Published online Sep 7, 2010. doi: 10.3748/wjg.v16.i33.4169
Revised: April 7, 2010
Accepted: April 14, 2010
Published online: September 7, 2010
AIM: To characterize the histopathologic specifications of non-alcoholic steatohepatitis (NASH) according to age and gender.
METHODS: An analytical cross-sectional study was conducted in two private gastroenterology clinics on biopsy proven patients suffering from NASH. Biopsy histopathologic findings as well as demographic and laboratory data of the patients at the time of biopsy were gathered retrospectively from clinical records. The grading and staging of histopathologic findings were performed according to the Brunt method after reevaluation of the slides by a pathologist. Patients were divided into two groups according to age (below and above 55 years). Mean quantitative grade of all pathologic findings were also calculated according to Brunt scoring values.
RESULTS: A total number of 77 NASH patients, consisting of 58 males (75.3%) and 19 (24.7%) females with a mean age of 41.99 ± 11.80 years (range, 18-70 years), were enrolled. The mean age (48.72 ± 13.99 years vs 39.74 ± 10.16 years, P = 0.004) and aspartate aminotransferase level (75.11 ± 29.68 U/L vs 52.78 ± 25.00 U/L, P = 0.002) was significantly higher in female patients. Mean quantitative grade of hepatosteatosis was significantly higher in females (2.00 ± 0.82 vs 1.59 ± 0.68, P = 0.031) compared to males. Fifty four percent (34/65) of young patients had mild hepatosteatosis (Grade I) while only one patient (11.2%) in the older group had grade I hepatosteatosis. Patients aged ≥ 55 had significantly more severe hepatosteatosis (Grade III) (44.4% vs 9.5%, P = 0.007) and the mean quantitative grade of hepatosteatosis was significantly higher among them (2.33 ± 0.71 vs 1.56 ± 0.67, P = 0.002). Multivariate analysis after omitting the confounding role of age revealed a higher grade of hepatosteatosis in female patients (P = 0.010).
CONCLUSION: These findings point toward the possible influence of age in the severity of steatohepatitis, portal and lobar inflammation in patients suffering from NASH while gender independently might contribute to the level of steatohepatitis.
- Citation: Daryani NE, Daryani NE, Alavian SM, Zare A, Fereshtehnejad SM, Keramati MR, Pashaei MR, Habibollahi P. Non-alcoholic steatohepatitis and influence of age and gender on histopathologic findings. World J Gastroenterol 2010; 16(33): 4169-4175
- URL: https://www.wjgnet.com/1007-9327/full/v16/i33/4169.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i33.4169
Nonalcoholic steatohepatitis (NASH) is a common clinicopathological entity with a worldwide distribution[1] which is defined as a cryptogenic form of liver disease that is often observed in obese patients in the absence of identifiable causes such as alcohol abuse, drug toxicity or viral infection[2]. Although the disease is often indolent, 15%-20% of patients may progress to fibrosis and 7%-17% may insidiously develop cirrhosis which is a strong warning finding in patients’ liver biopsies. Additionally, NASH is responsible for the majority of cryptogenic cirrhosis in patients who are at risk of complications, such as portal hypertension and hepatocellular carcinoma[3-7]. According to survival rates, population-based studies in the United States revealed lower overall survival for patients with nonalcoholic fatty liver disease compared to the general population[8].
Although the etiology of NASH is unknown, a number of co-morbid conditions have been suggested as predisposing factors for the development of NASH such as obesity, type 2 diabetes mellitus, and hyperlipidemia[6,7,9-13]. It is assumed that the emergence of NASH closely resembles the epidemiology of obesity and diabetes mellitus[14].
Recently, there was a parallel increase in the prevalence of obesity, metabolic syndrome (hyperglycemia, visceral obesity, hyperlipidemia and hypertension) and NASH. As a result, NASH is considered part of metabolic syndrome[15]. Pathogenesis of metabolic syndrome is based on insulin resistance. Aging and reduced physical activity, which cause hyperinsulinemia and insulin resistance, result in obesity and initiate metabolic syndrome which in turn further increases insulin resistance[16]. Considerable difference in the prevalence of metabolic syndrome between genders has been attributed to sex hormones. Many studies have shown that women tend to gain weight following the menopause and distribution of their body fat changes toward visceral adiposity[17]. There is a considerable increase in the prevalence of metabolic syndrome in women after menopause.
