Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4467
Revised: June 28, 2010
Accepted: July 5, 2010
Published online: September 21, 2010
AIM: To determine the role of diabetes mellitus (DM) and other associated factors in Chinese hepatocellular carcinoma (HCC) patients with cirrhosis, compared with those HCC patients without cirrhosis, in the single setting of hepatitis B virus (HBV) infection, after other known concomitant diseases were excluded.
METHODS: A total of 482 patients, treated at the China-Japan Friendship Hospital, Ministry of Health (Beijing, China), in the period January 2003 to June 2009, and with a hospital discharge diagnosis of HCC, were included. Demographic, clinical, laboratory, metabolic and instrumental features were analyzed.
RESULTS: Of the total, 310 patients were diagnosed with HBV infection and, following the inclusion and exclusion criteria, 224 were analyzed, including 122 patients (54.5%) with cirrhosis (the case group) and 102 patients without cirrhosis (the control group). Twenty-seven patients (12.1%) were diabetic, including 19 in the case group and 8 in the control group (19/122 = 15.6% vs 8/102 = 7.8%, P = 0.077). Thirty-one possible relevant parameters were compared by univariate analysis, and 9 variables were selected for multivariable analysis, including DM (P = 0.077), past history of HBV infection (P = 0.005), total bilirubin (P < 0.001), albumin level (P < 0.001), international normalized ratio (INR) (P < 0.001), alanine aminotransferase (P = 0.050), platelet (P < 0.001), total cholesterol (P = 0.047), and LDL cholesterol (P = 0.002) levels. Diabetes showed a statistical difference by multivariable analysis [odds ratio (OR) 4.88, 95% confidence interval (CI): 1.08-21.99, P = 0.039], although no significant difference was found in univariate analysis. In addition, three cirrhosis-related parameters remained statistically different, including INR (OR 117.14, 95% CI: 4.19-3272.28, P = 0.005), albumin (OR 0.89, 95% CI: 0.80-0.99, P = 0.027), and platelet count (OR 0.992, 95% CI: 0.987-0.999, P = 0.002).
CONCLUSION: Besides the three cirrhosis-related parameters, DM was found to be the sole independent factor associated with HCC in patients with HBV-related cirrhosis, compared with those without cirrhosis.
- Citation: Gao C, Zhao HC, Li JT, Yao SK. Diabetes mellitus and hepatocellular carcinoma: Comparison of Chinese patients with and without HBV-related cirrhosis. World J Gastroenterol 2010; 16(35): 4467-4475
- URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4467
Hepatocellular carcinoma (HCC) is a worldwide malignancy, and the incidence rate has increased significantly over the past two decades in China, Japan, the USA and other countries[1-3]. The reason for this increase in HCC has not yet been explained clearly, although more than 50% of this increase has been attributed to hepatitis virus or alcoholic liver disease, especially to hepatitis C virus (HCV)[1,4]. Risk factors for HCC that have been identified include hepatitis B virus (HBV), HCV, cirrhosis, heavy alcohol consumption, non-alcoholic steatohepatitis (NASH), aflatoxin exposure, increasing age, male sex, and positive family history; however, in 15%-50% of HCC patients no specific risk factor has been found[4-6].
Diabetes mellitus (DM) has been put forward as a potential risk factor for HCC by some studies; however, no consensus has been reached about the “true” role of DM in HCC, at least as a “true” independent risk factor in patients with HBV infection, for example, whether DM itself directly predisposes to HCC[1]. Earlier epidemiologic studies showed no association between HCC and DM[7,8], but some recent studies have identified DM as a risk factor for HCC[4,5,9,10], especially two cohort studies conducted in Sweden and the USA[5,11]. In the Swedish cohort study, 153 852 patients diagnosed with diabetes were identified by use of the Swedish In-patient Register. The observations showed that patients with DM were at increased risk of developing primary liver cancers[11]. This conclusion was supported by another subsequent study conducted in the USA[5].
However, some limitations in these studies, which would influence the interpretation of the results and conclusion, could not be omitted[1]. The first was that the studies were performed by use of specific medical systems and large samples were extracted using ICD-9 codes and computers. The special medical systems functioned for specific people and the study population could not be regarded as representative of the overall population. The second was that the number of studies was very limited and performed in the USA or European countries, not in the areas with higher incidence of HCC, such as China and other Asian countries. The third was that the values of the odds ratio (OR) or relative risk (RR) were very much lower, indicating no strong relationship between DM and HCC, which could be modified by other possible risk factors; for example, some factors related to hepatitis or cirrhosis. The fourth was that healthy or non-liver cancer patients served as controls in almost all of the studies, and thus the possible influencing factors which were related to carcinoma could not be adjusted easily. No information was available about the role of DM in HCC patients with cirrhosis, when HCC patients without cirrhosis served as controls.
Moreover, one recent study which was conducted in the Taiwan province of China showed that neither DM nor being overweight were risk factors for HCC in this area, which has a higher incidence of HCC on the basis of a series of community-based cross-sectional and case-controlled studies[12]. These aforementioned issues indicate that it is not appropriate to draw a final conclusion about the relationship between DM and HCC before related questions are resolved and further related issues should be clarified by future research. Our study was designed to determine: (1) the role of DM and other associated factors in Chinese HCC patients with liver cirrhosis, compared with HCC patients without cirrhosis, in the single setting of HBV infection, after other known concomitant diseases were excluded; (2) the role of some factors associated with the metabolic syndrome in these patients, including body weight, height, serum glucose, blood pressure and blood lipid levels; and (3) any relationships of DM, past history of HBV infection and cirrhosis in these Chinese HCC patients; for example, the influence of courses of HBV infection and DM.
