Published online Sep 14, 2010. doi: 10.3748/wjg.v16.i34.4297
Revised: September 14, 2009
Accepted: September 21, 2009
Published online: September 14, 2010
AIM: To evaluate whether symptoms of inflammatory bowel disease (IBD), before diagnosis modify dietary habits, and to investigate the pre-illness diet in patients with recent IBD in comparison with an age-matched healthy control group.
METHODS: Overall, 83 new cases of IBD (41 ulcerative colitis, 42 Crohn’s disease) and 160 healthy controls were studied. Portions per week of 34 foods and beverages before onset of symptoms were recorded using a validated questionnaire. Duration of symptoms before IBD diagnosis, presence of specific symptoms and their impact on subjective changes in usual dietary habits were also recorded. The association between diet and IBD was investigated by multiple logistic regression and dietary patterns were assessed by factor analysis.
RESULTS: Changes in dietary habits, due to the presence of symptoms, were reported by 38.6% of patients and were not significantly related to specific symptoms, rather to long duration of symptoms, only in Crohn’s disease patients. In IBD patients who did not change dietary habits, moderate and high consumption of margarine (OR = 11.8 and OR = 21.37) was associated with ulcerative colitis, whilst high consumption of red meat (OR = 7.8) and high intake of cheese were associated with Crohn’s disease.
CONCLUSION: More than one third of IBD patients change dietary habits before diagnosis. Margarine, red meat and cheese increase the risk of ulcerative colitis and Crohn’s disease.
- Citation: Maconi G, Ardizzone S, Cucino C, Bezzio C, Russo AG, Porro GB. Pre-illness changes in dietary habits and diet as a risk factor for inflammatory bowel disease: A case-control study. World J Gastroenterol 2010; 16(34): 4297-4304
- URL: https://www.wjgnet.com/1007-9327/full/v16/i34/4297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i34.4297
The aetiology of inflammatory bowel disease (IBD) is still unknown. Since genetically determined mechanisms have remained unchanged over historical time periods, the increase in incidence of ulcerative colitis (UC) and Crohn’s disease (CD) during the 20th century suggests environmental influences. Epidemiological and clinical evidence support an association between IBD and many apparently unrelated environmental factors, including diet, smoking, geographical and social status, occupation, and microbial factors[1-4]. In particular, the role of dietary factors or components of diet in the pathophysiology have long since been taken into consideration, and immunological mechanisms linking food antigens to development of inflammation have also been postulated. However, this attractive explanation is far from proven, and studies investigating this potential link are few and unconvincing. In some reports, it has been suggested that increased consumption of sugar and refined carbohydrates might be a risk factor for CD[5-10], and has also been demonstrated in some UC patients[7,9,11-13], whereas protein and fat intake, as well as decreased consumption of fruit, vegetables and fibre, appear to increase the risk of IBD, but data reported have been more controversial[5,14-16]. Indeed, a causal relationship between diet and IBD is difficult to define, due to the possibility that early symptoms of the disease may lead to a modification in dietary habits and the inability of the patients to accurately remember their diet before the onset of symptoms. To date, only a few studies have examined the pre-illness diet, in incident cases, and have shown conflicting results[5,15-18].
The present study aimed to evaluate whether the signs and symptoms of IBD in patients before diagnosis led to a modification in their diet, and to investigate pre-illness dietary habits in patients with recent IBD in comparison to those in an age-matched control group.
Between June and September 2003, and between September 2007 and June 2008, incident cases of UC and CD (diagnosis < 12 mo, median duration of symptoms: 5 mo, range: 0-84 mo) consecutively observed at the IBD Unit of the Gastroenterology Department of L. Sacco University Hospital were interviewed. Controls were recruited among healthy blood donors of the same Hospital and were frequency-matched (by quinquennial of age and sex) with the cases. None of the controls reported serious abdominal diseases that could have influenced their dietary habits. All patients were contacted by two L. Sacco University gastroenterologists (Cucino C and Bezzio C), who interviewed patients and controls, asking questions in an identical manner, with interviews lasting approximately 30 min each during which a validated questionnaire was employed which had been previously used for epidemiological studies concerning the relationship between cancer and diet[19].
This questionnaire included information on socio-demographic characteristics, anthropometric measures, lifestyle, including both tobacco smoking and alcohol habits, and personal and family medical history. Attention was focused, in particular, on long-term drinking, smoking, and dietary habits before diagnosis and onset of symptoms in IBD patients. Patients and healthy controls were asked about the number and size of portions of different food items consumed per week and whether 3 alcoholic and 4 non-alcoholic drinking items were consumed per day. IBD patients were also investigated to determine the duration of symptoms before diagnosis and the presence of specific symptoms such as diarrhoea, abdominal pain, rectal bleeding and weight loss > 3 kg. In IBD patients, an attempt was made to establish whether they had changed their usual dietary habits due to symptoms. The categorical answers to these questions were “yes”, “no” or “don’t know”. The validated food frequency questionnaire investigated the usual dietary habits during the 5 years prior to diagnosis and onset of symptoms for IBD patients or during the last 5 years in healthy controls. The questionnaire included 34 food items and beverages commonly used in the Italian diet, grouped as follows: (1) bread and cereal dishes (first courses); (2) red meat and other main dishes such as fish, pork, poultry or rabbit (second courses); (3) vegetables (side dishes); (4) fruit; (5) sweets (including refined carbohydrates and sugar), desserts and soft drinks; (6) milk and hot beverages; and (7) alcoholic beverages. Furthermore, the consumption of coffee (non decaffeinated and decaffeinated), tea, olives and seed oils, margarine and butter were also investigated. Questions were also asked concerning the average weekly frequency of consumption of each dietary item; occasional intakes (< once a week but at least once a month) were coded as 0.5/wk.