Despite the high prevalence of NASH, its natural history is not well understood. To the best of our knowledge, only about 30 cases of NASH have been described with sequential liver biopsies[3,6,7]. Because different sections of metabolic syndrome show significant differences that are related to age and gender, there is speculation that the clinicopathological features of NASH may also vary in relation to these factors. The following study was performed to evaluate the relationship between age and gender with pathologic findings on liver biopsies of NASH patients.
This analytic cross-sectional study was conducted in two private gastroenterology clinics in Tehran, Iran during 2005-2009. Data were retrospectively collected using the medical records of all eligible subjects who were biopsy proven NASH patients that met the following inclusion criteria: (1) Histopathologic confirmation of nonalcoholic steatohepatitis; (2) Non-alcohol consumers or intake of less than 100 g ethanol per week which was confirmed by the attending physician and family members who were in close contact with the patient; (3) Negative serologic markers of viral or autoimmune hepatitis, including hepatitis B surface antigen, hepatitis C virus antibody (ELISA), human immunodeficiency virus antibody (ELISA), antinuclear antibodies, anti-smooth muscle antibodies, anti-liver/kidney microsomes type 1 antibodies and negative α-1 antitrypsin; and (4) Not having any other liver disorders including metabolic liver diseases (e.g. Wilson’s disease and hemochromatosis), drug induced liver disease, primary biliary cirrhosis, primary sclerosing cholangitis, and biliary obstruction. Moreover, patients with any history of jejunoileal bypass surgery, having received hormone replacement therapy for menopause, any usage of other drugs known to result in steatosis (e.g. glucocorticoids, synthetic estrogens, aspirin, tamoxifen, amiodarone, calcium-channel blockers, and methotrexate), pregnancy or incomplete medical records were all excluded. Finally, 77 NASH patients were eligible to enroll in this study.
Levels of aminotransferases, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), alkaline phosphatase (Alk-P), total serum cholesterol and triglycerides, low density lipoprotein, high density lipoprotein, fasting blood sugar, partial thromboplastin time, serum platelet count and serum level of γ-glutamyl transpeptidase were measured using standard techniques. In addition, other baseline characteristics such as patients’ age and gender and body mass index were recorded for all patients.
According to the guidelines concerning the indications for liver biopsy, all patients who show no improvement of liver function after several months of diet and exercise treatment are recommended to undergo a liver biopsy. Due to the retrospective nature of the study, all biopsy specimens were recovered from the laboratory and reevaluated for histopathologic findings. In order to definitely diagnose NASH, all liver biopsy specimens were examined for fibrosis, steatosis, hepatocyte ballooning, lobar and portal inflammation. The grading and staging of all biopsy specimens were determined based on the method proposed by Brunt et al[18]. The categorization of the histopathologic findings regarding this method is presented in Table 1. Mild and moderate steatohepatitis were defined as conditions in which < 33% and 33%-66% of hepatocytes were affected, respectively; whereas, severe steatohepatitis was characterized with > 66% involvement of hepatocytes. Additionally, advanced fibrosis consisted of bridging fibrosis (grade III) and cirrhosis. In order to restrict the information bias, a single pathologist reported all histopathologic findings. The mean grade of all pathologic findings were also calculated according to Brunt scoring values.
