Published online Sep 21, 2010. doi: 10.3748/wjg.v16.i35.4422
Revised: June 28, 2010
Accepted: July 5, 2010
Published online: September 21, 2010
AIM: To investigate the influence of chronic pancreatitis (CP) on serum concentrations of amino acids.
METHODS: Thirty-five male patients with alcoholic CP and 21 healthy male subjects were examined. Serum concentrations of amino acids were assayed by ion-pair high-performance liquid chromatography with mass detection.
RESULTS: Serum glutamate concentration was increased in CP patients as compared to controls. In contrast, serum concentrations of glutamine, histidine, tyrosine, proline, tryptophan and threonine were significantly decreased in CP patients. A trend towards decreasing concentrations of serum lysine, alanine, methionine and valine as well as for total serum amino acids was observed. The sum of aromatic and the sum of essential amino acid concentrations were significantly lower in CP patients than in controls.
CONCLUSION: CP leads to decreased serum concentrations of several amino acids, such as essential and aromatic serum amino acids, most likely due to decreased exocrine function.
- Citation: Adrych K, Smoczynski M, Stojek M, Sledzinski T, Slominska E, Goyke E, Smolenski RT, Swierczynski J. Decreased serum essential and aromatic amino acids in patients with chronic pancreatitis. World J Gastroenterol 2010; 16(35): 4422-4427
- URL: https://www.wjgnet.com/1007-9327/full/v16/i35/4422.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i35.4422
Chronic pancreatitis (CP) is characterized by progressive depletion of acinar structures and subsequent replacement of endo- and exocrine pancreatic cells with fibrous tissue[1]. In the course of CP, pancreatic exocrine insufficiency usually develops earlier than endocrine insufficiency[2]. The secretion and, consequently, the activity of digestive enzymes decrease gradually, resulting in maldigestion. CP often causes weight loss due to reduced food intake (caused by pain and/or persistent alcohol abuse) and maldigestion of proteins as well as complex lipids and carbohydrates[3]. Malnutrition is thus common in patients with CP and its severity is one of the major factors predicting complications and outcome of the disease[1,3]. In a recent study, we demonstrated that several nutritional parameters are significantly decreased in patients with advanced CP[4]. It has been reported that protein calorie malnutrition is strongly associated with decreased serum concentrations of amino acids[5].
Amino acids are important substrates for: (1) protein synthesis; (2) glucose and urea synthesis; (3) energy production; and (4) synthesis of biologically active substances (e.g. nitric oxide, catecholamines, thyroid hormones), creatinine and carnitine[6]. They also play an important role in specialized metabolic and regulatory functions such as modulation of the carcinogenic pathway (proline)[7], regulation of insulin and glucagon secretion (leucine, lysine)[8,9], or synaptic maintenance and plasticity (glutamate)[10]. Moreover, some amino acids are cell signaling molecules and regulators of gene expression[6]. Thus, an adequate serum concentration of amino acids is necessary for the maintenance of human health and prevention of disease. The serum amino acid concentration depends on their influx to the blood (from food and/or muscle) and the capacity of the body to dispose of them (via uptake from the blood by various organs). It is influenced by both hormonal activity (mainly insulin secretion) and food intake. Insulin stimulates uptake and inhibits release of amino acids from muscle[11]. Recently, we reported that serum insulin concentration decreases in patients with CP[12,13]. Collectively, the above data suggest that maldigestion of protein and changes in serum insulin concentration during the course of CP may, in theory, have a significant impact on serum amino acid concentration. These processes, in turn, may influence the course of the disease. A recent study demonstrated significant abnormalities in serum amino acid profile in patients with acute pancreatitis[14,15]. In particular, the serum concentrations of arginine, citrulline, ornithine and glutamine were significantly decreased compared to the level after recovery. These changes may influence the inflammatory events and organ function in the course of acute pancreatitis[15]. Moreover, it has been suggested that supplementation of selected amino acids can be of value in severe acute pancreatitis[15]. Several studies assessing the serum amino acid concentration in patients with CP have been reported[16-23]. However, there is no clear consensus regarding the effect of CP on serum amino acid concentration. These reports concentrated mainly on the amino acid consumption test as a method of detecting exocrine pancreatic insufficiency. Most of these papers showed that total serum amino acid concentrations in patients with CP did not significantly differ from controls[16,18-23]. However, Dzieniszewski et al[17] showed that patients with advanced CP have lower total serum amino acid concentrations than control subjects. These contradictory results and the fact that most authors except Dzieniszewski et al[17] used the ninhydrin method, which is less accurate, for determination of total serum amino acid concentration, led us to revaluate serum amino acid concentration in patients with advanced CP using high-performance liquid chromatography with mass detection, a much more reliable method for measurement of amino acid concentration in biological samples.
