Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4455
Revised: June 13, 2010
Accepted: June 20, 2010
Published online: September 21, 2010
AIM: To assess the antacid effects of the tonic Chinese herbal prescriptions, Si-Jun-Zi-Tang (SJZT) and Shen-Ling-Bai-Zhu-San (SLBZS).
METHODS: Decoctions of the tonic Chinese herbal prescriptions, SJZT and SLBZS, were prepared according to Chinese original documents. The pH of the prescription decoctions and their neutralizing effects on artificial gastric acids were determined and compared with water and the active controls, sodium bicarbonate and colloidal aluminum phosphate. A modified model of Vatier’s artificial stomach was used to determine the duration of consistent neutralization effect on artificial gastric acids. The neutralization capacity in vitro was determined with the titration method of Fordtran’s model.
RESULTS: The results showed that both SJZT and SLBZS have antacid effects in vitro. Compared with the water group, SJZT and SLBZS were found to possess significant gastric acid neutralizing effects. The duration for consistent neutralization of SLBZS was significantly longer than that of water. Also, SLBZS and SJZT exhibited significant antacid capacities compared to water.
CONCLUSION: SJZT and SLBZS were consistently active in the artificial stomach model and are suggested to have antacid effects similar to the active control drugs.
- Citation: Wu TH, Chen IC, Chen LC. Antacid effects of Chinese herbal prescriptions assessed by a modified artificial stomach model. World J Gastroenterol 2010; 16(35): 4455-4459
- URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4455
Peptic ulcer (PU) is one of the most common gastrointestinal disorders[1-3]. The goals of treatment for PU are to relieve pain, enhance ulcer healing, prevent complications, and prevent ulcer recurrence. Current drug therapy for PU is oriented primarily toward neutralizing (e.g. antacids)[4-6] or reducing the amount of acid secreted (e.g. H2 receptor antagonists, proton pump inhibitors)[7,8] or protecting the gastric mucosa from the effects of acid (e.g. sucralfate)[9]. As the role of Helicobacter pylori is becoming better understood, treatment with antibiotics is becoming an important part of PU therapy and recurrence prevention[10].
Although effectiveness can be obtained with these clinical drugs, their potential side effects and drug interactions represent a major problem in therapy[11]. Moreover, newer drugs introduced for the treatment of PU are expensive and the cost-effectiveness is also an important consideration[12]. Clinically, many people cannot use chemosynthetic drugs because of the potential side effects. Therefore, traditional herbal drugs possessing fewer side effects should be investigated as an ideal alternative for the treatment of PU.
Traditional Chinese medicine has been clinically used in China for thousands of years for the treatment of many chronic diseases. Si-Jun-Zi-Tang (SJZT) is a basic tonic prescription consisting of six herb components: Panax ginseng, Atractylodes ovata, Poria cocos, Glycyrrhiza uralensis, Zingiber officinale, and Zizyphus jujube. Shen-Ling-Bai-Zhu-San (SLBZS) is also an energy tonic prescription consisting of eleven herb components: Atractylodes ovata, Poria cocos, Panax ginseng, Glycyrrhiza uralensis, Dioscorea batatas, Dolichos lablab, Coix lachryma-jobi, Amomum xanthioides, Platycodon grandiflorum, Nelumbo nucifera and Citrus erythrosa. SJZT and SLBZS, the common Chinese herbal prescriptions tonifying the spleen and stomach, are traditionally used for the treatment of gastrointestinal diseases in oriental countries. Their anti-ulcer activities have also been mentioned in recent research.
The secretion of gastric acid (HCl) is intimately related to PU disease. Antacids heal ulcers through elimination of gastric acid by neutralization and have been used in the treatment of PU for many years. Common antacid preparations include sodium bicarbonate (SB), calcium carbonate, and salts of aluminum and magnesium. Since some people cannot use chemosynthetic drugs because of their side effects, the tonic Chinese herbal prescriptions SJZT and SLBZS should be considered as an alternative for the treatment of PU. To reveal the anti-ulcer effects of SJZT and SLBZS, therefore, the present study aimed to assess their antacid effects on gastric acid neutralization compared with water and the active controls, SB and colloidal aluminum phosphate (CAP). The antacid effects were assessed in vitro using the titration method of Fordtran’s model[13], and the modified model of Vatier’s artificial stomach[14-17] was utilized to determine the effects on gastric acid secretion and emptying.