| Finding | Grading |
| Hepatosteatosis | |
| Grade I | < 33% of hepatocytes affected |
| Grade II | 33%-66% of hepatocytes affected |
| Grade III | > 66% of hepatocytes affected |
| Fibrosis | |
| No fibrosis | No fibrosis |
| Grade I | Zone 3 perisinusoidal pericellular fibrosis, focally or extensively present |
| Grade II | Zone 3 perisinusoidal pericellular fibrosis, with focal or extensive periportal fibrosis |
| Grade III | Zone 3 perisinusoidal pericellular fibrosis and portal fibrosis with extensive or focal bridging fibrosis |
| Cirrhosis | Cirrhosis |
| Hepatocyte ballooning | |
| No ballooning | No ballooning |
| Grade I | Sometimes, zone 3 |
| Grade II | Evident, zone 3 |
| Grade III | Symptomatic, more dominant in zone 3 |
| Lobar inflamation | |
| No change | No change |
| Grade I | Diffuse neutrophils, monocytes at 1 or 2 points in a 20 × microscopic field |
| Grade II | PMN with ballooning hepatocytes, chronic inflammation at 2 to 4 points in a 20 × microscopic field |
| Portal inflamation | |
| No change | No change |
| Grade I | Mild, some portal areas |
| Grade II | Mild to moderate, most portal areas |
| Grade III | Moderate to severe, most portal areas |
All laboratory and histopathologic features of enrolled NASH patients were evaluated and compared between two gender (male vs female) and age (< 55 years as younger vs≥ 55 years as older patients) groups.
The data were analyzed using SPSS v.15 software for Windows (Chicago, USA). Descriptive data were reported using parameters such as frequency, mean, mode and SD. Kolmogorov-Smirnov test was performed to evaluate normal distribution of the quantitative variables. To test the differences between parametric and non-parametric variable means in the two study groups, independent T-test and Mann-Whitney U-test were used.
To compare the differences between the frequency of qualitative variables between study groups Chi square test was performed. Moreover, in order to evaluate the confounding effects of age on the association between histopathologic findings and gender, univariate analysis was used. Receiver operating characteristics (ROC) curve analysis was also performed to assess the predictability of advances in histopathologic findings with patients’ age, and the area under curve (AUC) and the best cutoff value were determined. The appropriate diagnostic values of each cutoff point were reported, including sensitivity and specificity.
A 5% probability of a type I error (two-tailed), and a power of 80% were considered in the analysis. All reported P-values are two-tailed.
A total of 77 NASH patients were enrolled in this study, consisting of 58 males (75.3%) and 19 (24.7%) females with a mean age of 41.99 ± 11.80 years (range, 18-70 years). Baseline characteristics including paraclinical data are listed in Table 2. As shown, the mean serum levels of liver enzymes including ALT, AST and Alk-P were 117.73 ± 237.52 U/L, 58.29 ± 27.77 U/L and 176.48 ± 96.95 U/L, respectively.
| Variable | Total (n = 77) | Sex groups | P-value | Age groups | P-value | ||
| Male (n = 58) | Female (n = 19) | < 55 yr (n = 65) | ≥ 55 yr (n = 12) | ||||
| Age (yr) | 41.99 ± 11.80 | 39.74 ± 10.16 | 48.72 ± 13.99 | 0.004a | - | - | - |
| Gender, n (%) | |||||||