All patients treated at our hospital (China-Japan Friendship Hospital, Ministry of Health, Beijing, China) in the period January 2003 to June 2009, and with a hospital discharge diagnosis of HCC, were included. Those patients fulfilling the diagnostic criteria, inclusion and exclusion criteria were analyzed. HBV infection was defined as serum hepatitis B surface antigen (HBsAg)-positive for at least 6 mo or HBsAg-positive when diagnosed with HCC. Patients fulfilling the following criteria were excluded: (1) confirmed diagnosis of HCC for more than 15 d or had been treated at entry; (2) duration of DM was less than 1 year before confirmed diagnosis of HCC; (3) confirmed HCV, hepatitis D virus or human immunodeficiency virus infection; (4) heavy alcohol consumption (> 80 g/d in males and > 40 g/d in females for more than 10 years); (5) confirmed exposure to Aspergillus flavus, confirmed diagnosis of drug- or poison-induced liver damage; (6) presence of other malignancies; and (7) presence of hemachromatosis, Wilson’s disease, autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, and beriberoid diseases or allergic disorder. The Human Research Ethics Committee at the China-Japan Friendship Hospital approved the study and our study was carried out in accordance with the Helsinki Declaration.
The diagnosis of HCC was histologically confirmed by needle biopsy or based on the findings of typical radiological features in at least two image examinations, including ultrasound scan (US), contrast-enhanced dynamic computerized tomography (CT), magnetic resonance imaging (MRI), and hepatic angiography (showing tumor stain), or by a single positive imaging technique associated with serum α-fetoprotein (AFP) level > 400 ng/mL[13]. Liver cirrhosis was diagnosed based on the histological findings of needle biopsy, or the findings of typical radiological features in at least two image examinations (a non-homogenous hepatic texture or surface, rarefied hepatic central vein, an enlarged caudate lobe, splenomegaly, or collateral veins), or by a single image technique associated with typical manifestations of decompensated liver function and portal hypertension (jaundice, spider angiomata, caput medusae, palmar erythema, anorexia, fatigue, weight loss, anemia, leucopenia, thrombocytopenia, increased liver enzymes, lowered albumin, elevated serum bilirubin or prolonged prothrombin time)[6]. The definition of DM was a fasting plasma glucose level of 126 mg/dL or greater on at least two occasions, plasma glucose of 200 mg/dL or greater at 2 h for a 75-g oral glucose tolerance test (OGTT), or the need for insulin or an oral hypoglycemic drug to control glucose levels. These data were re-evaluated carefully by at least two authors.
The possibly relevant demographic, clinical, laboratory, metabolic and instrumental features of patients were recorded, including age, sex, past history of HBV infection, history of cirrhosis and diabetes, body weight, height, systolic blood pressure (SBP), diastolic blood pressure (DBP), smoking, alcohol consumption, presence of hepatitis B e antigen (HBeAg), hepatitis B virus load (HBV-DNA), AFP, blood glucose, total bilirubin (TBil), albumin, international normalized ratio (INR), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), platelet count, triglycerides (TG), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, creatinine (Cr) and blood urea nitrogen (BUN).
These data were obtained when patients were diagnosed with HCC, and data obtained 15 d before or after diagnosis of HCC were excluded. If the same patient was admitted to our hospital for more than one time, the data from the first time of hospitalization were recorded; if multiple values for one parameter could be obtained from one hospitalization, the first measured or detected value was regarded as the final value. Patients having missing values which would play some role for statistical results would be excluded from the study population. Body-mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. Overweight was defined as BMI ≥ 23 kg/m2, and obesity BMI ≥ 25 kg/m2, according to the Asian and Chinese criteria. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg, and mean artery pressure (MAP) was calculated as 1/3 SBP plus 2/3 DBP. Child-Pugh score were calculated based on 5 associated parameters, including ascites, hepatic encephalopathy, TBil, serum albumin and INR.
Information regarding smoking and alcohol consumption was obtained from the medical records; those smoking less than one pack of cigarettes in a week were regarded as non-smoking; those whose alcohol consumption was < 140 g/wk in males and < 70 g/wk in females were regarded as non-alcohol use; those having stopped smoking or alcohol use for more than 5 years were also considered as non-smoking or non-alcohol use. The findings from physical examinations and image techniques, including ultrasound scan, contrast-enhanced dynamic CT, MRI, and hepatic angiography, were re-assessed carefully for clinical classification, clinical stage and tumor nodus metastasis (TNM) stage. The criteria for classification or stages were derived from the anticancer committee of China and the International Union against Cancer. For the clinical classification, massive-type HCC was defined as the diameter of carcinoma ≥ 5 cm; nodular-type HCC was defined as the diameter < 5 cm; small-type HCC was defined as the diameter < 3 cm for one single or two nodules.