The study was performed in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the Medical University of Gdansk Ethics Committee. All patients signed an informed consent form for this investigation. Of the patients treated for CP in the Department of Gastroenterology and Hepatology, Medical University of Gdansk during 2006-2008, we selected 35 males aged 33-72 years (mean age, 50 ± 10 years), with a history of alcoholic CP. All patients included in the study met diagnostic criteria for CP[24]. The diagnosis was based on clinical symptoms and typical results on imaging studies. Most patients included in the study were found to have the following abnormalities: pancreatic parenchymal calcifications, pancreatic duct stones, irregular dilation and/or stenosis of the pancreatic duct, fibrosis and parenchymal inhomogeneity. As determined by the results of endoscopic retrograde pancreatography (ERP), 28 patients displayed marked (grade 5 according to the Cambridge classification), 5 moderate (grade 4) and 2 mild (grade 3) stage of disease[25]. Patients with CP who had an exacerbation of the disease (based on clinical symptoms accompanied by significantly increased serum amylase and lipase, and urine amylase) and patients with liver cirrhosis were excluded from the study. Patients with CP were moderate or heavy drinkers. Twenty-one healthy male volunteers aged 23-61 years (mean age, 34 ± 13 years) formed the control group. Selected laboratory values in both groups are presented in Table 1. The body mass index (BMI) was calculated for all study participants. Fasting blood samples, from patients and healthy controls, were collected at 8 a.m.
| Control | Chronic pancreatitis | Statistical significance | |
| No. of patients | 21 | 35 | |
| Age (yr) | 34 ± 13 | 50 ± 10 | < 0.01 |
| Body weight (kg) | 76 ± 4 | 64 ± 10 | < 0.01 |
| Body mass index (kg/m2) | 25 ± 2.7 | 22 ± 3.4 | < 0.01 |
| Serum total protein (g/L) | 76 ± 4 | 72 ± 8 | < 0.05 |
| Serum albumin (g/L) | 46 ± 3.6 | 42 ± 8 | < 0.05 |
| Serum triacylglycerol (mg/dL) | 107 ± 30 | 119 ± 43 | NS |
| Serum cholesterol (mg/dL) | 195 ± 26 | 175 ± 44 | < 0.05 |
| Glucose (mmol/L) | 5 ± 0.64 | 7.7 ± 2.8 | < 0.01 |
| Insulin (μU/mL) | 12 ± 5.2 | 9.1 ± 4.4 | < 0.05 |
| Homeostasis model assessment | 2.7 ± 1.3 | 2.6 ± 1.6 | NS |
| Leptin (ng/mL) | 6.6 ± 2.9 | 3.9 ± 2.5 | < 0.01 |
| Hemoglobin (mg/dL) | 15 ± 2.9 | 13 ± 1.8 | < 0.01 |
| Blood urea nitrogen (mg/dL) | 11 ± 2.8 | 14 ± 6 | NS |
| Creatinine (mg/dL) | 0.9 ± 0.13 | 0.89 ± 0.22 | NS |
| Serum amylase (U/L) | 54 ± 31 | 65 ± 35 | NS |
| Urine amylase (U/L) | 174 ± 66 | 344 ± 336 | < 0.05 |
| Serum lipase (U/L) | 28 ± 16 | 69 ± 75 | < 0.05 |
| Serum alanine aminotransferase (U/L) | 21 ± 9 | 38 ± 38 | < 0.05 |
| Serum aspartate aminotransferase (U/L) | 19 ± 5 | 41 ± 45 | < 0.05 |
| Serum alkaline phosphatase (U/L) | 80 ± 28 | 104 ± 65 | NS |
| Serum-glutamyl transpeptidase (U/L) | 26 ± 14 | 112 ± 152 | < 0.05 |