| Female | 19 (24.7) | - | - | - | 14 (21.5) | 5 (41.6) | 0.214 |
| Male | 58 (75.3) | 51 (78.5) | 7 (58.4) | ||||
| BMI (kg/m2) | 28.62 ± 3.42 | 28.86 ± 3.25 | 27.81 ± 4.00 | 0.336 | 28.74 ± 3.55 | 26.87 ± 2.52 | 0.186 |
| Fasting blood sugar (mg/dL) | 107.37 ± 47.60 | 103.16 ± 47.39 | 121.14 ± 47.14 | 0.195 | 107.88 ± 52.06 | 114.12 ± 25.85 | 0.742 |
| Total cholesterol (mg/dL) | 197.29 ± 47.81 | 192.07 ± 45.52 | 212.68 ± 52.26 | 0.105 | 197.07 ± 46.98 | 200.67 ± 37.82 | 0.827 |
| Triglyceride (mg/dL) | 202.26 ± 118.13 | 201.72 ± 97.26 | 203.89 ± 169.65 | 0.947 | 206.40 ± 121.92 | 202.33 ± 100.36 | 0.876 |
| LDL-cholesterol (mg/dL) | 119.09 ± 39.20 | 119.87 ± 35.00 | 117.13 ± 49.64 | 0.822 | 118.27 ± 38.74 | 134.43 ± 40.13 | 0.313 |
| HDL-cholesterol (mg/dL) | 46.61 ± 24.53 | 44.97 ± 25.20 | 50.87 ± 22.97 | 0.301 | 47.86 ± 26.45 | 40.12 ± 13.68 | 0.891 |
| PTT (s) | 12.47 ± 1.50 | 12.39 ± 1.66 | 12.72 ± 0.86 | 0.846 | 12.45 ± 1.58 | 12.40 ± 1.52 | 0.842 |
| Platelet count (/L) | 358 505 ± 48 852 | 357 505 ± 52 403 | 361 125 ± 39 954 | 0.122 | 395 325 ± 55 385 | 237 385 ± 63 753 | 0.964 |
| ALT (U/L) | 117.73 ± 237.52 | 92.05 ± 60.72 | 196.11 ± 466.82 | 0.943 | 123.21 ± 260.49 | 100.44 ± 91.57 | 0.772 |
| AST (U/L) | 58.29 ± 27.77 | 52.78 ± 25.00 | 75.11 ± 29.68 | 0.002a | 57.28 ± 28.17 | 57.89 ± 20.52 | 0.951 |
| Alk-P (U/L) | 176.48 ± 96.95 | 171.14 ± 92.19 | 192.21 ± 111.00 | 0.417 | 176.11 ± 94.50 | 183.44 ± 128.40 | 0.837 |
| γ-GTP (U/L) | 29.55 ± 28.40 | 28.69 ± 30.95 | 31.42 ± 24.47 | 0.852 | 28.74 ± 3.55 | 15.17 ± 3.76 | 0.333 |
As mentioned before, all patients underwent liver biopsy and the results are shown in Table 3. The most common grade of hepatosteatosis was grade I in 36 patients (46.8%) and liver cirrhosis was detected in 5 patients (6.7%).
| Finding | Total (n = 77) | Sex groups | P-value | Age groups | P-value | ||
| Male (n = 58) | Female (n = 19) | < 55 yr (n = 65) | ≥ 55 yr (n = 12) | ||||
| Hepatosteatosis | |||||||
| Grade I | 36 (46.8) | 30 (51.7) | 6 (31.6) | 34 (54) | 1 (11.2) | ||
| Grade II | 29 (37.7) | 22 (37.9) | 7 (36.8) | 0.068 | 23 (36.5) | 4 (44.4) | 0.007a |
| Grade III | 12 (15.6) | 6 (10.3) | 6 (31.6) | 6 (9.5) | 4 (44.4) | ||
| Mean grade | 1.69 ± 0.73 | 1.59 ± 0.68 | 2.00 ± 0.82 | 0.031a | 1.56 ± 0.67 | 2.33 ± 0.71 | 0.002a |
| Fibrosis | |||||||
| No fibrosis | 31 (41.3) | 24 (42.1) | 7 (38.9) | 27 (44.3) | 2 (22.2) | ||
| Grade I | 29 (38.7) | 24 (42.1) | 5 (27.8) | 22 (36.1) | 4 (44.4) | ||
| Grade II | 7 (9.3) | 4 (7) | 3 (16.7) | 0.563 | 6 (9.8) | 1 (11.1) | 0.307 |
| Grade III | 3 (4) | 2 (3.5) | 1 (5.6) | 3 (4.9) | - | ||
| Cirrhosis | 5 (6.7) | 3 (5.3) | 2 (11.1) | 3 (4.9) | 2 (22.2) | ||
| Mean grade | 0.96 ± 1.13 | 0.88 ± 1.05 | 1.22 ± 1.35 | 0.263 | 0.91 ± 1.09 | 1.56 ± 1.51 | 0.115 |
| Hepatocyte ballooning | |||||||
| No ballooning | 52 (72.2) | 41 (74.5) | 11 (64.7) | 42 (71.2) | 6 (75) | ||
| Grade I | 12 (16.7) | 8 (14.5) | 4 (23.5) | 0.224 | 11 (18.6) | 1 (12.5) | 0.935 |
| Grade II | 7 (9.7) | 6 (10.9) | 1 (5.9) | 5 (8.5) | 1 (12.5) | ||
| Grade III | 1 (1.4) | - | 1 (5.9) | 1 (1.7) | - | ||
| Mean grade | 0.40 ± 0.72 | 0.36 ± 0.68 | 0.53 ± 0.87 | 0.448 | 0.41 ± 0.72 | 0.37 ± 0.74 | 0.864 |
| Lobar inflammation | |||||||
| No change | 25 (36.8) | 23 (42.6) | 2 (14.3) | 18 (31.6) | 6 (100) | ||