In one case-control study conducted in the USA, 823 patients were included and the incidence rate of DM was 33%. However, among the HCC patients, 34% had HCV infection and 47% had alcoholic cirrhosis[14]. Considering the effect of HCV and alcohol intake on diabetes and cirrhosis, we assessed that the incidence rate of DM in HCC patients with HBV-related cirrhosis should be reduced to less than 30%. Our deduction was supported by two studies performed in Japan[15] and Taiwan (China)[16]. These studies showed that 40 (16%) of 245 HCC patients and 92 (15.8%) of 581 patients were diabetic. Taken together[1,9], we assumed that the incidence rate of DM in HCC patients with HBV-related cirrhosis would be 15%, whereas the rate in patients without cirrhosis would be 5%. With 80% power and at a 5% significance level, we calculated a sample size of 187 patients would be needed (94 in each group).
The analysis was performed using SPSS for Windows, version 13.0 (SPSS, Chicago, IL, USA). For continuous variables with normal curve distribution, mean ± SD was described and Independent-Samples t-test was used. For continuous variables with skewed distribution, the medians and inter-quartile ranges were described and Mann-Whitney non-parametric U-test was used. For the categorical variables, the numbers and proportions of patients in each group were described, and Pearson χ2 test, continuity correction χ2 tests or Fisher’s exact test were used. P < 0.05 was considered statistically significant and selected for multivariable analysis, including DM and ALT with marginal differences.
Based on the results from univariable analysis, nine variables were included in the unconditional logistic regression analysis, including DM, presence of past history of HBV infection, TBil, albumin, INR, ALT, platelet count, total cholesterol, and LDL cholesterol. For better understanding of the role of Child-Pugh score, TBil, albumin and INR were included in the multivariable analysis as 3 independent variables, and then Child-Pugh score was excluded for dependence of variables. We expressed results as odds ratios (ORs) and their 95% confidence intervals (CIs). For all tests, P < 0.05 was considered statistically significant and all P values quoted are two-sided.
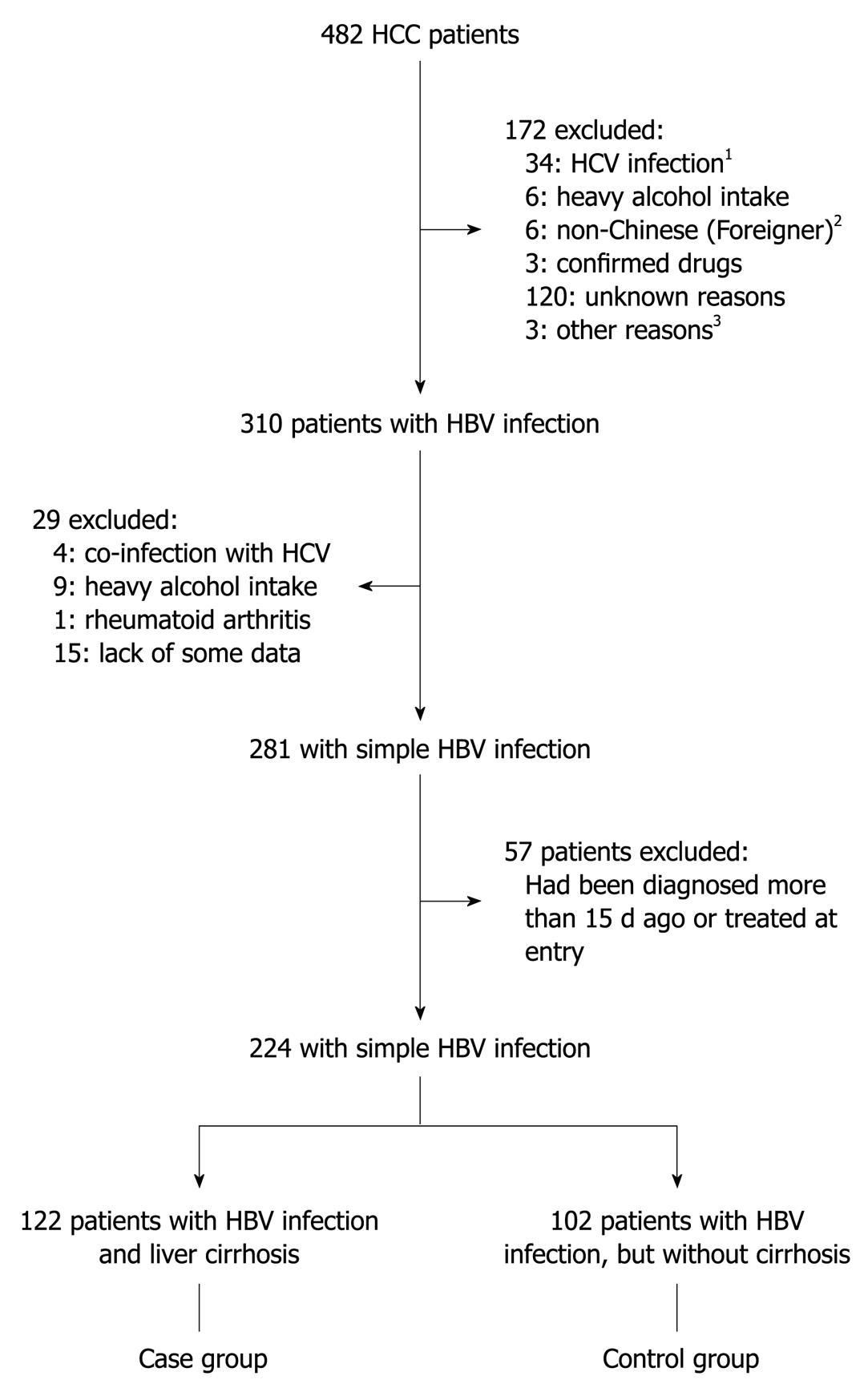
A total of 482 HCC patients were treated at our hospital in the study period and 258 were excluded for the following reasons (Figure 1): 38, HCV infection (4 co-infection with HBV); 15, heavy alcohol consumption (9 in the HBV infection group); 120, unknown reasons (cryptogenic); 6, non-Chinese (foreigners); 3, confirmed drug- or poison-induced liver damage; 57, confirmed diagnosis of HCC for more than 15 d or had been treated at entry; 15, lack of some data which influenced the statistical analysis; and 4, other reasons. Of the total, 310 patients were diagnosed with HBV infection and 224 patients were subsequently analyzed, including 122 patients (54.5%) with cirrhosis (the case group) and 102 patients without cirrhosis (the control group).