Data were reported as mean and SD or as median and range, where appropriate. The association between symptoms and changes in dietary habit was evaluated by the Fisher exact test.
Consumption of foods and beverages, as well as other continuous variables, were subdivided into tertiles (low, moderate and high consumptions).
Odds ratios (OR) and corresponding 95% CI were defined using multiple logistic regression models. All the regression equations included terms for age (5-year groups), sex, years of education, tobacco consumption (never, ex-smoker, current smoker of < 15, 15-24, ≥ 25 cigarettes/d) and body mass index (BMI) (quintiles). Tests for trends were based on the likelihood ratio test between models with and without a linear term for the diet score.
Dietary patterns, based on nutrient intake, associated with IBD, were defined using factor analysis, a multivariate technique which analyses the underlying structure of a set of data in order to explain observed relationships between a large number of variables in terms of simpler relations.
Within a factor, negative loading indicates that foods are inversely associated with the factor, while positive loading indicates a direct association with the factor. After varimax rotation, factor scores were saved from the principal components analysis for each individual. Factor scores were categorized into tertiles based on the distribution of the control.
The study population comprised 83 IBD patients (41 UC, 42 CD) and 160 sex- and age-matched healthy controls, comparable for social status, years of education, BMI and smoking habits. CD patients showed more frequent ileal localisation of disease and, as expected, an inflammatory behaviour, whilst UC patients showed more frequent left sided colitis (Table 1).
| Cases | Controls | |
| (n = 83) | (n = 160) | |
| Sex (M/F) | 49/34 | 97/63 |
| Age (mean ± SD) | 37.5 ± 15.2 | 40.4 ± 14.6 |
| Social status, n (%) | ||
| Unmarried | 50 (60.3) | 83 (51.9) |
| Married | 26 (31.3) | 69 (43.1) |
| Divorced/widowed | 7 (8.4) | 8 (5.0) |
| Years of education (mean-SD) | 12.4-3.5 | 11.5-4.2 |
| Body mass index, kg2/cm (mean-SD) | 26.1-9.3 | 29.1-10.8 |
| Smoking habit, n (%) | ||
| Never | 32 (38.6) | 80 (50.0) |
| Smokers | 29 (34.9) | 41 (35.6) |
| Ex-smokers | 22 (26.5) | 39 (24.4) |
| No. cigarettes/d (smokers) mean (range) | 13.4 (3-50) | 14.7 (5-30) |
| Disease location (Crohn’s disease) n = 42 | ||
| Ileum, n (%) | 18 (42.9) | |
| Ileum and colon | 13 (31.0) | / |
| Colon | 11 (26.2) | |
| Disease location (ulcerative colitis) n = 41 | ||
| Proctitis | 5 (12.2) | |
| Left sided colitis | 22 (53.7) | / |
| Pancolitis | 14 (34.1) | |
| Disease behaviour (Crohn’s disease) | ||
| Inflammatory (B1) | 27 (64.3) | |
| Stricturing (B2) | 8 (19.0) | / |
| Penetrating (B3) | 7 (16.7) |
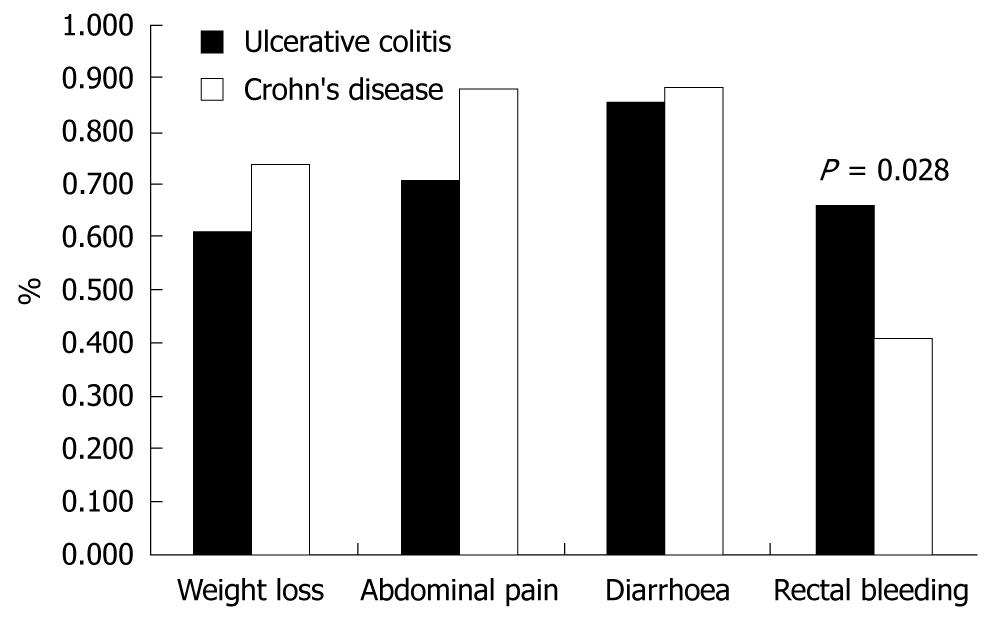
The duration of symptoms before diagnosis was comparable in UC and CD patients [median (range): 5 (1-84) vs 6 (0-48) mo]. Weight loss, diarrhoea and abdominal pain at diagnosis were comparable, judging from the complaints referred to by the UC and CD patients. As expected, UC patients presented rectal bleeding more frequently than CD patients (Figure 1).