Serum amino acid concentrations were determined using liquid chromatography/mass spectrometry (LC/MS) as described recently[26]. Briefly, an aliquot of plasma (0.4 mL) was deproteinized with 0.4 mL of 10% trichloroacetic acid (TCA). The tubes were centrifuged at 4°C, 12 000 ×g for 5 min. The supernatant was collected and TCA removed by diethyl ether extraction followed by freeze-drying. The material obtained was dissolved in 0.1 mL of 10 mmol/L nonafluoropentanoic acid in H2O and analyzed by ion-pair high-performance liquid chromatography with mass detection. Chromatographic separation was performed using a 3 μm Hypersil BDS 150 mm × 2.0 mm column. The mobile phase was delivered at 0.2 mL/min in a gradient from 0% to 60% acetonitrile in 12 min. The mass detector (Thermo-Finnigan LCQ Advantage, Waltham, MA, USA) with electrospray (ESI) ion source was operated in positive MS2 mode for detection of amino acids with the collision energy setting at 25%. Electrospray cone voltage was set at 4.5 kV and the heated capillary temperature was 275°C. Sheath gas flow was set for 35 arbitrary units. Post-column sheet flow of methanol with 0.05% formic acid at 0.2 mL/min was used to improve ionization efficiency. The identity of individual amino acids was confirmed by the similarity of molecular weights, fragmentation pattern and chromatographic retention time.
Serum leptin and insulin concentrations were analyzed as previously described[12,13]. Serum alanine aminotransferase, aspartate aminotransferase, amylase, lipase, alkaline phosphatase, γ-glutamyl transpeptidase, glucose, triacylglycerols, total cholesterol, hemoglobin, total protein and albumin, blood urea nitrogen (BUN), creatinine as well as urine amylase were analyzed using standard procedures. The homeostasis model assessment score (HOMA score) was used to assess insulin resistance as described by Matthews et al[27].
Statistical analysis was performed using Microsoft Excel. The statistical significance of differences observed between patients with CP and controls was assessed using the two-tailed t-test.
Table 1 shows selected nutritional and laboratory parameters in patients with CP and controls. Body weight, BMI and hemoglobin concentration were significantly lower in patients with advanced CP as compared to control subjects. Moreover, CP patients had lower serum concentrations of total protein and albumin. Serum total cholesterol concentration was lower (P <0.05) in patients with CP compared to controls. Serum glucose concentration was higher in patients with CP. BUN and creatinine concentrations were essentially similar in CP patients and control subjects. Table 1 also shows that serum insulin concentrations in patients with CP were significantly lower than in control subjects. The changes in serum insulin concentration observed in patients with CP essentially paralleled changes in serum leptin concentration (Table 1). Table 1 also shows that no significant differences were observed in the HOMA score between patients with CP and control subjects. We also studied serum amylase, lipase, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and γ-glutamyl-transpeptidase activity in patients with CP and control subjects. Serum lipase, alanine aminotransferase, aspartate aminotransferase and γ-glutamyl-transpeptidase activity were slightly elevated in patients with CP. Urine amylase activity in patients with CP was also higher than in control subjects (Table 1).
