quadrant biopsy protocols, because in 11/19 patients, dysplasia was detected only on random sampling[31]. The combined use of WLE, AFI, and NBI is possible with commercially available endoscopes with magnification (Figure 1). The value of this so called ‘trimodal imaging’ was investigated in a multicenter study. AFI was superior to WLE in detecting dysplastic lesions, but the false positive rate was high as reported before (81%). This was reduced to 26% after inspection with NBI[22]. Contrary to these findings, a recent smaller study found that the sensitivity of AFI was suboptimal, but that NBI had a good negative predictive value[32]. Thus, the current available evidence is inconsistent, but on balance, trimodal imaging seems to improve the dysplasia detection rates in BE.
Figure 1 Trimodal imaging of Barrett’s esophagus.
A: High resolution endoscopy showing Barrett’s segment with no conspicuous lesions; B: Autofluorescence imaging shows a low intensity abnormal area in magenta (arrow) suggestive of dysplasia; C: Narrow band imaging with magnification showing irregular vascular patterns (arrows) consistent with dysplasia.
FICE AND I-SCAN
These techniques are based on a new computed spectral estimation technology. FICE (Fujinon endoscopy®) and I-Scan (Pentax Medical®) transforms an ordinary endoscopic image taken from the video processor and arithmetically processes the reflected photons to reconstitute virtual images by increasing the relative intensity of narrowed blue light to a maximum and by decreasing narrowed red and green light to a minimum. This leads to better delineation of microvasculature and mucosal pit patterns due to the differential absorption of light by hemoglobin in the mucosa. A recent study has found that Barrett’s esophagus can be easily diagnosed with FICE compared to standard endoscopy, with a clear demarcation between the Barrett’s segment and gastric mucosa[33]. A randomized crossover trial by Pohl et al[34] compared the accuracy of FICE to acetic acid chromoscopy (AAC) in detection of HGD/early cancer and found that FICE is comparable to AAC. I-Scan was studied in patients with reflux symptoms and was noted to help in identifying reflux associated lesions[35]. More studies are necessary in Barrett’s esophagus to assess the utility of these new techniques.
CONFOCAL LASER ENDOMICROSCOPY
The concept of “optical biopsy” had been achieved in its true sense by the confocal laser endomicroscopy (CLE). An integrated confocal microscope (Pentax Medical®), and a probe based confocal microscope that can be passed through the working channel of an ordinary endoscope (Mauna Kea technologies®) are available commercially. To create confocal images, blue laser light is focused on the desired tissue via the distal end of confocal endoscope. Fluorescent materials are used intravenously, which are excited by laser lights and the confocal optical unit detects this in a defined horizontal level. Extreme magnification (up to 1000 times) is obtained with this technology acquiring images at the cellular/nuclear level, mimicking histopathology sections, thereby allowing targeted biopsy and reducing the number of random biopsies.
During endomicroscopy, the columnar lined epithelium could be easily identified and goblet cells appear as dark cells within the intestinal metaplasia. It is possible to distinguish the gastric type epithelium from the intestinal type, and any suspicious areas could be targeted[36]. CLE was used in Barrett’s esophagus to study the mucosal morphology and predict dysplasia. The sensitivity in predicting intestinal metaplasia and dysplasia compared to targeted histology was 98% and 93%, with a specificity of 94% and 98%[37]. They have proposed a classification for detection of Barrett’s esophagus and associated neoplasia comprising of criteria for vessel and crypt architecture. CLE with optical biopsies or targeted biopsies have been shown to improve the yield of endoscopically inapparent BE dysplasia in a randomized trial, compared to non-targeted biopsies[38]. However, scanning a long segment of Barrett’s with this technique is challenging and may not be appropriate in routine surveillance.
All the above modalities could take significant additional time during the procedure and in routine clinical practice this needs to be considered against resources. In our experience, “trimodal imaging” adds around 5-10 min to routine examination and biopsies. The dysplasia detection studies described are potentially assessing sensitivities of various techniques against four quadrant or targeted biopsies. However, this is not the true sensitivity of the modality, as we know that random biopsies are associated with significant sampling error. Nevertheless, most studies have investigated the additional value of these new techniques over WLE in improving dysplasia detection.
CONCLUSION
In summary, the detection of dysplasia in Barrett’s esophagus has been improved by the newer imaging techniques, such as autofluorescence endoscopy and narrow band imaging. NBI with magnification is particularly useful in characterizing suspicious lesions. However, most of these studies are conducted in centers of excellence and whether similar results could be reproduced in centers with less experience needs to be ascertained. Large randomized trials are necessary before advocating these techniques for routine surveillance. Nevertheless, we believe that a thorough examination using a high resolution endoscope and a high definition monitor with the use of mucolytics should be the minimum standard for routine Barrett’s surveillance.
Peer reviewers: José Liberato Ferreira Caboclo, Professor, Rua Antônio de Godoy, 4120, São José do Rio Preto, Brazil; Marco Giuseppe Patti, MD, Professor of Surgery, Director, Center for Esophageal Diseases, University of Chicago Pritzker School of Medicine, 5841 S. Maryland Avenue, MC 5095, Room G 201, Chicago, IL 60637, United States; Joel H Rubenstein, MD, MSc, Assistant Professor, Division of Gastroenterology, University of Michigan Medical School, 3912 Taubman Center, SPC 5362, Ann Arbor, MI 48109, United States
S- Editor Wang JL L- Editor Stewart GJ E- Editor Ma WH