| Grade I | 37 (54.4) | 27 (50) | 10 (71.4) | 0.138 | 34 (59.6) | - | 0.005a |
| Grade II | 6 (8.8) | 4 (7.4) | 2 (14.3) | 5 (8.8) | - | ||
| Mean grade | 0.72 ± 0.62 | 0.65 ± 0.62 | 1.00 ± 0.55 | 0.052 | 0.77 ± 0.60 | - | 0.004a |
| Portal inflammation | |||||||
| No change | 41 (55.3) | 33 (58.9) | 8 (44.4) | 35 (58.3) | 3 (33.3) | ||
| Grade I | 25 (33.8) | 19 (33.9) | 6 (33.3) | 19 (31.7) | 5 (55.6) | 0.045a | |
| Grade II | 6 (8.1) | 3 (5.4) | 3 (16.7) | 0.192 | 5 (8.3) | - | |
| Grade III | 2 (2.8) | 1 (1.8) | 1 (5.6) | 1 (1.7) | 1 (11.1) | ||
| Mean grade | 0.59 ± 0.81 | 0.52 ± 0.76 | 0.83 ± 0.92 | 0.163 | 0.55 ± 0.79 | 0.89 ± 0.93 | 0.200 |
Baseline and clinical characteristics of NASH patients are listed in Table 2. As shown, the mean age of female patients was significantly higher than male patients (48.72 ± 13.99 years vs 39.74 ± 10.16 years, P = 0.004). In addition, the mean serum level of AST was also significantly higher in females (75.11 ± 29.68 U/L vs 52.78 ± 25.00 U/L, P = 0.002). Other baseline and clinical characteristics were not significantly different between the two gender groups.
Different histopathologic findings of the patients are presented in Table 3. The most common grade of hepatosteatosis in males was grade I in 51.7% (30/58); whereas, the commonest grade in females was grade II with a prevalence of 36.8% (7/19). Moreover, the mean quantitative grade of hepatosteatosis was significantly higher in females (2.00 ± 0.82 vs 1.59 ± 0.68, P = 0.031). Advanced fibrosis (consisting of grade III and cirrhosis) was reported in 8.8% (5/58) and 16.7% (3/19) of males and females, respectively. The mean grade of fibrosis was also higher in females (1.22 ± 1.35 vs 0.88 ± 1.05). However, these differences were not statistically significant (P = 0.563 and 0.263). As shown in Table 3, other histopathologic findings were not statistically different between the two gender groups of the NASH patients.
Based on the data in Table 2, the mean serum level of ALT in patients aged < 55 years and ≥ 55 years was 123.21 ± 260.49 U/L and 100.44 ± 91.57 U/L, respectively, which showed no significant difference (P = 0.772). Similarly, the mean serum level of AST was not statistically different between the two age groups of patients (57.28 ± 28.17 U/L vs 57.89 ± 20.52 U/L, P = 0.951). Other baseline and clinical characteristics were not significantly different between the two age groups.
Table 3 shows the histopathologic findings of NASH patients regarding different age groups. While 54% (34/65) of younger patients had mild hepatosteatosis (Grade I), only one patient (11.2%) in the older group had grade I hepatosteatosis. On the other hand, the older patients had suffered significantly more severe hepatosteatosis (Grade III) (44.4% vs 9.5%, P = 0.007). Moreover, the mean quantitative grade of hepatosteatosis was significantly higher among patients with an age of ≥ 55 years (2.33 ± 0.71 vs 1.56 ± 0.67, P = 0.002). Although the prevalence of any grade of fibrosis was also higher in older patients, this difference was not statistically significant (77.8% vs 54.7%, P = 0.307). By contrast, lobar and portal inflammation were reported significantly more often in histopathologic findings of younger NASH patients (P = 0.005 and 0.045, Table 3).