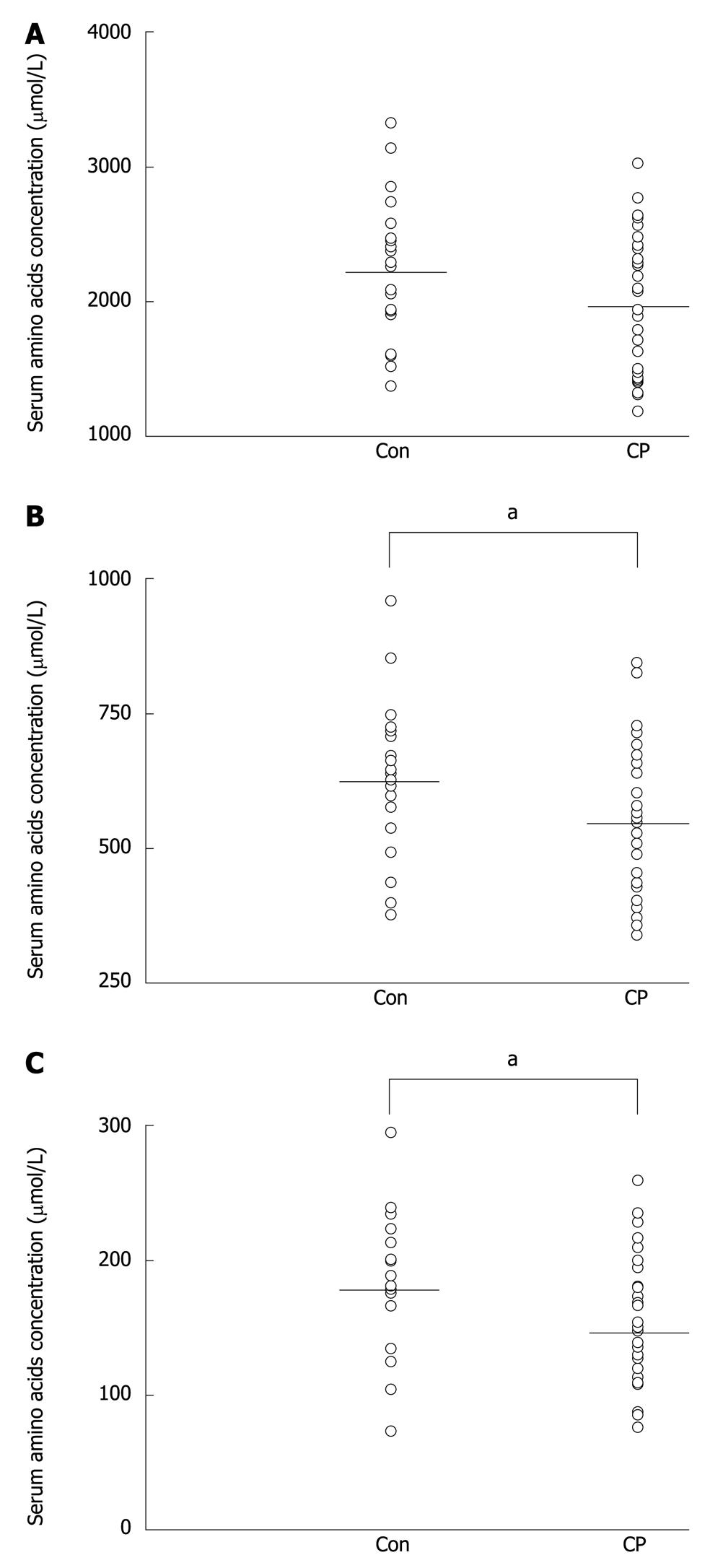
Table 2 shows the serum concentrations of amino acids in control subjects and patients with advanced CP. Surprisingly, the serum concentration of glutamate, as opposed to other amino acids, was significantly increased in patients with CP. In contrast, there were significant decreases in serum glutamine, histidine, tryptophan, tyrosine, proline and threonine in patients with CP. A trend towards decreased concentrations of serum alanine, methionine, valine and lysine was also observed, however, the differences did not reach statistical significance. We did not find significant decreases in the serum concentrations of other amino acids such as: glycine, isoleucine, cysteine, serine, leucine, phenylalanine, arginine, aspartate and asparagine (Table 2). The total serum amino acid concentration was slightly lower in CP patients than in healthy subjects, however, the differences did not reach statistical significance (Figure 1A). A significant decrease in the sum of selected amino acids such as essential (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine) (Figure 1B) and aromatic (phenylalanine, tryptophan, tyrosine) (Figure 1C) amino acid concentration in the serum of patients with advanced CP was found.