All the Chinese herbal materials used in the study were purchased from the Shun-yuan Herbal Pharmacy, Taipei and authenticated at Taipei Medical University. The prescription SJZT contains the following six herbal components: Panax ginseng, Atractylodes ovata, Poria cocos, Glycyrrhiza uralensis, Zingiber officinale, and Zizyphus jujube (6:6:6:3:3:2). The prescription SLBZS contains the following eleven herbal components: Atractylodes ovata, Poria cocos, Panax ginseng, Glycyrrhiza uralensis, Dioscorea batatas, Dolichos lablab, Coix lachryma-jobi, Amomum xanthioides, Platycodon grandiflorum, Nelumbo nucifera and Citrus erythrosa (3:3:3:3:3:2.3:1.5:1.5:1.5:1.5:1.5). The prescriptions were prepared according to Chinese original documents. Prepared daily quantities of SJZT and SLBZS weighed 26 g and 24.8 g, respectively. The herb materials weighed as a daily quantity were extracted twice by refluxing with boiling water (1:20, w/v) for 1 h. The decoction was filtered and then concentrated to 270 mL. Concentrations of SJZT and SLBZS decoctions were 96.30 mg/mL and 91.85 mg/mL, respectively. The volume of each test dose was 90 mL.
Sodium chloride and pepsin were purchased from Sigma (St. Louis, MO, USA) and 1 mol/L hydrochloric acid was obtained from Merck (Darmstadt, Germany). SB and CAP, the active control drugs, were purchased from Astar Pharmaceutical Co. Ltd (Taipei, Taiwan).
The experimental instruments consisted of an adjustable electrode stand, a series flatbed recorder, a micro tubing pump, a standard pH meter, a stirrer/hot plate, and a series multi-functional temperature controller.
Two grams of salt and 3.2 mg of pepsin enzymes were dissolved in 500 mL water; 7.0 mL hydrochloric acid and adequate water were added to make a 1000 mL solution of the artificial gastric acid at pH 1.20. Nine grams of sodium chloride were dissolved in adequate water to make 1000 mL saline.
One dose of prescription decoction (90 mL) was used for the pH determination at temperatures ranging from 25°C to 37°C. The pH values of the control solutions, SB and CAP, were also determined for comparison.
Ninety milliliters of each test solution was added to 100 mL artificial gastric juices at pH 1.2. The pH values were determined to examine the neutralizing effect.
As previously described, the apparatus is made up of three elements: a pH recording system (R), a stomach (S) and a peristaltic pump (P). The stomach is made up of three portions, S1, S2 and S3. S1 is a reservoir (container), S2 models the secretory flux (F-IN), and S3 models the gastric emptying flux (F-OUT).
Ninety milliliters of each test sample was added to 100 mL of artificial gastric juice at pH 1.2 in the container of the artificial stomach at 37°C and continuously stirred (30 rpm) with a 2.5-cm magnetic stirring apparatus. Artificial gastric juice at pH 1.2 was pumped at 3 mL/min into the container of the artificial stomach, and it was pumped out at 3 mL/min at the same time. A pH meter was connected to continuously monitor the changes of pH in the container of the artificial stomach. The duration of neutralization effect was determined when the pH value was returned to its initial value (pH 1.2).
Ninety milliliters of the test sample was placed in a 250-mL beaker and warmed to 37°C. A magnetic stirrer was continuously run at 30 rpm to imitate the stomach movements. The test samples were titrated with artificial gastric juice to the end point of pH 3. The consumed volume (V) of artificial gastric juice was measured. The total consumed hydrogen ion (mmol) was measured as 0.063096 (mmol/mL) × V (mL).
Experimental data were expressed as mean ± SD. Comparisons between groups were analyzed by unpaired Student’s t-test. The differences were considered to be statistically significant when P < 0.05.