More detailed analysis was performed to evaluate the best cutoff points of patients’ age to predict advances in histopathologic findings. First, the results of ROC curve analysis showed that the values of patients’ age could significantly differentiate NASH patients with or without advanced fibrosis (AUC = 0.734, P = 0.032). Additionally, the cutoff value of 50 years had 62.5% sensitivity and 78.1% specificity to predict advanced fibrosis; whereas, the cutoff point of 45.5 years had a sensitivity of 75% and specificity of 69.7%.
According to the above, female patients were significantly older than males (P = 0.004); therefore, multivariate analysis was performed to evaluate the confounding role of age in the association between gender and histopathologic findings (especially the mean grade of hepatosteatosis). The results emphasized the higher grade of hepatosteatosis in female patients even when considering patients’ age as a fixed covariate between two genders (P = 0.010).
After omitting the patients aged > 50 years, the mean age of two gender groups were matched (36.87 ± 11.11 years in females vs 36.40 ± 7.25 years in males); and again the mean grade of hepatosteatosis was higher in female NASH patients (1.87 ± 0.83 vs 1.49 ± 0.62).
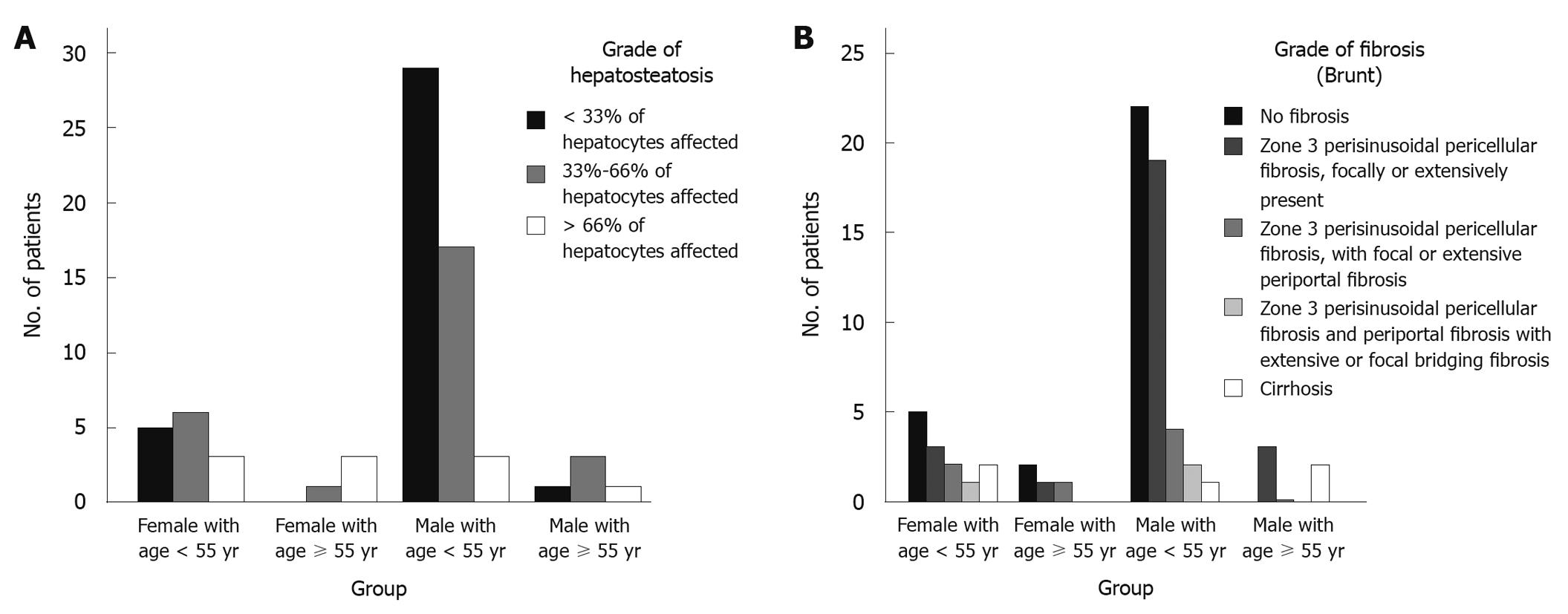
Furthermore, when both age and sex were considered (by dividing the patients into four groups), the more severe hepatosteatosis were reported in female patients aged ≥ 55 years (Figure 1A) while the mildest histopathologic findings were found in younger males (Figure 1A and B).