| Amino acid (μmol/L) | Control | Chronic pancreatitis | Statistical significance |
| Threonine | 125 ± 26 | 99 ± 33 | 0.0037 |
| Tryptophan | 67 ± 21 | 52 ± 16 | 0.0043 |
| Tyrosine | 59 ± 21 | 45 ± 17 | 0.0071 |
| Histidine | 48 ± 16 | 37 ± 14 | 0.0097 |
| Proline | 201 ± 82 | 157 ± 61 | 0.026 |
| Glutamate | 46 ± 24 | 66 ± 39 | 0.034 |
| Glutamine | 307 ± 97 | 256 ± 86 | 0.044 |
| Lysine | 114 ± 36 | 95 ± 36 | 0.059 |
| Alanine | 605 ± 128 | 535 ± 158 | 0.093 |
| Methionine | 15 ± 5.8 | 12 ± 5.7 | 0.13 |
| Valine | 93 ± 28 | 84 ± 23 | 0.17 |
| Isoleucine | 34 ± 11 | 31 ± 8 | 0.3 |
| Glycine | 154 ± 52 | 142 ± 46 | 0.37 |
| Arginine | 67 ± 37 | 61 ± 31 | 0.55 |
| Cysteine | 119 ± 42 | 112 ± 43 | 0.56 |
| Serine | 43 ± 13 | 41 ± 14 | 0.57 |
| Asparagine | 51 ± 22 | 47 ± 24 | 0.58 |
| Leucine | 67 ± 20 | 70 ± 16 | 0.62 |
| Aspartate | 22 ± 8 | 23 ± 11 | 0.67 |
| Phenylalanine | 57 ± 20 | 58 ± 24 | 0.87 |
We have demonstrated, for the first time, higher serum glutamate concentration, and lower serum glutamine, histidine, tryptophan, tyrosine, proline and threonine concentrations in patients with CP as compared to healthy subjects. The serum concentrations of alanine, methionine, valine and lysine, were lower in CP patients, as was the total serum amino acid concentration, but the differences did not reach statistical significance (Figure 1A). Moreover, the results presented here suggest that the measurement of selected amino acids such as essential and aromatic amino acids could help to assess exocrine pancreatic insufficiency. Our measurements of total serum amino acid concentrations in patients with CP are generally consistent with the results of previous studies[16,18,21].
Since the secretion of digestive enzymes gradually decreases in the course of CP, resulting in protein maldigestion, a decrease in serum concentrations of some amino acids would be expected. It is not clear why serum glutamate concentration is higher in patients with CP than in control subjects. The serum glutamate concentration was also increased, whereas serum glutamine concentration decreased during the first 5 d after the onset of symptoms in patients with acute pancreatitis[15]. This phenomenon is probably due to the increase in intracellular conversion of glutamine to glutamate[15]. In patients with CP, the ratio of serum glutamine to glutamate was 7:1 and was 4:1 in controls (Table 2). It is therefore likely that increased intracellular metabolism of glutamine to glutamate is also present during the course of CP. Another possible explanation for the increase in serum glutamate concentration could be an increase in intracellular conversion of 2-oxoglutarate to glutamate or inhibition of glutamate oxidation.