A conscious change of dietary habits due, before diagnosis, to the presence of symptoms, was reported by 32 patients (38.6%), 15 with UC and 17 with CD. The main dietary changes were reduction of fat and calorie intake (12 patients), while other patients reduced or stopped the consumption of fibre (18 patients) and milk or cheese (9 patients).
Change in dietary habits was not due to specific symptoms. However, in CD patients the long duration of symptoms was significantly correlated with changes in dietary habits (Table 2).
| Changes in diet | ||||
| Ulcerative colitis | Crohn’s disease | |||
| No (26) | Yes (15) | No (25) | Yes (17) | |
| Duration of symptoms | ||||
| Short ( ≤ 3 mo) | 14 (53.85) | 4 (26.67) | 8 (32.00) | 4 (23.53) |
| Intermediate (4-10 mo) | 7 (26.92) | 4 (26.67) | 13 (52.00) | 4 (23.53) |
| Long (> 10 mo) | 5 (19.23) | 7 (46.66) | 4 (16.00) | 9 (52.94)a |
| Median, range (mo) | 3 (1-15) | 7 (1-84) | 5 (1-30) | 12 (1-48)a |
| Weight loss | ||||
| Absent/low (< 2 kg) | 12 (46.15) | 4 (26.67) | 8 (32.00) | 3 (16.65) |
| Moderate (2-6 kg) | 8 (30.77) | 3 (20.00) | 11 (44.00) | 8 (47.06) |
| Severe (> 6 kg) | 6 (23.08) | 8 (53.33) | 6 (24.00) | 6 (35.29) |
| Abdominal pain | ||||
| No | 9 (34.61) | 3 (20.00) | 4 (16.00) | 1 (5.88) |
| Yes | 17 (65.39) | 12 (80.00) | 21 (84.00) | 16 (94.12) |
| Diarrhoea | ||||
| No | 5 (19.23) | 1 (6.67) | 4 (16.00) | 1 (5.88) |
| Yes | 21 (80.77) | 14 (93.33) | 21 (84.00) | 16 (94.12) |
| Rectal bleeding | ||||
| No | 9 (34.62) | 5 (33.33) | 13 (52.00) | 12 (70.59) |
| Yes | 17 (65.38) | 10 (66.67) | 12 (48.00) | 5 (29.41) |
To exclude any potential confounding influence of symptom-induced changes in diet (i.e. intake of milk in UC and fibre in CD) only patients who did not change dietary habits (26 UC and 25 CD patients) were taken into consideration (Tables 3 and 4). In these patients, an increased risk of UC (although not significant) was found for those reporting a high consumption of pasta and rice (OR = 3.38, 95% CI: 0.99-11.47) and with moderate or high consumption of margarine (OR = 11.8 and OR = 21.37, respectively). CD was significantly associated with moderate consumption of meat (OR = 7.8, 95% CI: 1.61-37.89) and high consumption of cheese (OR = 3.7, 95% CI: 1.14-12.01). High intake of fish and potatoes reduced the risk of IBD while the consumption of vegetables and tuna fish was negatively correlated with the risk of CD and high intake of eggs was negatively correlated with risk of UC.