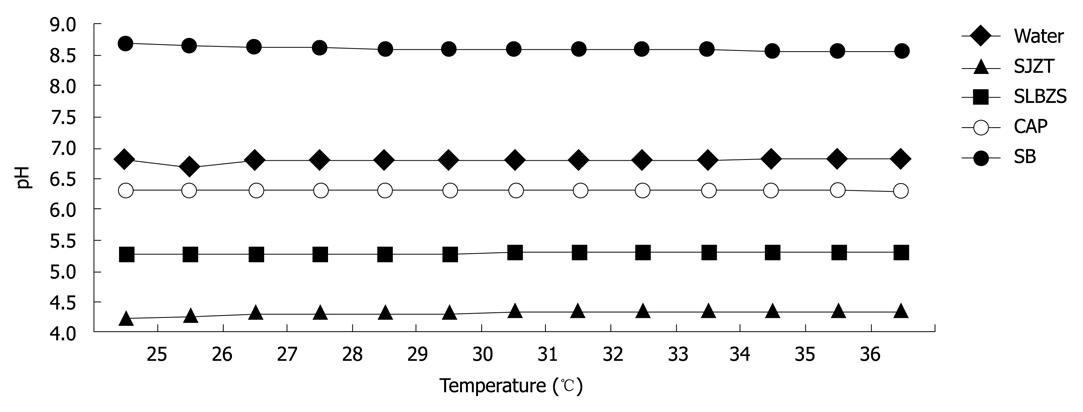
The pH values of the SJZT and SLBZS solutions at temperatures from 25°C to 37°C ranged from 4.25 to 4.34 and 5.25 to 5.30, respectively. The pH values of water, CAP and SB solutions at temperatures from 25°C to 37°C ranged from 6.79 to 6.81, 6.32 to 6.27 and 8.70 to 8.56, respectively. The results indicated that temperature did not affect pH significantly (Figure 1).
When 90 mL of the test solution was added to 100 mL of the artificial gastric juice (pH 1.2), the pH values of SJZT and SLBZS solutions were found to be 1.57 ± 0.04 and 1.76 ± 0.03, respectively. The pH values of water, CAP and SB solutions were 1.44 ± 0.03, 2.16 ± 0.03 and 1.83 ± 0.03, respectively. This result shows that the neutralizing capacity of CAP was better than those of SB, SLBZS and SJZT. The neutralizing capacity of SLBZS was similar to that of SB (Table 1).
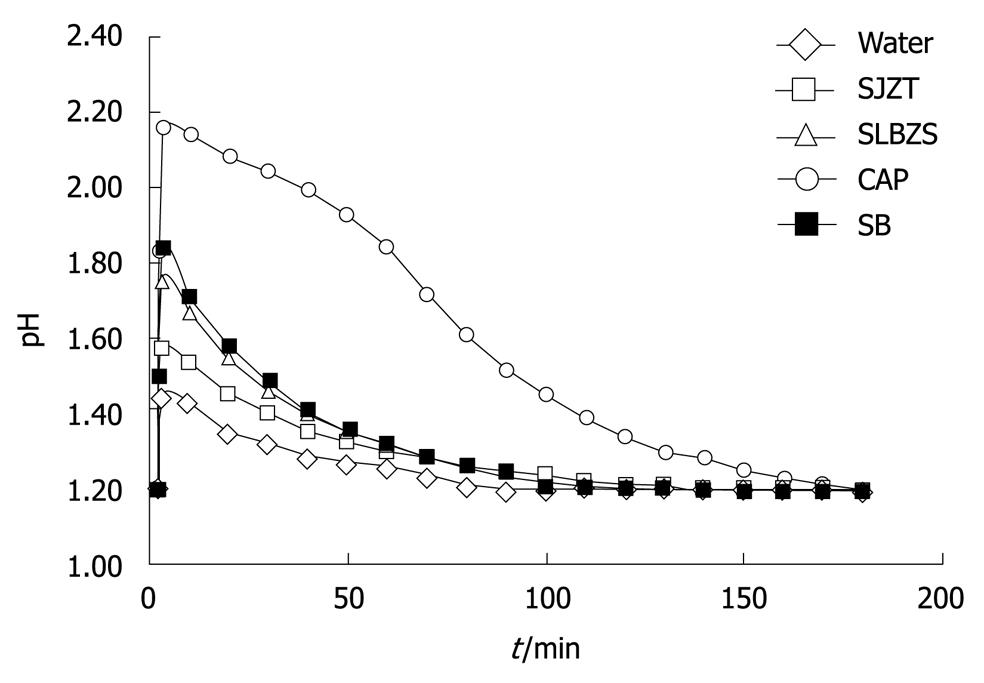
The durations for consistent neutralizing effects of SJZT and SLBZS solutions were 123 ± 24 min and 127 ± 5 min, respectively. Those of water, CAP and SB solutions were 87 ± 6, 172 ± 3 and 121 ± 14 min, respectively. The action duration of CAP was the longest, followed by the SLBZS and SJZT (Table 2, Figure 2).