The significant decrease in serum concentration of some amino acids, especially aromatic and essential amino acids suggests that protein maldigestion due to exocrine insufficiency could be one possible mechanism leading to this deficit. Among pancreatic proteases, chymotrypsin catalyzes the hydrolysis of only those peptide bonds in which the carboxyl (carbonyl) group is contributed by aromatic amino acids. Thus, one explanation of decreased concentration of aromatic amino acids would be that chymotrypsin is the most deficient of all pancreatic proteases during the course of CP. The unchanged concentration of phenylalanine and significant decrease in tyrosine (aromatic amino acids) suggest that the activity of phenylalanine hydroxylase is diminished, resulting in an increased serum phenylalanine to tyrosine ratio [1.0 ± 0.4 in control subjects vs 1.4 ± 0.7 (P < 0.05) in patients with CP]. Since tetrahydrobiopterin (BH4), as a cofactor of phenylalanine hydroxylase, is necessary for the conversion of phenylalanine to tyrosine, one can suppose that the intracellular BH4 concentration is diminished in patients with CP. Diminished phenylalanine hydroxylase activity, and consequently a lower rate of conversion of phenylalanine to tyrosine, should lead to an increase in serum phenylalanine. This was not the case. It is possible that the impact of diminished phenylalanine hydroxylase activity on the serum concentration of phenylalanine is counterbalanced by the effects of protein malnutrition in the course of CP. However, this is just one possible explanation for this phenomenon and other mechanisms are not excluded.
Another mechanism affecting the serum concentration of amino acids could be an increased catabolism of skeletal muscle protein. CP, being an inflammatory disease, can shift muscle protein from anabolism to catabolism. Consequently, the decrease in the serum concentration of some amino acids due to protein maldigestion could be counterbalanced by release of amino acids from muscle. This is probably the reason why the serum concentrations of several amino acids remain unchanged (or only slightly decreased) despite protein maldigestion during the course of CP. The diminished serum concentration of some amino acids (glutamine, histidine, tryptophan, proline and tyrosine) may have profound effects on the rate of protein synthesis. This may explain the lower concentration of total serum protein (Table 1). Moreover, lower serum concentration of some amino acids may influence the course of CP. Therefore, it is reasonable to postulate that supplementation of selected amino acids could be of value in advanced CP.
The reason for the decrease in serum histidine concentration in patients with CP is unknown. However, one can suppose that the decrease of this amino acid could be disadvantageous because histidine is a precursor of histamine (besides being a substrate for protein synthesis). Consequently, decreased histidine concentration can lead to a decrease in histamine concentration. It is well known that binding of histamine to its receptor located on the surface of parietal cells (H2 receptor) stimulates gastric acid secretion. Diminished histamine production can lead to diminished protein digestion. Moreover, histidine inhibits production of proinflammatory cytokines by human monocytes[28]. It is therefore likely that diminished histidine concentration can promote inflammation. Collectively, the decrease in serum histidine concentration may worsen the course of CP.
The results reported by Pitkänen et al[29] indicate that the decrease in serum amino acid concentrations occurs in groups of subjects who are older than 60 years, but not in individuals under 60. Thus, it is unlikely that the different age of controls (34 ± 13 years old men) and CP patients (50 ± 10 years old men) could be a reason for the results presented in this paper.
Finally, it should be emphasized that the application of tandem mass spectrometry for analysis of non-derivatized amino acids used in this study, represents a unique approach with numerous advantages as compared to the techniques used so far[16,18-23]. Our technique has better sensitivity and specificity. It requires only a small volume of serum and limited sample preparation. This procedure can be highly recommended not only for research applications but also for clinical use.
In conclusion, the results reported here indicate that advanced CP interferes with serum amino acid concentrations. Surprisingly, serum glutamate concentration increases during the course of CP, whereas serum concentrations of glutamine, histidine, tryptophan, tyrosine, proline and threonine were significantly lower in patients with CP. A tendency towards a decreased concentration of total serum amino acids was observed without reaching statistical significance. A significant decrease in selected amino acids such as essential and aromatic serum amino acid concentrations in patients with advanced CP was found. Decreased serum concentration of some amino acids is probably due to protein maldigestion. However, the effect of malnutrition and systemic chronic inflammation on serum amino acids cannot be excluded. It is tempting to speculate that, first, accurate measurement of selected amino acids such as aromatic and/or essential amino acids could help assess pancreatic exocrine insufficiency in the course of advanced CP, and, second, that supplementation of selected amino acids could be of therapeutic value in advanced CP.