Table 1 shows the demographic, clinical, laboratory, metabolic and instrumental features of the 224 HCC patients. Of them, 27 patients (12.1%) were diabetic [12 patients had been excluded because they were diagnosed at the same time of diagnosis of HCC in 10 patients and diagnosed for less than 1 year before the diagnosis of HCC in 2 patients, (27 + 12)/224 = 17.4%]; 173 patients (77.2%) had confirmed past history of HBV infection, and pathological diagnosis from needle biopsy was obtained from 32 patients (18/122 = 14.8% vs 14/102 = 13.7%, P > 0.05). The mean age of the 224 HCC patients was 54.6 (± 10.7) years old and 190 patients (84.8%) were male sex. Ninety-five patients (95/162 = 58.6%) were overweight or obese, and 59 patients (26.3%) had hypertension.
| Variable | HCC patients (n = 224)1 |
| Mean age (yr) | 54.6 ± 10.7 |
| Male sex | 190 (84.8) |
| Liver cirrhosis | 122 (54.5) |
| Diabetes mellitus | 27 (12.1) |
| Overweight or obesitya | 95 (58.6) |
| Hypertension | 59 (26.3) |
| Body weight (kg) | 68.8 ± 11.0 |
| Body heighta (cm) | 169.4 ± 6.4 |
| Mean body mass indexa (kg/m2) | 23.9 ± 3.4 |
| Systolic blood pressure (mmHg) | 128 ± 16 |
| Diastolic blood pressure (mmHg) | 79 ± 9 |
| Mean artery pressure (mmHg) | 95 ± 10 |
| Past history of HBV infection | 173 (77.2) |
| Smoking | 99 (44.2) |
| Alcohol intake | 69 (30.8) |
| Positive for HBeAgb | 52 (26.1) |
| HBV-DNAc, Log10 | 5.32 ± 1.40 |
| AFPd, > 400 ng/mL | 110 (50.9) |
| Child-Pugh Score2 | 6 (5-8) |
| Blood Glucose (mmol/L) | 5.88 ± 2.22 |
| Total Bilirubin2 (mg/L) | 13 (8-23) |
| Albumin level (g/L) | 36.6 ± 6.3 |
| International normalized ratio | 1.22 ± 0.24 |
| Alanine aminotransferase2 (U/L) | 53 (34-83) |
| γ-glutamyl transferase2 (U/L) | 113 (58-254) |
| Platelet count2 (× 109/L) | 125 (81-183) |
| Triglyceridese (mmol/L) | 1.02 ± 0.52 |
| Total cholesterole (mmol/L) | 4.27 ± 1.33 |
| HDL cholesterolf (mmol/L) | 0.93 ± 0.44 |
| LDL cholesterolf (mmol/L) | 2.44 ± 1.06 |
| Creatinine2 (mg/dL) | 0.9 (0.8-1.0) |
| Blood urea nitrogen2 (mmol/L) | 4.98 (3.92-6.28) |
The clinical classification, clinical stage and TNM stage are shown in Table 2. Regarding the clinical classification, massive-type HCC (134, 59.8%) and nodular-type HCC (61, 27.2%) were the two major types, which accounted for nearly 90% of the total patients. The massive-type HCC was more likely to appear in HCC patients without cirrhosis (52/122 = 42.6% vs 82/102 = 80.4%, P < 0.001), whereas the nodular-type HCC was more likely to appear in the group with cirrhosis (49/122 = 40.2% vs 12/102 = 11.8%, P < 0.001). Regarding the clinical stage, a statistical difference between groups was only found for stage II (44/122 = 36.1% vs 51/102 = 50.0%, P = 0.036). Regarding TNM stage, a difference was observed for T stage, whereas no differences were shown for N stage or M stage.