The consumed volumes of artificial gastric juices to titrate to pH 3.0 for water, SJZT, SLBZS, CAP and SB solutions were 1.2 ± 0.17, 22.4 ± 1.10, 33.3 ± 0.85, 58.9 ± 0.79 and 69.2 ± 0.96 mL, respectively. The consumed hydrogen ions were 0.08 ± 0.01, 1.41 ± 0.07, 2.10 ± 0.05, 3.72 ± 0.05 and 4.37 ± 0.06 mmol, respectively (Table 3). The active controls, SB and CAP, exhibited significant antacid potency. Simultaneously, neutralization capacities of the tonic Chinese herbal prescriptions, SJZT, SLBZS, were better than that of water.
There have recently been extraordinary advances in the understanding of the pathophysiology and treatment of PU[1-3]. It is generally accepted that it results from an imbalance between gastric aggressive factors and maintenance of the mucosal integrity through an endogenous defense mechanism[5]. The main aggressive factors reported for several decades are acid and pepsin. Therefore, PU disease has been predominantly treated with antacids, H2 receptor antagonists and proton pump inhibitors. Among these, antacids have been widely used in the treatment of ulcer disease for many years.
Antacids heal ulcers through elimination of gastric acid by neutralization; however, they do not decrease the volume of gastric secretions. Different antacid drugs vary markedly in their in vivo and in vitro potency, and that should be taken into account when antacids are prescribed. On the other hand, potency is not the only factor that should be considered in the choice of an antacid. Cost, taste, salt content, bowel habit, and side effects are also important.
Although antacids have been used frequently, their side effects and drug interactions represent a major clinical problem. Consequently, traditional Chinese herbal medicines have recently generated increasing interest for the treatment of ulcer disease. The common Chinese herbal prescriptions nourishing the spleen and stomach, SJZT and SLBZS, are traditionally used for the treatment of gastrointestinal diseases in oriental countries. Therefore, the present study applied the titration method of Fordtran’s model and the modified model of Vatier’s artificial stomach, which mimic the regular physiological functioning of a human stomach, to explore the antacid effects of the tonic Chinese herbal prescriptions, SJZT and SLBZS.
In the present study, the Chinese herbal prescriptions SJZT and SLBZS were found to have antacid effects in vitro. Compared with the water group, all the treatments including SJZT, SLBZS, CAP and SB were shown to possess significant gastric acid neutralizing effects (Table 1). With regard to the duration for consistent neutralization of gastric acids, the neutralization duration of CAP and SLBZS were significantly longer than that of water (Table 2). Also, SB, CAP, SLBZS and SJZT exhibited significant antacid capacities compared to water (Table 3). According to these findings, the Chinese tonic prescriptions, SJZT and SLBZS, are suggested to have antacid effects similar to CAP and SB.
Efficacious, intensive antacid therapy is often unacceptable because of the common side effects, especially altered bowel functions. Aluminum salts may cause constipation and magnesium salts cause diarrhea. SB should be avoided even though it is a potent neutralizer of acid because it contains significant amounts of sodium and may alter the systemic pH. In addition, antacid drug interactions have been frequently reported and this is a problem worthy of being noticed. The most clinically significant interactions occur with ferrous sulfate, tetracycline and quinolone antibiotics. Other interactions are potentially significant because they involve drugs with narrow therapeutic ranges. Considering the side effects and interactions of antacids, the traditional Chinese prescriptions possessing fewer side effects should be looked to as an alternative for the treatment of PU.