| Controls | Ulcerative colitis | ||
| n (%) | n (%) | OR1 (95% CI) | |
| Pasta and/or rice | |||
| Low | 67 (41.88) | 7 (26.92) | 2 |
| Moderate | 68 (42.50) | 12 (46.15) | 1.89 (0.66-5.41) |
| High | 25 (15.63) | 7 (26.92) | 3.38 (0.99-11.47) |
| Bread | |||
| Low | 60 (37.50) | 6 (23.08) | 2 |
| Moderate | 55 (34.38) | 9 (34.62) | 1.31 (0.42-4.13) |
| High | 45 (28.13) | 11 (42.31) | 2.40 (0.80-7.26) |
| Sweets and cakes | |||
| Low | 52 (32.50) | 10 (38.46) | 2 |
| Moderate | 49 (30.63) | 4 (15.38) | 0.37 (0.10-1.33) |
| High | 59 (36.88) | 12 (46.15) | 0.96 (0.36-2.51) |
| Red meat | |||
| Low | 73 (45.63) | 12 (46.15) | 2 |
| Moderate | 38 (23.75) | 8 (30.77) | 1.22 (0.45-3.32) |
| High | 49 (30.63) | 6 (23.08) | 0.63 (0.20-1.94) |
| White meat | |||
| Low | 45 (28.13) | 5 (19.23) | 2 |
| Moderate | 70 (43.75) | 17 (65.38) | 2.04 (0.69-6.05) |
| High | 45 (28.13) | 4 (15.38) | 0.75 (0.19-3.04) |
| Tuna fish | |||
| Low | 37 (23.13) | 10 (38.46) | 2 |
| Moderate | 48 (30.00) | 6 (23.08) | 0.43 (0.13-1.37) |
| High | 75 (46.88) | 10 (38.46) | 0.49 (0.18-1.36) |
| Fish | |||
| Low | 61 (38.13) | 17 (65.38) | 2 |
| Moderate | 39 (24.38) | 3 (11.54) | 0.33 (0.09-1.26) |
| High | 60 (37.50) | 6 (23.08) | 0.33 (0.11-0.92) |
| Processed meat | |||
| Low | 50 (31.25) | 9 (34.62) | 2 |
| Moderate | 57 (35.63) | 10 (38.46) | 0.82 (0.28-2.36) |
| High | 53 (33.13) | 7 (26.92) | 0.63 (0.21-1.91) |
| Milk | |||
| Low | 45 (28.13) | 13 (50.00) | 2 |
| Moderate | 36 (22.50) | 3 (11.54) | 0.30 (0.08-1.19) |
| High | 79 (49.38) | 10 (38.46) | 0.55 (0.21-1.40) |
| Cheese | |||
| Low | 43 (26.88) | 8 (30.77) | 2 |
| Moderate | 70 (43.75) | 11 (42.31) | 0.93 (0.33-2.63) |
| High | 47 (29.38) | 7 (26.92) | 0.98 (0.31-3.12) |
| Eggs | |||
| Low | 44 (27.50) | 11 (42.31) | 2 |
| Moderate | 51 (31.88) | 11 (42.31) | 0.78 (0.30-2.06) |
| High | 65 (40.63) | 4 (15.38) | 0.21 (0.06-0.73) |
| Potatoes | |||
| Low | 25 (15.63) | 10 (38.46) | 2 |
| Moderate | 78 (48.75) | 11 (42.31) | 0.39 (0.14-1.09) |
| High | 57 (35.63) | 5 (19.23) | 0.24 (0.09-0.83) |
| Vegetables | |||
| Low | 44 (27.67) | 11 (42.31) | 2 |
| Moderate | 67 (42.14) | 10 (38.46) | 0.49 (0.18-1.35) |
| High | 48 (30.19) | 5 (19.23) | 0.37 (0.11-1.21) |
| Fruits | |||
| Low | 48 (30.00) | 9 (34.62) | 2 |
| Moderate | 61 (38.13) | 11 (42.31) | 1.12 (0.42-3.01) |
| High | 51 (31.88) | 6 (23.08) | 0.41 (0.13-1.35) |
| Refined sugar | |||
| Low | 57 (35.63) | 9 (34.62) | 2 |
| Moderate | 45 (28.13) | 12 (46.15) | 1.52 (0.57-4.09) |
| High | 58 (36.25) | 5 (19.23) | 0.57 (0.17-1.87) |
| Butter | |||
| Low | 28 (17.50) | 1 (3.85) | 2 |
| Moderate | 88 (55.00) | 18 (69.23) | 6.02 (0.75-47.97) |
| High | 44 (27.50) | 7 (26.92) | 5.18 (0.58-46.20) |
| Margarine | |||
| Low | 57 (35.63) | 1 (3.85) | 2 |
| Moderate | 84 (52.50) | 19 (73.08) | 11.80 (1.51-91.99) |
| High | 19 (11.88) | 6 (23.08) | 21.37 (2.32-196.6) |
| Olive oil | |||
| Low | 47 (29.38) | 6 (23.08) | 2 |
| Moderate | 88 (55.00) | 16 (61.54) | 1.40 (0.50-3.93) |
| High | 25 (15.63) | 4 (15.38) | 1.16 (0.29-4.63) |
| Seed oil | |||
| Low | 33 (20.63) | 1 (3.85) | 2 |
| Moderate | 97 (60.63) | 22 (84.62) | 7.26 (0.93-56.53) |
| High | 30 (18.75) | 3 (11.54) | 3.82 (0.36-40.14) |
| Controls | Crohn’s disease | |||
| n (%) | n (%) | OR1 (95% CI) | ||
| Pasta and/or rice | ||||
| Low | 67 (41.88) | 8 (32.00) | 2 | |
| Moderate | 68 (42.50) | 14 (56.00) | 1.59 (0.61-4.17) | |
| High | 25 (15.63) | 3 (12.00) | 1.01 (0.24-4.34) | |
| Bread | ||||
| Low | 60 (37.50) | 6 (24.00) | 2 | |
| Moderate | 55 (34.38) | 8 (32.00) | 1.25 (0.38-4.04) | |