In the course of chronic pancreatitis (CP), the secretion of digestive enzymes gradually decreases, resulting in maldigestion. In the advanced stage of disease, in turn, maldigestion of proteins could lead to a decrease in serum amino acid concentrations.
Malnutrition is common in patients with CP and its severity is a factor predicting complications and outcome of the disease. However, there is no clear consensus regarding the effect of CP on serum amino acid concentration. In this study, the authors demonstrate that CP is associated with a decrease in the serum concentration of essential and aromatic amino acids, most likely due to decreased pancreatic exocrine function.
This is the first study to report that serum glutamate concentration increases and serum essential and aromatic amino acid concentrations decrease in the course of CP. The decrease in amino acids concentration is probably the result of protein maldigestion typical in advanced CP. A new method: ion-pair high-performance liquid chromatography with mass detection was used to assay non-derivatized amino acids.
Measurement of aromatic and essential amino acids could help to assess pancreatic exocrine insufficiency in the course of advanced CP. Moreover, supplementation of selected amino acids could be of therapeutic value in advanced CP.
Essential amino acids are those that have to be supplied in the diet as they cannot be synthesized in the human body. Aromatic amino acids contain aromatic structures in the side chain (i.e. phenylalanine, tyrosine, tryptophan). Ion-pair high-performance liquid chromatography with mass detection - an analytical technique based on the separation of molecules and selective detection of its ions.
The authors describe the decreased essential and aromatic amino acid content in the sera of patients with chronic pancreatitis and give several possible explanations. The manuscript is well written and conveys the message appropriately.
Peer reviewer: Tatjana Crnogorac-Jurcevic, MD, PhD, Cancer Research UK, Molecular Oncology Unit, Barts and The London School of Medicine and Dentistry, John Vane Science Centre, Charterhouse Square, London EC1M 6BQ, United Kingdom
S- Editor Wang YR L- Editor Webster JR E- Editor Ma WH
| 1. | Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682-707. |
| 2. | Schrader H, Menge BA, Schneider S, Belyaev O, Tannapfel A, Uhl W, Schmidt WE, Meier JJ. Reduced pancreatic volume and beta-cell area in patients with chronic pancreatitis. Gastroenterology. 2009;136:513-522. |
| 3. | Meier R, Ockenga J, Pertkiewicz M, Pap A, Milinic N, Macfie J, Loser C, Keim V. ESPEN Guidelines on Enteral Nutrition: Pancreas. Clin Nutr. 2006;25:275-284. |
| 4. | Adrych K, Smoczynski M, Sledzinski T, Dettlaff-Pokora A, Goyke E, Swierczynski J. Increased serum resistin concentration in patients with chronic pancreatitis: possible cause of pancreatic fibrosis. J Clin Gastroenterol. 2009;43:63-68. |
| 5. | Polge A, Bancel E, Bellet H, Strubel D, Poirey S, Peray P, Carlet C, Magnan de Bornier B. Plasma amino acid concentrations in elderly patients with protein energy malnutrition. Age Ageing. 1997;26:457-462. |
| 6. | Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1-17. |
| 7. | Phang JM, Donald SP, Pandhare J, Liu Y. The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids. 2008;35:681-690. |
| 8. | Kalogeropoulou D, Lafave L, Schweim K, Gannon MC, Nuttall FQ. Leucine, when ingested with glucose, synergistically stimulates insulin secretion and lowers blood glucose. Metabolism. 2008;57:1747-1752. |
| 9. | Kalogeropoulou D, LaFave L, Schweim K, Gannon MC, Nuttall FQ. Lysine ingestion markedly attenuates the glucose response to ingested glucose without a change in insulin response. Am J Clin Nutr. 2009;90:314-320. |