It has been suggested that some patients with NASH may manifest a benign course where others follow a more aggressive course which results in cirrhosis and final liver failure. The basis for this unusual course in patients suffering from the identical situation proposes that the disease might have a benign and non-aggressive course in some patients whereas, in patients with more aggressiveness some factors may contribute and aggravate the consequent liver damage and fibrosis which can finally result in cirrhosis. For the last two decades, several studies have considered different issues as possible risk factors for NASH such as hyperlipidemia, hypertension and insulin resistance. Unlike these studies, we did not focus on metabolic syndrome because these risk factors have been studied in many previous reports[19,20] but we have tried to demonstrate some of the factors which are believed to play a role in disease severity and pathological findings in Iranian patients suffering from NASH. However, it is noted that the prevalence of the known risk factors of metabolic disorders were not significantly different between the two age and gender groups in our study (Table 2).
This study has revealed some important points which need to be discussed further. First, the severity of hepatosteatosis and portal inflammation increases with age. Second, female patients being affected are older. Third, hepatosteatosis is more severe in female patients even after age-adjustment and finally, female patients have higher AST levels.
Early studies had considered female gender as a risk factor for NASH but this was not identified by recent studies[9,21]. Angulo et al[22] reported that there is a trend toward higher levels of fibrosis in female patients with NASH but in another study on children suffering from NASH such a relationship was not reported[23]. Ratziu et al[24] studying the clinical and pathological aspects of NASH in Japanese patients concluded that older patients have more severe fibrosis compared to younger adults where the older group consisted of significantly more females. According to these findings they considered female gender as a possible risk factor for NASH severity but according to multivariate analysis on both age groups, gender had no role in the severity of the liver involvement. Likewise, level of fibrosis was higher in female patients in this study, but there was not a significant difference among different genders. The only significant difference considering age and pathological findings was the level of hepatosteatosis. In contrast to the study of Yatsuji et al[25], our findings highlighted the significantly higher grade of hepatosteatosis in female patients despite the elimination of the confounding role of patients’ age in this association.
Patients aged ≥ 55 years of age had significantly higher levels of hepatosteatosis compared to younger patients. Although the level of fibrosis was not significantly different, older patients had considerably more severe portal inflammation and less lobar inflammation. In addition, results of ROC curve analysis showed that the cutoff values of 50 or 45.5 years for patients’ age could significantly differentiate NASH patients with or without advanced fibrosis.
Previous studies suggested a number of factors associated with more severe and progressive liver fibrosis. In one study focusing on obese patients, age ≥ 50 years [odds ratio (OR) 14.1], a body mass index ≥ 28 kg/m2 (OR 5.7), triglycerides ≥ 1.7 mmol/L (OR 5), and an ALT concentration ≥ 2 × normal (OR 4.6) were associated with more severe fibrosis[24]. In another study on 733 patients, the authors presented a model consisting of age, body mass index, platelet count, albumin, and AST/ALT ratio which had a good predictive value for advanced fibrosis[26].
As mentioned before, in a similar study on Japanese patients, the older patients had much more advanced fibrosis and worse deterioration of liver function compared to the younger group[25]. Age related mitochondrial dysfunction has been suggested recently as a contributing factor in developing insulin resistance[27]. Pathophysiological considerations, laboratory investigations and clinical association studies have supported the central role of mitochondrial dysfunction in developing insulin resistance and subsequent liver disease[20,28-30].
More recently, a systematic review was performed for assessment of factors associated with fibrosis progression in NASH patients by Argo et al[31]. They involved patients with one initial liver biopsy and one or more biopsies during the course of disease and revealed that age and initial level of fibrosis are the only predictors of fibrosis progression. Compared to their findings our results also showed that advanced age contributed to higher grades of hepatosteatosis and portal inflammation but such a relationship was not found with gender. However, the most severe grade of hepatosteatosis was found in older female patients.
Unfortunately previous studies comparing different components of Brunt classification of liver pathology according to independent risk factors are scarce and more precise studies with a greater study population are needed to correctly evaluate the relationship between these factors and liver pathology.