| Total patients (n = 224) | Patients with LC (n = 122) | Patients without LC (n = 102) | P value1 | |
| Clinical classification | ||||
| Massive-type | 134 (59.8) | 52 (42.6) | 82 (80.4) | < 0.001 |
| Nodular-type | 61 (27.2) | 49 (40.2) | 12 (11.8) | < 0.001 |
| Small-type | 15 (6.7) | 13 (10.7) | 2 (2.0) | 0.010 |
| Diffuse-type | 14 (6.3) | 8 (6.6) | 6 (5.9) | 0.835 |
| Clinical Stage | ||||
| Stage I | 15 (6.7) | 9 (7.4) | 6 (5.9) | 0.656 |
| Stage II | 95 (42.4) | 44 (36.1) | 51 (50.0) | 0.036 |
| Stage III | 114 (50.9) | 69 (56.6) | 45 (44.1) | 0.064 |
| TNM stage | ||||
| Stage I | 11 (4.9) | 8 (6.6) | 3 (2.9) | 0.212 |
| Stage II | 43 (19.2) | 34 (27.9) | 9 (8.8) | < 0.001 |
| Stage III | 76 (33.9) | 30 (24.6) | 46 (45.1) | 0.001 |
| Stage IV | 94 (42.0) | 50 (41.0) | 44 (43.1) | 0.745 |
| T Stage | ||||
| Stage 1-2 | 81 (36.2) | 61 (50.0) | 20 (19.6) | < 0.001 |
| Stage 3-4 | 143 (63.8) | 61 (50.0) | 82 (80.4) | - |
| N Stage | ||||
| Stage 0 | 194 (86.6) | 110 (90.2) | 84 (82.4) | 0.087 |
| Stage 1 | 30 (13.4) | 12 (9.8) | 18 (17.6) | - |
| M stage | ||||
| Stage 0 | 130 (58.0) | 72 (59.0) | 58 (56.9) | 0.745 |
| Stage 1 | 94 (42.0) | 50 (41.0) | 44 (43.1) | - |
Of the 27 diabetic patients, 19 patients were in the case group and 8 patients in the control group (19/122 = 15.6% vs 8/102 = 7.8%, P = 0.077): not significant, but a marginal difference was shown for DM by Pearson Chi-Square test. In univariable analysis (Table 3), compared with HCC patients without cirrhosis, HCC patients with cirrhosis had a higher percentage of past history of HBV infection (P = 0.005), higher Child-Pugh score (P < 0.001), higher total biliribin (P < 0.001), lower albumin level (P < 0.001), prolonged prothrombin time/INR (P < 0.001), lower platelet count (P < 0.001), lower total cholesterol (P = 0.047), and lower LDL cholesterol (P = 0.002). In addition, a marginal statistical difference was found for the comparison of serum ALT level (P = 0.050). No statistical differences were shown for the other 21 variables.
| Variable | Patients with LC1 (n = 122) | Patients without LC1 (n = 102) | P value2 |
| Mean age (yr) | 54.7 ± 10.0 | 54.6 ± 11.4 | 0.909 |
| Male sex | 106 (86.9) | 84 (82.4) | 0.346 |
| Diabetes mellitus | 19 (15.6) | 8 (7.8) | 0.077 |
| Overweight or obesitya | 54 (60.7) | 41 (56.2) | 0.562 |
| Hypertension | 33 (27.0) | 26 (25.5) | 0.792 |
| Body weight (kg) | 69.7 ± 11.4 | 67.7 ± 10.4 | 0.168 |
| Body heighta (cm) | 169.4 ± 6.2 | 169.5 ± 6.6 | 0.912 |
| Mean body mass indexa (kg/m2) | 24.1 ± 3.6 | 23.7 ± 3.1 | 0.441 |
| Systolic blood pressure (mmHg) | 128 ± 15 | 130 ± 16 | 0.323 |
| Diastolic blood pressure (mmHg) | 78 ± 9 | 79 ± 9 | 0.255 |
| Mean artery pressure (mmHg) | 95 ± 10 | 96 ± 10 | 0.243 |
| Past history of HBV infection | 103 (84.4) | 70 (68.6) | 0.005 |
| Smoking | 57 (46.7) | 42 (41.2) | 0.405 |
| Alcohol intake | 43 (35.2) | 26 (25.5) | 0.115 |
| Positive for HBeAgb | 32 (29.4) | 20 (22.2) | 0.254 |
| HBV-DNAc, Log10 | 5.35 ± 1.37 | 5.26 ± 1.51 | 0.829 |
| AFPd, > 400 ng/mL | 59 (50.9) | 51 (51.0) | 0.984 |
| Child-Pugh Score3 | 7 (6-10) | 5 (5-6) | < 0.001 |
| Blood glucose (mmol/L) | 5.99 ± 2.44 | 5.73 ± 1.93 | 0.362 |
| Total bilirubin3 (mg/L) | 17 (9-31) | 10 (8-17) | < 0.001 |
| Albumin level (g/L) | 34.8 ± 6.3 | 38.8 ± 5.6 | < 0.001 |
| International normalized ratio | 1.31 ± 0.27 | 1.12 ± 0.15 | < 0.001 |
| Alanine aminotransferase3 (U/L) | 58 (37-90) | 45 (30-81) | 0.050 |
| γ glutamyl transferase3 (U/L) | 110 (52-257) | 117 (61-257) | 0.816 |
| Platelet count3 (× 109/L) | 94 (61-152) | 162 (115-217) | < 0.001 |
| Triglyceridese (mmol/L) | 1.02 ± 0.54 | 1.02 ± 0.49 | 0.995 |
| Total cholesterole (mmol/L) | 4.08 ± 1.44 | 4.53 ± 1.13 | 0.047 |
| HDL cholesterolf (mmol/L) | 0.89 ± 0.48 | 0.99 ± 0.38 | 0.152 |
| LDL cholesterolf (mmol/L) | 2.21 ± 1.10 | 2.78 ± 0.92 | 0.002 |
| Creatinine3 (mg/dL) | 0.9 (0.8-1.0) | 0.9 (0.8-1.0) | 0.839 |
| Blood urea nitrogen3 (mmol/L) | 4.95 (3.85-6.32) | 5.02 (3.96-6.19) | 0.927 |
Based on the results from univariable analysis, nine variables were included in the unconditional logistic regression analysis, including DM, presence of past history of HBV infection, TBil, albumin, INR, ALT, platelet count, total cholesterol, and LDL cholesterol levels. For better understanding of the role of Child-Pugh score, TBil, albumin and INR were included as three independent variables and Child-Pugh score was excluded for dependence of variables. In multivariable analysis (Table 4), diabetes mellitus (OR 4.88, 95% CI: 1.08-21.99, P = 0.039), INR (OR 117.14, 95% CI: 4.19-3272.28, P = 0.005), albumin level (OR 0.89, 95% CI: 0.80-0.99, P = 0.027), and platelet count (OR 0.992, 95% CI: 0.987-0.999, P = 0.002) showed statistical differences, whereas the other five variables did not differ significantly.