| 10. | Tapiero H, Mathe G, Couvreur P, Tew KD. II. Glutamine and glutamate. Biomed Pharmacother. 2002;56:446-457. |
| 11. | Fukagawa NK, Minaker KL, Young VR, Rowe JW. Insulin dose-dependent reductions in plasma amino acids in man. Am J Physiol. 1986;250:E13-E17. |
| 12. | Adrych K, Smoczynski M, Goyke E, Stelmanska E, Swierczynski J. Decreased serum leptin concentration in patients with chronic pancreatitis. Pancreas. 2007;34:417-422. |
| 13. | Adrych K, Smoczynski M, Stelmanska E, Korczynska J, Goyke E, Swierczynski J. Serum adiponectin and leptin concentrations in patients with chronic pancreatitis of alcoholic and nonalcoholic origin. Pancreas. 2008;36:120-124. |
| 14. | Sandstrom P, Gasslander T, Sundqvist T, Franke J, Svanvik J. Depletion of serum L-arginine in patients with acute pancreatitis. Pancreas. 2003;27:261-266. |
| 15. | Sandstrom P, Trulsson L, Gasslander T, Sundqvist T, von Dobeln U, Svanvik J. Serum amino acid profile in patients with acute pancreatitis. Amino Acids. 2008;35:225-231. |
| 16. | Domschke S, Heptner G, Kolb S, Sailer D, Schneider MU, Domschke W. Decrease in plasma amino acid level after secretin and pancreozymin as an indicator of exocrine pancreatic function. Gastroenterology. 1986;90:1031-1038. |
| 17. | Dzieniszewski J, Jarosz M, Kunachowicz H, Klys W. [Level of amino acids in blood plasma as a test for exocrine pancreatic function]. Wiad Lek. 1990;43:387-396. |
| 18. | Gullo L, Pezzilli R, Ventrucci M, Barbara L. Caerulein induced plasma amino acid decrease: a simple, sensitive, and specific test of pancreatic function. Gut. 1990;31:926-929. |
| 19. | Heptner G, Domschke S, Schneider MU, Kolb S, Domschke W. [Amino acid level in plasma--expressed as alpha-amino-nitrogen--reaction to stimulation of the exocrine pancreas: approaches to a new pancreatic function test]. Klin Wochenschr. 1987;65:1054-1061. |
| 20. | Kemmer TP, Malfertheiner P, Haberle H, Pohlandt F, Friess H, Buchler M, Ditschuneit H. [The diagnostic value of the amino acid absorption test in detection of a disorder of exocrine pancreatic function]. Z Gastroenterol. 1992;30:391-396. |
| 21. | Lembcke B, Konle O, Duan LP, Caspary WF. Lack of accuracy of plasma alpha-amino nitrogen profiles as an indicator of exocrine pancreatic function both after continuous and bolus stimulation of the pancreas with secretin and cholecystokinin-pancreozymin. Z Gastroenterol. 1994;32:679-683. |
| 22. | Mårtensson J, Bolin T. Sulfur amino acid metabolism in chronic relapsing pancreatitis. Am J Gastroenterol. 1986;81:1179-1184. |
| 23. | Schneider MU, Meister R, Domschke S, Zirngibl H, Strebl H, Heptner G, Gebhardt C, Gall FP, Domschke W. Whipple’s procedure plus intraoperative pancreatic duct occlusion for severe chronic pancreatitis: clinical, exocrine, and endocrine consequences during a 3-year follow-up. Pancreas. 1987;2:715-726. |
| 24. | Homma T. Criteria for pancreatic disease diagnosis in Japan: diagnostic criteria for chronic pancreatitis. Pancreas. 1998;16:250-254. |
| 26. | Swierczynski J, Sledzinski T, Slominska E, Smolenski R, Sledzinski Z. Serum phenylalanine concentration as a marker of liver function in obese patients before and after bariatric surgery. Obes Surg. 2009;19:883-889. |
| 27. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. |
| 28. | Andou A, Hisamatsu T, Okamoto S, Chinen H, Kamada N, Kobayashi T, Hashimoto M, Okutsu T, Shimbo K, Takeda T. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology. 2009;136:564-574.e2. |
| 29. | Pitkänen HT, Oja SS, Kemppainen K, Seppä JM, Mero AA. Serum amino acid concentrations in aging men and women. Amino Acids. 2003;24:413-421. |