| High | 45 (28.13) | 11 (44.00) | 2.47 (0.81-7.56) | |
| Sweets and cakes | ||||
| Low | 52 (32.50) | 8 (32.00) | 2 | |
| Moderate | 49 (30.63) | 4 (16.00) | 0.43 (0.11-1.63) | |
| High | 59 (36.88) | 13 (52.00) | 1.21 (0.44-3.29) | |
| Red meat | ||||
| Low | 73 (45.63) | 8 (32.00) | 2 | |
| Moderate | 38 (23.75) | 6 (24.00) | 1.25 (0.38-4.07) | |
| High | 49 (30.63) | 11 (44.00) | 2.42 (0.85-6.85) | |
| White meat | ||||
| Low | 45 (28.13) | 8 (32.00) | 2 | |
| Moderate | 70 (43.75) | 15 (60.00) | 1.33 (0.50-3.52) | |
| High | 45 (28.13) | 2 (8.00) | 0.25 (0.05-1.27) | |
| Tuna fish | ||||
| Low | 37 (23.13) | 13 (52.00) | 2 | |
| Moderate | 48 (30.00) | 6 (24.00) | 1.31 (0.35-4.87) | |
| High | 75 (46.88) | 6 (24.00) | 0.25 (0.08-0.77) | |
| Fish | ||||
| Low | 61 (38.13) | 16 (64.00) | 2 | |
| Moderate | 39 (24.38) | 6 (24.00) | 0.57 (0.19-1.72) | |
| High | 60 (37.50) | 3 (12.00) | 0.18 (0.05-0.67) | |
| Processed meat | ||||
| Low | 50 (31.25) | 2 (8.00) | 2 | |
| Moderate | 57 (35.63) | 18 (72.00) | 7.80 (1.61-37.89) | |
| High | 53 (33.13) | 5 (20.00) | 1.97 (0.35-11.03) | |
| Milk | ||||
| Low | 45 (28.13) | 7 (28.00) | 2 | |
| Moderate | 36 (22.50) | 2 (8.00) | 0.41 (0.07-2.18) | |
| High | 79 (49.38) | 16 (64.00) | 1.55 (0.57-4.19) | |
| Cheese | ||||
| Low | 43 (26.88) | 5 (20.00) | 2 | |
| Moderate | 70 (43.75) | 5 (20.00) | 0.54 (0.14-2.06) | |
| High | 47 (29.38) | 15 (60.00) | 3.70 (1.14-12.01) | |
| Eggs | ||||
| Low | 44 (27.50) | 10 (40.00) | 2 | |
| Moderate | 51 (31.88) | 9 (36.00) | 0.85 (0.30-2.40) | |
| High | 65 (40.63) | 6 (24.00) | 0.42 (0.14-1.29) | |
| Potatoes | ||||
| Low | 25 (15.63) | 7 (28.00) | 2 | |
| Moderate | 78 (48.75) | 13 (52.00) | 0.60 (0.19-1.83) | |
| High | 57 (35.63) | 5 (20.00) | 0.24 (0.06-0.91) | |
| Vegetables | ||||
| Low | 44 (27.67) | 14 (56.00) | 2 | |
| Moderate | 67 (42.14) | 7 (28.00) | 0.26 (0.09-0.78) | |
| High | 48 (30.19) | 4 (16.00) | 0.21 (0.05-0.78) | |
| Fruits | ||||
| Low | 48 (30.00) | 9 (36.00) | 2 | |
| Moderate | 61 (38.13) | 8 (32.00) | 0.78 (0.26-2.30) | |
| High | 51 (31.88) | 8 (32.00) | 0.76 (0.26-2.21) | |
| Refined sugar | ||||
| Low | 57 (35.63) | 8 (32.00) | 2 | |
| Moderate | 45 (28.13) | 12 (48.00) | 1.61 (0.58-4.42) | |
| High | 58 (36.25) | 5 (20.00) | 0.58 (0.17-1.97) | |
| Butter | ||||
| Low | 28 (17.50) | 1 (4.00) | 2 | |
| Moderate | 88 (55.00) | 20 (80.00) | 6.07 (0.75-49.20) | |
| High | 44 (27.50) | 4 (16.00) | 1.84 (0.18-18.19) | |
| Margarine | ||||
| Low | 57 (35.63) | 8 (32.00) | 2 | |
| Moderate | 84 (52.50) | 13 (52.00) | 1.07 (0.40-2.84) | |
| High | 19 (11.88) | 4 (16.00) | 1.17 (0.29-4.74) | |
| Olive oil | ||||
| Low | 47 (29.38) | 10 (40.00) | 2 | |
| Moderate | 88 (55.00) | 10 (40.00) | 0.60 (0.22-1.61) | |
| High | 25 (15.63) | 5 (20.00) | 0.98 (0.29-3.35) | |
| Seed oil | ||||
| Low | 33 (20.63) | 6 (24.00) | 2 | |
| Moderate | 97 (60.63) | 15 (60.00) | 0.84 (0.29-2.43) | |
| High | 30 (18.75) | 4 (16.00) | 0.66 (0.16-2.76) | |
In particular, as far as any possible correlation of diet with disease location in CD is concerned, tuna fish consumption was negatively correlated with both pure colonic location (OR = 0.33, 95% CI: 0.11-0.98) and ileal or ileocolonic CD (OR = 0.48, 95% CI: 0.26-0.91), while vegetable consumption showed protective effects in patients with ileal or ileocolonic location of CD (OR = 0.43, 95% CI: 0.21-0.88). Interestingly, we also found that an increased risk of ileal or ileocolonic (but not pure colonic) CD associated with high bread or cheese consumption (OR = 2.61, 95% CI: 1.26-5.41 and OR = 2.61, 95% CI: 1.26-5.41, respectively) (Table 5).