Factors associated with metabolic syndrome in our study included DM, overweight or obesity, hypertension, triglycerides, and HDL cholesterol. Because waist circumference measurements could not be obtained, total cholesterol and LDL cholesterol levels were included in the final analysis. DM has been described and will be omitted in this section. Univariable analysis (Table 3) showed that significant differences were found for the total cholesterol and LDL cholesterol levels (P = 0.047 and P = 0.002, respectively). Multivariable analysis (Table 4) showed no variables remained statistically different, indicating that the statistical differences in the univariable analysis were probably modified by the presence of diabetes mellitus and/or the factors associated with HBV-related cirrhosis (INR, platelet count and albumin).
Our study was designed to determine the role of DM and other associated factors in Chinese HCC patients with liver cirrhosis, compared with those HCC patients without cirrhosis, in the setting of HBV infection. Next, we further demonstrated some relationships between DM, HBV infection and cirrhosis in the HCC patients. Of the 224 HCC patients, 173 patients had a confirmed past history of HBV infection (103/122 = 84.4% vs 70/102 = 68.6%, P = 0.005), indicating the presence of past history of HBV infection was more likely to occur in those HCC patients with hepatic cirrhosis. Only a marginal statistical difference (P = 0.06) was observed when the courses of HBV infection were compared between the two groups. However, no significant difference (P = 0.798) was found after the 51 patients without past history of HBV infection were excluded. Of the 27 diabetics, 19 patients (70.4%) were in the case group, and 14 of the 19 patients (73.7%) were diagnosed with DM before the diagnosis of cirrhosis, whereas the remaining 5 patients were diagnosed after cirrhosis. When the courses of DM between the 2 groups were compared in these 27 patients, no significant difference (P = 0.389) was found.
No consensus has been reached about the role of DM in HCC, at least as a “true” independent risk factor in patients with HBV infection, for example, whether DM itself directly predisposes to HCC. Our study provided some answers for this question and showed: (1) besides the 3 cirrhosis-related parameters (INR, albumin and platelet count), DM was found to be the sole factor associated with HCC in Chinese patients with cirrhosis, compared with those HCC patients without cirrhosis, in the single setting of HBV infection; (2) some available factors associated with metabolic syndrome (MS), apart from DM, produced no obvious effects in these patients, indicating that the role of DM was independent of other factors of MS; and (3) between the 2 groups, a marginal statistical difference was observed for the courses of past HBV infection whereas no significant difference was found for the courses of DM.
Our positive conclusion that DM is an independent associated factor for HCC has been put forward by some previous studies[1,4,9,10], however, we answered this question from another viewpoint; based on our available literature and current knowledge, this was the first time that HCC patients, not healthy or non-liver cancer patients, served as controls. Therefore, some possible factors associated with carcinoma could be controlled easily. To address this question, we included a new patient cohort: hospital-based Chinese HCC patients with HBV-related cirrhosis were studied. Although the entire study population came from one single tertiary referral hospital and the overall numbers were not very large, we provided enough numbers of patients based on our sample estimation for statistical analysis. Moreover, one of our big strengths was the strict inclusion and exclusion criteria, allowing some potential influencing factors to be controlled. Maybe it was because of the number of patients and samples that a marginal statistical difference was found for the comparison of DM in the univariable analysis and a very wide 95% CI was observed in the multivariable analysis. We hope our results and conclusion will be validated in greater numbers of patients and more referral hospitals.
Cirrhosis is one of the most important risk factors for HCC; to determine the role of DM in HCC, comparison of the cirrhotic patients with HCC patients was necessary and we have conducted this comparison (data not shown). Twenty-seven patients (12.1%) were diabetic in our study population which was lower than previous reports and the sample estimation. The reasons were deduced to be as follows: (1) when considering the influence of the course of DM for HCC, 12 diabetic patients were excluded because the diagnosis of DM was less than 1 year before the diagnosis of HCC. In fact, the total number was 39 cases and the overall percentage of diabetic patients was 17.4% which was consistent with other studies; (2) it has been confirmed that HCV and heavy alcohol consumption play important roles in the onset and development of DM, however, these patients had been excluded to control any possible influence; and (3) our diagnosis was mainly based on the serum glucose; OGTT was only used when necessary. Although the aforementioned issues perhaps play some role in affecting the results and conclusion, we provide relatively sufficient evidence to answer the study question, especially as no statistical difference was found for the role of DM in the univariate analysis.