In traditional Chinese medicine, the spleen and stomach are the principal organs in tonification and are responsible for receiving, digesting and transforming food and drink into Qi, Blood and Body Fluids[18]. Qi is a type of refined Essence produced by the internal organs. Qi insufficiency may manifest in specific organs. For instance, Spleen-Qi deficiency manifests as a poor appetite, abdominal distension, loose stools or diarrhea, a feeling of heaviness in the limbs and fatigue[18,19]. The herbs that tonify the Qi are very effective for improving the general condition and treating general weakness. Traditionally, the Chinese herbal prescriptions tonifying the spleen and stomach are widely used for the treatment of gastrointestinal diseases in oriental countries[20]. SJZT and SLBZS are common Chinese herbal prescriptions tonifying the spleen and stomach. As described above, SJZT consists of six herbal medicines and SLBZS consists of eleven herb components. Among these, Panax ginseng, Atractylodes ovata, Poria cocos and Glycyrrhiza uralensis are the major herbs in SJZT and SLBZS. Since these herbs are commonly used for tonifying Qi in clinical practice, further studies for the identification of the active components and to elucidate their mode of action are in progress.
In conclusion, the Chinese herbal prescriptions SJZT and SLBZS are consistently active in the artificial stomach model. There may be a potential benefit in offering these as an alternative for the treatment of PU. The mechanism of antacid activity by SJZT and SLBZS is not proven from the present results and requires further investigations.
Antacids, H2 receptor antagonists and proton pump inhibitors are the predominant drug therapies for the treatment of peptic ulcer (PU). Although effectiveness can be obtained with these drugs, their potential side effects and drug interactions represent a major problem in therapy. Traditional Chinese herbal medicines have recently generated increasing interest for the treatment of ulcer disease.
Si-Jun-Zi-Tang (SJZT) and Shen-Ling-Bai-Zhu-San (SLBZS), the common Chinese herbal prescriptions tonifying the spleen and stomach, are traditionally used for the treatment of gastrointestinal diseases in oriental countries. The present study aimed to assess the antacid effects of SJZT and SLBZS on gastric acid neutralization compared with water and the active controls. The antacid effects were assessed in vitro using the titration method of Fordtran’s model, and the modified model of Vatier’s artificial stomach was utilized to determine the effects on gastric acid secretion and emptying.
Clinical experience indicates the Chinese herbal prescriptions tonifying the spleen and stomach are traditionally used for the treatment of gastrointestinal diseases in oriental countries. Their anti-ulcer effects needed to be proven by scientific studies. This research is the first study using a modified artificial stomach model to reveal the antacid effects of SJZT and SLBZS. The related findings might contribute to clinical applications in future.
The study highlighted the antacid activities of SJZT and SLBZS in the artificial stomach model. There may be a potential benefit in offering an alternative for the treatment of PU. Further investigations for the identification of active components and to elucidate the mechanism of action are in progress.
SJZT is a Chinese prescription consisting of six herb components: Panax ginseng, Atractylodes ovata, Poria cocos, Glycyrrhiza uralensis, Zingiber officinale, and Zizyphus jujube. SLBZS is a Chinese prescription consisting of eleven herb components: Atractylodes ovata, Poria cocos, Panax ginseng, Glycyrrhiza uralensis, Dioscorea batatas, Dolichos lablab, Coix lachryma-jobi, Amomum xanthioides, Platycodon grandiflorum, Nelumbo nucifera and Citrus erythrosa.
Antacid effects of Chinese herbal prescriptions assessed by a modified artificial stomach model. This paper comprises an antacid and anti-ulcer study on two Chinese herbal preparations evaluated in an artificial stomach model. The study design is simple and easy to understand and useful in evaluating antacid studies. It is a neat and good study.