| Small bowel | Colon | |
| Pasta and/or rice | 1.44 (0.71-2.92) | 0.65 (0.20-2.13) |
| Bread | 2.61 (1.26-5.41)2 | 0.27 (0.06-1.10) |
| Sweets and cakes | 1.17 (0.64-2.14) | 1.39 (0.53-3.63) |
| Red meat | 1.58 (0.88-2.82) | 1.23 (0.49-3.12) |
| White meat | 0.74 (0.38-1.46) | 0.40 (0.12-1.29) |
| Tuna fish | 0.48 (0.26-0.91)2 | 0.33 (0.11-0.98)2 |
| Fish | 0.48 (0.24-0.93)2 | 0.41 (0.13-1.29) |
| Processed meat | 1.31 (0.67-2.58) | 0.74 (0.27-2.01) |
| Milk | 1.30 (0.71-2.36) | 1.13 (0.46-2.78) |
| Cheese | 2.61 (1.26-5.41)2 | 1.94 (0.62-6.09) |
| Eggs | 0.67 (0.36-1.23) | 0.69 (0.27-1.80) |
| Potatoes | 0.50 (0.23-1.07) | 0.46 (0.15-1.41) |
| Vegetables | 0.43 (0.21-0.88)2 | 0.60 (0.20-1.83) |
| Fruits | 0.82 (0.42-1.58) | 0.83 (0.29-2.36) |
| Refined sugar | 0.80 (0.44-1.46) | 0.77 (0.31-1.95) |
| Butter | 0.83 (0.39-1.79) | 1.44 (0.41-5.12) |
| Margarine | 1.10 (0.51-2.36) | 1.83 (0.58-5.76) |
| Olive oil | 1.20 (0.58-2.51) | 0.24 (0.06-1.00)2 |
| Seed oil | 0.81 (0.37-1.79) | 1.05 (0.32-3.42) |
No correlation was found between beverages and risk of IBD.
Factor analysis identified three dietary-intake factors that, overall, explained 94% of total variability, accounting for 59%, 20% and 14%, respectively. A first dietary pattern, called “refined”, was mainly correlated to pasta, sweets, red and processed meat, butter and margarine. The second pattern (prudent) loaded heavily on white meat, tuna fish, fish, eggs and potatoes. The last pattern, designated “healthy”, loaded heavily bread, cheese and, in particular, fruit and vegetables, as well as olive oil (which was found to be negatively correlated with the two previously described patterns). Evaluation of the association between these specific dietary intake patterns and IBD showed that a “refined” diet was associated with an increased risk of UC and CD. In contrast, the “prudent” pattern was significantly associated with a decreased risk (Table 6). The “healthy” pattern was not consistently associated with UC, moreover, a non-significant increase in risk was present for CD.
| Factor 1 | Factor 2 | Factor 3 | |
| Pasta and/or rice | 0.30 | -0.25 | 0.32 |
| Bread | -0.01 | -0.40 | 0.51 |
| Soft, sweets and cakes | 0.45 | -0.28 | 0.09 |
| Red meat | 0.57 | 0.30 | 0.23 |
| White meat | 0.45 | 0.49 | -0.02 |
| Tuna fish | 0.43 | 0.60 | -0.11 |
| Fish | 0.33 | 0.58 | -0.12 |
| Processed meat | 0.46 | 0.04 | -0.02 |
| Milk | 0.03 | -0.33 | 0.04 |
| Cheese | 0.02 | 0.19 | 0.56 |
| Eggs | -0.05 | 0.69 | 0.08 |
| Potatoes | 0.09 | 0.58 | 0.01 |
| Vegetables | -0.52 | 0.22 | 0.32 |
| Fruit | -0.53 | -0.01 | 0.25 |
| Refined sugar | -0.01 | -0.03 | 0.60 |
| Butter | 0.47 | 0.20 | 0.27 |
| Margarine | 0.46 | 0.29 | -0.02 |
| Olive oil | -0.17 | -0.32 | 0.44 |
| Seed oil | 0.39 | 0.13 | 0.07 |
| Refined | Prudent | Healthy | |
| OR (95% CI) | OR1 (95% CI) | OR (95% CI) | |
| Ulcerative colitis | |||
| Low2 | 13 | 13 | 13 |
| Moderate | 2.29 (0.70-7.32) | 0.32 (0.11-0.90) | 0.80 (0.28-2.28) |
| High | 2.46 (0.77-7.83) | 0.15 (0.04-0.51) | 0.95 (0.33-2.76) |
| Crohn’s disease | |||
| Low | 13 | 13 | 13 |
| Moderate | 1.51 (0.45-4.99) | 0.47 (0.17-1.27) | 0.57 (0.18-1.78) |
| High | 2.15 (0.67-6.87) | 0.13 (0.03-0.51) | 1.39 (0.49-3.95) |
In this case-control study analysing the relationship between pre-illness diet and the risk of IBD, in incident cases of UC and CD, results showed a positive relationship between the intake of margarine and a non-significant trend for pasta and rice in UC, and meat and cheese in CD. IBD patients reported a significant reduction in intake of potatoes and fish, and CD patients also have reduced consumption of vegetables and tuna fish compared with healthy controls. An association between pasta, sweets, red and processed meat, butter and margarine and IBD was also observed by factor analysis.