In conclusion, our data points toward the possible influence of age in the severity of steatohepatitis, portal and lobar inflammation in patients suffering from NASH and also indicates that gender independently contributed to the level of steatohepatitis. Moreover, according to the lower cutoff values of 50 or 45.5 years of age for advanced fibrosis in Iranian patients, it might be possible to consider progression toward cirrhosis earlier (before 55 years) in an Iranian population.
Prevalence of obesity, metabolic syndrome and non-alcoholic steatohepatitis (NASH) has increased recently in a parallel manner. NASH has been considered as a possible part of metabolic syndrome. Insulin resistance is the basis of metabolic syndrome pathogenesis. Hyperinsulinemia and insulin resistance occurs with aging and reduced physical activity. Additionally, due to sex hormones the prevalence of metabolic syndrome is different between genders.
NASH is an important clinicopathological entity which is usually distributed among obese patients who show considerable insulin resistance. This study has investigated the possible difference in pathological findings of patients suffering from NASH according to age and gender.
These findings point toward the possible influence of age in the severity of steatohepatitis, portal and lobar inflammation in patients suffering from NASH while gender independently might contribute to the level of steatohepatitis. Gender might contribute to the level of steatohepatitis. Severity of steatohepatitis, portal and lobar inflammation is higher in older patients suffering from NASH.
Physicians should be more cautious while treating older and female patients as these groups might have more severe pathologic findings in their liver biopsies.
This manuscript is interesting. The number of patients included in the study is small and may underestimate the frequency of NASH.
Peer reviewer: Michel M Murr, MD, Professor of Surgery, USF Health, Director of Bariatric Surgery, Tampa General Hospital, 1 Tampa General Circle, Tampa, FL 33647, United States
S- Editor Wang JL L- Editor O'Neill M E- Editor Lin YP
| 1. | Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042-2047. |
| 2. | Rivera CA. Risk factors and mechanisms of non-alcoholic steatohepatitis. Pathophysiology. 2008;15:109-114. |
| 3. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. |
| 4. | Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056-1062. |
| 5. | Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538-1544. |
| 6. | Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20:594-598. |
| 7. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. |
| 8. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. |
| 9. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. |
| 10. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. |
| 11. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. |
| 12. | Pinto HC, Baptista A, Camilo ME, Valente A, Saragoça A, de Moura MC. Nonalcoholic steatohepatitis. Clinicopathological comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci. 1996;41:172-179. |
| 13. | Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106-1110. |
| 14. | Charlton M. Cirrhosis and liver failure in nonalcoholic fatty liver disease: Molehill or mountain? Hepatology. 2008;47:1431-1433. |
| 15. | Nonomura A, Mizukami Y, Unoura M, Kobayashi K, Takeda Y, Takeda R. Clinicopathologic study of alcohol-like liver disease in non-alcoholics; non-alcoholic steatohepatitis and fibrosis. Gastroenterol Jpn. 1992;27:521-528. |
| 16. | Bechtold M, Palmer J, Valtos J, Iasiello C, Sowers J. Metabolic syndrome in the elderly. Curr Diab Rep. 2006;6:64-71. |
| 17. | Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, Shimomura I, Tarui S, Matsuzawa Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207-212. |
| 18. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. |
| 19. | Marchesini G, Forlani G. NASH: from liver diseases to metabolic disorders and back to clinical hepatology. Hepatology. 2002;35:497-499. |
| 20. | Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367-372. |
| 21. | Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99-S112. |
| 22. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. |
| 23. | Ko JS, Yoon JM, Yang HR, Myung JK, Kim HR, Kang GH, Cheon JE, Seo JK. Clinical and histological features of nonalcoholic fatty liver disease in children. Dig Dis Sci. 2009;54:2225-2230. |
| 24. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. |
| 25. | Yatsuji S, Hashimoto E, Tobari M, Tokushige K, Shiratori K. Influence of age and gender in Japanese patients with non-alcoholic steatohepatitis. Hepatol Res. 2007;37:1034-1043. |
| 26. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. |
| 27. | Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140-1142. |
| 28. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. |
| 29. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. |
| 30. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. |