Besides DM, three cirrhosis-related factors were shown to be possible associated factors for HCC in these patients, including prolonged prothrombin time/INR, lower platelet count and lower albumin level. As for other factors, it is well known that increasing age and male sex are risk factors for HCC; however, no differences were found in our study. The reason was deduced to be that similar HCC patients, not non-cancer or non-liver cancer patients, were used as controls and the role of other risk factors was limited.
Non-alcoholic fatty liver disease (NAFLD), including its most severe form, NASH, has been considered as a risk factor for HCC[17-19]. Unfortunately, biopsies were obtained from a minority of our patients (32, 14.3%) because it was unnecessary for most of the confirmed HCC patients, and re-evaluation cannot be performed. Another limitation was that most of the cirrhotic patients were diagnosed clinically rather than on a biopsy and the role of cirrhosis could be underestimated. However, we followed strictly the diagnostic criteria which were recommended by the authorized institutes and used widely in clinical practice. We believed that our results are most likely applicable to clinical practice.
The pathogenesis mechanisms remain unclear and some are suggested as follows. The first is regarding obesity, diabetes and NAFLD in HCC. Obesity can lead to insulin resistance (IR) and steatosis, which are associated with the release of inflammatory mediators and production of cytokines. NASH can result in some typical histologic characteristics[10]. Therefore, diabetes and obesity can cause hepatic inflammation, leading to oxidative stress and lipid peroxidation, resulting in hepatocyte injury and necrosis, and subsequently HCC. The second suggested mechanism is that IR leads to a state of hyperinsulinemia which, via interaction with the insulin receptor, promotes increased phosphorylation and activation of downstream pathways[20]. The latter have been shown to play some role in tumorigenesis by decreasing apoptosis and increasing mitogenesis. The third mechanism involves insulin-like growth factor (IGF) which, while acting through separate binding proteins and receptors, has the same downstream intracellular mediators as the insulin receptor pathway[21,22]. Recent studies have reported altered IGF signaling in 90% of HCCs, including the autocrine production of IGFs, IGF binding proteins, and IGF binding protein proteases as well as IGF receptor expression[23-25]. The clarification of these mechanisms would be helpful to address the question of an association of DM with HCC.
In addition, considering that cirrhotic patients with HCV infection are twice as likely to have type-2 DM than patients with HBV infection[26,27], a very obvious difference was presumed between HCV infection and HBV infection regarding the role of DM in HCC and the reason remains unclear. One study has shown that more severe insulin resistance was present in non-cirrhotic patients with HCV infection than in patients with HBV infection[26]. IR was associated with the presence of serum HCV core antigen, the severity of hepatic fibrosis and decreased expression of hepatic insulin receptor substrate (IRS) 1 and IRS2 in patients with HCV infection. HCV core down-regulated the expression of IRS1 and IRS2 in human hepatoma cell lines as well as in whole animals. These observations suggested that HCV causes changes in specific hepatic molecules regulating glucose metabolism and results in severe insulin resistance. A possible mechanism suggested was that HCV core-induced suppressor of cytokine signaling (SOCS) 3 promotes proteosomal degradation of IRS1 and IRS2 through ubiquitination[26]. However, another study reported an association of exogenous insulin or sulphonylurea treatment with an increased incidence of HCC in patients with HCV infection, especially in those without cirrhosis[27]. These findings make the DM question more difficult to answer and further studies are required.
In addition to the aforementioned issues, some limitations should be acknowledged. The first limitation was due to the nature of our hospital-based case-control study, in that some data could not be obtained and some possible factors could not be adjusted. For example, NAFLD and NASH have been regarded as risk factors for HCC, but we could not assess these changes. Actually, biopsy was unnecessary for these confirmed HCC patients and was not recommended. Secondly, the diagnosis of cirrhosis and HCC was mostly based on imaging findings, which could lead to some underestimation and diagnostic bias. This concern has been addressed above. The third constraint was limited generalizability, because the entire study population came from a single tertiary referral hospital and extending these results to the overall population may be a concern. In addition, some diagnostic parameters were unavailable from the clinical records for those diseases listed in the exclusion criteria. The setting was designed as the HBV infection; however, we believe that, except for HCV and heavy alcohol intake, co-existence with HBV as the cause or risk factor for HCC was limited.
In conclusion, besides the 3 cirrhosis-related parameters (INR, albumin and platelet count), DM was found to be the sole independent associated factor for HCC in Chinese patients with hepatic cirrhosis, compared with those HCC patients without cirrhosis, in the single setting of HBV infection. These patients should be especially closely monitored. Future research should clarify these issues: (1) the basic oncogenic mechanisms by which diabetes “directly” predisposes to HCC, especially in animal models and experiments; (2) the role of DM in the genesis of HCC and the factors involved in its complications; (3) the impact of DM on the natural history of patients with HCC; (4) the impact of early diagnosis and treatment of DM in HCC; and (5) the benefits of controlling DM in the management of HCC and subsequent complications.