Several studies have focused on the role of dietary factors in IBD, some of which were already implicated as influencing the development of IBD, starting from the study by Martini & Brandes which demonstrated a higher intake of refined carbohydrates in CD patients compared to controls[20]. This report was then followed by several studies in which other dietary factors, such as fibre, proteins, and total calorie intake, were evaluated in patients with UC and CD[5,6,8,11-17]. Despite the large number of papers on this issue, it has been shown that studying the association between diet and chronic disease presents methodological problems, since dietary habits could have already been influenced by the pathological condition itself. This has been demonstrated also by the present data, which revealed that UC and CD patients had already modified their dietary habits by themselves before diagnosis. We found that most IBD patients remembered having avoided vegetables, milk and/or cheese, or having reduced calorie intake before diagnosis and the onset of symptoms. The influence of symptoms on dietary habits and the difficulties encountered in recalling foods eaten 5 years before diagnosis are the main problems emerging in studies investigating diet and IBD.
In previous studies IBD patients, when asked about their dietary habits, described an increased consumption of refined sugar and a decreased intake of fruit and vegetables. These findings have also been interpreted as a consequence of the disease, rather than a factor which could be implicated in its aetiology. Indeed, refined sugar is rapidly absorbed in the small intestine, avoiding bulking effects and giving the possibility to compensate loss of energy and weight, which are typical characteristics of UC and CD. On the other hand, a decreased consumption of fibres could easily be the effect of trying to avoid symptoms caused by the bulk-rich food. Due to this methodological bias, in some case-control studies efforts have been made to retrieve data on the patients’ dietary habits many years before onset of the illness, unfortunately obtaining less reliable answers, due to the weakness of the memory of those replying as well as the influence that the symptoms themselves could have had on the pre-illness dietary habits, the so called “recall bias”. One condition which should be met is, therefore, to interview the patients about pre-illness dietary habits as soon as the diagnosis of IBD is made, in order to consider those subjects as incident cases. Even new patients might have had symptoms for several months or years prior to the diagnosis. To date, only a few studies by-passed the recall bias interviewing incident cases of IBD, as soon as possible after diagnosis, collecting information on pre-illness dietary habits[5,15-18].
The consumption of refined sugar was found to be positively associated with IBD in two of these studies[5,17], while another reported a positive association with CD but not with UC[20]. The total intake of proteins and a high consumption of eggs were shown to be positively related with the risk of IBD in one study[17]. This study also showed that a high consumption of fruit, vegetables and fibres, in general, helps to protect against the risk of IBD, while two other studies did not show any difference between patients and controls[5,20]. The study by Thornton et al[16] did not find any striking relationship in the intake of sugar, fibre, carbohydrates and proteins between the new cases of UC and controls.
In our study, only patients in whom the diagnosis had been made < 1 year prior to the interview and who had not modified dietary habits were considered. We found a significant intake of margarine and a high, although not significant, intake of rice and pasta in UC and a significant consumption of red meat and cheese in CD patients. We also identified a dietary pattern at higher risk of IBD correlated to pasta, sweets, red and processed meat, butter and margarine intake.
The differences in collecting and grouping foods and nutrients make it difficult to compare our results with those of other studies. On the other hand, while some authors reported an increased intake of protein in CD patients[21,22] others did not[17], furthermore, the pre-illness consumption of red meat, as well as cheese, has not been previously considered in detail. It is worthwhile pointing out that we found a relationship between both these nutrients and CD and that these foods are also related to Mycobacterium avium subspecies paratuberculosis, a candidate as an infectious aetiological agent in CD[23,24]. We also found a correlation between UC and margarine, pasta and rice intake, but not with other carbohydrates such as bread and sugar. These findings confirm the results of another Italian study showing that UC patients had a high intake of total carbohydrates but not with those of a Japanese study in which the intake of bread for breakfast tended to be positively associated with the risk of UC[25,26]. However, the latter study, together with a recent multicenter study, showed that margarine and polyunsatured fatty acid intake was positively and significantly associated with UC, an association also found in our study[26,27].
In conclusion, although based on only a small number of cases, our study is one of the few in the literature providing information on the changes in dietary habits before diagnosis in IBD patients, thus explaining the difficulties and uncertainties encountered in epidemiological studies on diet and IBD. Furthermore, the study also revealed the role played by some environmental dietary factors and identified, by factor analysis, a specific dietary pattern at risk of IBD.
The aetiology of inflammatory bowel disease (IBD) is still unknown, but the role of dietary factors in the pathophysiology has long since been taken into consideration. However, studies investigating this potential link are few and unconvincing.
To date, only a few studies have examined the pre-illness diet, in incident IBD cases, showing conflicting results. In the present study the authors showed that signs and symptoms of IBD in patients before diagnosis led to a modification in their diet, and confirmed that pre-illness dietary habits, namely margarine, red meat, and cheese significantly increased the risk of ulcerative colitis and Crohn’s disease.
A causal relationship between diet and IBD is difficult to define, due to the possibility that early symptoms of the disease may lead to a modification in dietary habits and the inability of the patients to accurately remember their diet before the onset of symptoms. The present case-control study evaluated whether the signs and symptoms of IBD patients before diagnosis led to a modification in their diet and assessed the association between diet and IBD onset in patients who did not change dietary habits by using multiple logistic regression and factor analysis.