Peer reviewer: Dr. Vandana Panda, Pharmacology and Toxicology, Prin. K. M. Kundnani College of Pharmacy, Jote Joy Building, Rambhau Salgaonkar Marg, Cuffe Parade, Colaba, Mumbai 400 005, India
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Mertz HR, Walsh JH. Peptic ulcer pathophysiology. Med Clin North Am. 1991;75:799-814. |
| 2. | Højgaard L, Mertz Nielsen A, Rune SJ. Peptic ulcer pathophysiology: acid, bicarbonate, and mucosal function. Scand J Gastroenterol Suppl. 1996;216:10-15. |
| 3. | Holle GE. Pathophysiology and modern treatment of ulcer disease. Int J Mol Med. 2010;25:483-491. |
| 4. | Maton PN, Burton ME. Antacids revisited: a review of their clinical pharmacology and recommended therapeutic use. Drugs. 1999;57:855-870. |
| 5. | Berstad A, Weberg R. Antacids in the treatment of gastroduodenal ulcer. Scand J Gastroenterol. 1986;21:385-391. |
| 6. | Fordtran JS, Collyns JA. Antacid pharmacology in duodenal ulcer. Effect of antacids on postcibal gastric acidity and peptic activity. N Engl J Med. 1966;274:921-927. |
| 7. | Deakin M, Williams JG. Histamine H2-receptor antagonists in peptic ulcer disease. Efficacy in healing peptic ulcers. Drugs. 1992;44:709-719. |
| 8. | Richardson P, Hawkey CJ, Stack WA. Proton pump inhibitors. Pharmacology and rationale for use in gastrointestinal disorders. Drugs. 1998;56:307-335. |
| 9. | Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979;77:761-767. |
| 10. | Sung JJ, Chung SC, Ling TK, Yung MY, Leung VK, Ng EK, Li MK, Cheng AF, Li AK. Antibacterial treatment of gastric ulcers associated with Helicobacter pylori. N Engl J Med. 1995;332:139-142. |
| 11. | Blume H, Donath F, Warnke A, Schug BS. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 2006;29:769-784. |
| 12. | Sonnenberg A, Schwartz JS, Cutler AF, Vakil N, Bloom BS. Cost savings in duodenal ulcer therapy through Helicobacter pylori eradication compared with conventional therapies: results of a randomized, double-blind, multicenter trial. Gastrointestinal Utilization Trial Study Group. Arch Intern Med. 1998;158:852-860. |
| 13. | Fordtran JS, Morawski SG, Richardson CT. In vivo and in vitro evaluation of liquid antacids. N Engl J Med. 1973;288:923-928. |
| 14. | Vatier J, Harman A, Castela N, Droy-Lefaix MT, Farinotti R. Interactions of cimetidine and ranitidine with aluminum-containing antacids and a clay-containing gastric-protective drug in an "artificial stomach-duodenum" model. J Pharm Sci. 1994;83:962-966. |
| 15. | Vatier J, Lionnet F, Vitré MT, Mignon M. A model of an 'artificial stomach' for assessing the characteristics of an antacid. Aliment Pharmacol Ther. 1988;2:461-470. |
| 16. | Vatier J, Vallot T, Vitre MT, Mignon M. New approach for the in vitro evaluation of antacids. Arzneimittelforschung. 1990;40:175-179. |
| 17. | Vatier JL, Gao Z, Fu-Cheng XM, Vitre MT, Levy DA, Cohen G, Mignon M. Evidence for the interaction between antacid and gastric mucosa using an "artificial stomach" model including gastric mucosa. J Pharmacol Exp Ther. 1992;263:1206-1211. |
| 18. | Wu XN. Current concept of Spleen-Stomach theory and Spleen deficiency syndrome in TCM. World J Gastroenterol. 1998;4:2-6. |
| 19. | Kuang YL, Tan RQ, Yan WM. A study on hemorrheology in patients with spleen Qi deficiency. J Tradit Chin Med. 1988;8:235-238. |
| 20. | Li S, Chen Z, Yan H, Wang Q. Clinical observation on 80 children with peptic ulcer treated primarily by traditional Chinese medicine. J Tradit Chin Med. 1995;15:14-17. |