Diabetes mellitus has been put forward as a potential risk factor for hepatocellular carcinoma (HCC) by some studies; however, no consensus has been reached about the “true” role of diabetes mellitus (DM) in HCC, at least as a “true” independent factor in patients with hepatitis B virus (HBV) infection.
The authors found that, besides the three cirrhosis-related parameters [international normalized ratio (INR), albumin and platelet count], DM was the sole independent factor for HCC in Chinese patients with cirrhosis, compared with those HCC patients without cirrhosis, in the single setting of HBV infection.
This is the first time that HCC patients, not healthy or non-liver cancer patients, served as controls, and some possible factors associated with carcinoma could thus be controlled easily.
HCC patients with diabetes should be especially closely monitored and future research should clarify the associated issues.
In this paper, Gao et al examined the role of diabetes mellitus and other associated factors in Chinese HCC patients with cirrhosis, compared with those HCC patients without cirrhosis, in the single setting of HBV infection. They found that besides the three cirrhosis-related parameters (INR, album in and platelet count), DM was the sole independent factor associated with HCC in patients with HBV-related cirrhosis. The presented data are excellent.
Peer reviewers: Astrid van der Velde, PhD, Team Wetenschap, Netherlands Heart Foundation, PO Box 300, 2501 CH, The Hague, The Netherlands; Takumi Kawaguchi, MD, PhD, Department of Digestive Disease Information and Research, Kurume University School of Medicine, 67 Asahi-machi, Kurume 830-0011, Japan
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Gao C, Yao SK. Diabetes mellitus: a “true” independent risk factor for hepatocellular carcinoma? Hepatobiliary Pancreat Dis Int. 2009;8:465-473. |
| 2. | Yuen MF, Hou JL, Chutaputti A. Hepatocellular carcinoma in the Asia pacific region. J Gastroenterol Hepatol. 2009;24:346-353. |
| 3. | El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823. |
| 4. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. |
| 5. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. |
| 7. | Kessler II. Cancer mortality among diabetics. J Natl Cancer Inst. 1970;44:673-686. |
| 8. | Lu SN, Lin TM, Chen CJ, Chen JS, Liaw YF, Chang WY, Hsu ST. A case-control study of primary hepatocellular carcinoma in Taiwan. Cancer. 1988;62:2051-2055. |
| 9. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. |
| 10. | Yuan JM, Govindarajan S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009-1017. |
| 11. | Adami HO, Chow WH, Nyrén O, Berne C, Linet MS, Ekbom A, Wolk A, McLaughlin JK, Fraumeni JF Jr. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472-1477. |
| 12. | Tung HD, Wang JH, Tseng PL, Hung CH, Kee KM, Chen CH, Chang KC, Lee CM, Changchien CS, Chen YD. Neither diabetes mellitus nor overweight is a risk factor for hepatocellular carcinoma in a dual HBV and HCV endemic area: community cross-sectional and case-control studies. Am J Gastroenterol. 2010;105:624-631. |
| 13. | Huo TI, Wu JC, Lui WY, Huang YH, Lee PC, Chiang JH, Chang FY, Lee SD. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99:1479-1487. |
| 14. | El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462-2467. |
| 15. | Toyoda H, Kumada T, Nakano S, Takeda I, Sugiyama K, Kiriyama S, Tanikawa M, Sone Y, Hisanaga Y. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer. 2001;91:957-963. |
| 16. | Huo TI, Lui WY, Huang YH, Chau GY, Wu JC, Lee PC, Chang FY, Lee SD. Diabetes mellitus is a risk factor for hepatic decompensation in patients with hepatocellular carcinoma undergoing resection: a longitudinal study. Am J Gastroenterol. 2003;98:2293-2298. |
| 17. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. |
| 18. | Cotrim HP, Paraná R, Braga E, Lyra L. Nonalcoholic steatohepatitis and hepatocellular carcinoma: natural history? Am J Gastroenterol. 2000;95:3018-3019. |
| 19. | Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, Takasaki K, Ludwig J. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37:154-160. |
| 20. | Dellon ES, Shaheen NJ. Diabetes and hepatocellular carcinoma: associations, biologic plausibility, and clinical implications. Gastroenterology. 2005;129:1132-1134. |
| 21. | Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6:103-111. |
| 22. | Lee HC, Tian B, Sedivy JM, Wands JR, Kim M. Loss of Raf kinase inhibitor protein promotes cell proliferation and migration of human hepatoma cells. Gastroenterology. 2006;131:1208-1217. |
| 23. | Nussbaum T, Samarin J, Ehemann V, Bissinger M, Ryschich E, Khamidjanov A, Yu X, Gretz N, Schirmacher P, Breuhahn K. Autocrine insulin-like growth factor-II stimulation of tumor cell migration is a progression step in human hepatocarcinogenesis. Hepatology. 2008;48:146-156. |
| 24. | Jeng YM, Chang CC, Hu FC, Chou HY, Kao HL, Wang TH, Hsu HC. RNA-binding protein insulin-like growth factor II mRNA-binding protein 3 expression promotes tumor invasion and predicts early recurrence and poor prognosis in hepatocellular carcinoma. Hepatology. 2008;48:1118-1127. |
| 25. | Longato L, de la Monte S, Kuzushita N, Horimoto M, Rogers AB, Slagle BL, Wands JR. Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant alterations in the liver. Hepatology. 2009;49:1935-1943. |
| 26. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. |