The results of this study could contribute to a better understanding of the impact and significance of diet in the pathogenesis of inflammatory bowel disease.
In the present study, the association between diet and dietary pattern and IBD has been assessed using multiple logistic regression and factor analysis. Factor analysis is a multivariate technique which analyses the underlying structure of a set of data in order to explain observed relationships between a large number of variables in terms of simpler relations.
Although the number of patients is rather small, it is a well written and interesting study with some weaknesses which need to be addressed in order to strengthen the manuscript.
Peer reviewer: Ioannis E Koutroubakis, MD, PhD, Assistant Professor of Medicine, University Hospital Heraklion, Department of Gastroenterology, PO Box 1352, 71110 Heraklion, Crete, Greece
S- Editor Li LF L- Editor O’Neill M E- Editor Ma WH
| 1. | Tobin MV, Logan RF, Langman MJ, McConnell RB, Gilmore IT. Cigarette smoking and inflammatory bowel disease. Gastroenterology. 1987;93:316-321. |
| 3. | Cucino C, Sonnenberg A. Occupational mortality from inflammatory bowel disease in the United States 1991-1996. Am J Gastroenterol. 2001;96:1101-1105. |
| 4. | Cashman KD, Shanahan F. Is nutrition an aetiological factor for inflammatory bowel disease? Eur J Gastroenterol Hepatol. 2003;15:607-613. |
| 5. | Mayberry JF, Rhodes J, Allan R, Newcombe RG, Regan GM, Chamberlain LM, Wragg KG. Diet in Crohn’s disease two studies of current and previous habits in newly diagnosed patients. Dig Dis Sci. 1981;26:444-448. |
| 6. | Järnerot G, Järnmark I, Nilsson K. Consumption of refined sugar by patients with Crohn’s disease, ulcerative colitis, or irritable bowel syndrome. Scand J Gastroenterol. 1983;18:999-1002. |
| 7. | Bianchi Porro G, Panza E. Smoking, sugar, and inflammatory bowel disease. Br Med J (Clin Res Ed). 1985;291:971-972. |
| 8. | Sonnenberg A. Geographic and temporal variations of sugar and margarine consumption in relation to Crohn’s disease. Digestion. 1988;41:161-171. |
| 9. | Husain A, Korzenik JR. Nutritional issues and therapy in inflammatory bowel disease. Semin Gastrointest Dis. 1998;9:21-30. |
| 10. | Russel MG, Engels LG, Muris JW, Limonard CB, Volovics A, Brummer RJ, Stockbrügger RW. Modern life’ in the epidemiology of inflammatory bowel disease: a case-control study with special emphasis on nutritional factors. Eur J Gastroenterol Hepatol. 1998;10:243-249. |
| 11. | Mayberry JF, Rhodes J, Newcombe RG. Increased sugar consumption in Crohn’s disease. Digestion. 1980;20:323-326. |
| 12. | Persson PG, Ahlbom A, Hellers G. Diet and inflammatory bowel disease: a case-control study. Epidemiology. 1992;3:47-52. |
| 13. | Thornton JR, Emmett PM, Heaton KW. Smoking, sugar, and inflammatory bowel disease. Br Med J (Clin Res Ed). 1985;290:1786-1787. |
| 14. | Kasper H, Sommer H. Dietary fiber and nutrient intake in Crohn’s disease. Am J Clin Nutr. 1979;32:1898-1901. |
| 15. | Thornton JR, Emmett PM, Heaton KW. Diet and Crohn’s disease: characteristics of the pre-illness diet. Br Med J. 1979;2:762-764. |
| 17. | Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754-760. |
| 18. | Geerling BJ, Dagnelie PC, Badart-Smook A, Russel MG, Stockbrügger RW, Brummer RJ. Diet as a risk factor for the development of ulcerative colitis. Am J Gastroenterol. 2000;95:1008-1013. |
| 19. | Bosetti C, Gallus S, Trichopoulou A, Talamini R, Franceschi S, Negri E, La Vecchia C. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2003;12:1091-1094. |
| 20. | Martini GA, Brandes JW. Increased consumption of refined carbohydrates in patients with Crohn‘s disease. Klin Wochenschr. 1976;54:367-371. |
| 21. | Gee MI, Grace MG, Wensel RH, Sherbaniuk RW, Thomson AB. Nutritional status of gastroenterology outpatients: comparison of inflammatory bowel disease with functional disorders. J Am Diet Assoc. 1985;85:1591-1599. |
| 22. | Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741-745. |
| 23. | Hermon-Taylor J. Treatment with drugs active against Mycobacterium avium subspecies paratuberculosis can heal Crohn’s disease: more evidence for a neglected public health tragedy. Dig Liver Dis. 2002;34:9-12. |
| 24. | Hermon-Taylor J. Protagonist. Mycobacterium avium subspecies paratuberculosis is a cause of Crohn’s disease. Gut. 2001;49:755-756. |
| 25. | Tragnone A, Valpiani D, Miglio F, Elmi G, Bazzocchi G, Pipitone E, Lanfranchi GA. Dietary habits as risk factors for inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7:47-51. |
| 26. | A case-control study of ulcerative colitis in relation to dietary and other factors in Japan. The Epidemiology Group of the Research Committee of Inflammatory Bowel Disease in Japan. J Gastroenterol. 1995;30 Suppl 8:9-12. |