Published online Aug 28, 2010. doi: 10.3748/wjg.v16.i32.4100
Revised: June 14, 2010
Accepted: June 21, 2010
Published online: August 28, 2010
AIM: To investigate the effects and mechanism of disruption of focal adhesion kinase (FAK) expression on collagen metabolism in rat hepatic stellate cells (HSC).
METHODS: The plasmids expressing FAK short hairpin RNA (shRNA) were transfected into HSC-T6 cells, and the level of FAK expression was determined by both real-time quantitative polymerase chain reaction (Q-PCR) and Western blotting analysis. The production of type I collagen and type III collagen in FAK-disrupted cells was analyzed by real-time Q-PCR. The level of collagen metabolism proteins, including matrix metalloproteinases-13 (MMP-13) and tissue inhibitors of metalloproteinases-1 (TIMP-1) was also determined by both real-time Q-PCR and Western blotting analysis.
RESULTS: The transfection of FAK shRNA plasmids into HSC resulted in disrupted FAK expression. Compared with the HK group, the levels of type I collagen and type III collagen mRNA transcripts in FAK shRNA plasmid group were significantly decreased (0.69 ± 0.03 vs 1.96 ± 0.15, P = 0.000; 0.59 ± 0.07 vs 1.62 ± 0.12, P = 0.020). The production of TIMP-1 in this cell type was also significantly reduced at both mRNA and protein levels (0.49 ± 0.02 vs 1.72 ± 0.10, P = 0.005; 0.76 ± 0.08 vs 2.31 ± 0.24, P = 0.000). However, the expression of MMP-13 mRNA could be significantly up-regulated by the transfection of FAK shRNA plasmids into HSC (1.74 ± 0.20 vs 1.09 ± 0.09, P = 0.000).
CONCLUSION: These data support the hypothesis that shRNA-mediated disruption of FAK expression could attenuate extracellular matrix (ECM) synthesis and promote ECM degradation, making FAK a potential target for novel anti-fibrosis therapies.
-
Citation: Dun ZN, Zhang XL, An JY, Zheng LB, Barrett R, Xie SR. Specific shRNA targeting of
FAK influenced collagen metabolism in rat hepatic stellate cells. World J Gastroenterol 2010; 16(32): 4100-4106 - URL: https://www.wjgnet.com/1007-9327/full/v16/i32/4100.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i32.4100
Liver fibrosis results from excessive deposition of extracellular matrix (ECM) components[1]. These components, which are mainly composed of type I collagen and type III collagen, are produced by hepatic stellate cells (HSC). The activation, proliferation and migration of HSC play a central role in liver fibrogenesis[2,3]. Activated HSCs are the main producers of collagens and matrix metalloproteinases (MMPs) in the fibrotic liver. The MMP which is able to degrade type I collagen and type III collagen is MMP-13. However, this can be specifically inhibited by tissue inhibitors of metalloproteinases-1 (TIMP-1), and its level is found to be high in the fibrotic liver of rats.
Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase, whose phosphorylation can promote the proliferation and collagen synthesis of HSC[4-8]. It had previously been shown that when FAK related non-kinase (FRNK) plasmids were transfected into fibronectin (FN)-stimulated HSC using liposome transfection, the over-expression of FRNK significantly decreased the collagen synthesis of HSC in vitro[9,10]. This led us to speculate that suppression of FAK expression may provide a new target in the treatment of liver fibrosis.
To date, RNA interference has been the most effective gene silencing technology. It can specifically inhibit the transcription of target genes, and in turn reduce the expression and function of the corresponding proteins[11]. We aim to inhibit FAK expression by transfecting FAK short hairpin RNA (shRNA) plasmids into HSC. To our knowledge, this is the first report that FAK expression is specifically inhibited in HSC cells. This allows us to further analyze the role of FAK in collagen synthesis and degradation in this cell type, and find out how FAK regulates the expression of MMP-13 and TIMP-1.
The shRNA-expressing plasmids, pEGFP-FAK shRNA, were purchased from Wuhan Genesil Biotechnology Co. Ltd. (Wuhan, China). One additional plasmid, p-EGFP-HK, was used to express nonsense shRNA and served as the control. Sofast™ Transfection Reagent was purchased from Xiamen Sunma Biological Engineering Co. Ltd. (Xiamen, China).
The cell line HSC-T6, which is the phenotypically activated HSC, was donated by Professor Xu LM, from Hepatopathy Institute of Shanghai University of Traditional Chinese Medicine. HSCs were cultured in HG-DMEM medium supplemented with 8% FBS, 100 IU/mL penicillin, 100 g/mL streptomycin, 4 mmol/L glutamine and 1 mol/L HEPES. Cells were cultured in a 5% CO2 humidified incubator at 37°C. All experiments were conducted when cells were at an exponential stage of growth. Cells were seeded into a 25 cm2 plastic culture flask with a total of 2-3 × 105 cells or were seeded in 96-well plates to a density of 3 × 104/mL × 200 μL/well. When cells were approximately 70%-80% confluent, shRNA plasmid was transfected into FN-stimulated HSC using a cationic polymer. The cells were divided into five groups: (1) blank control group (control); (2) FN stimulation group (FN); (3) transfection reagent group (Sofast); (4) pEGFP-HK shRNA group (HK); and (5) pEGFP-FAK shRNA group (FAK shRNA). FN was added to groups 2-5 at a concentration of 10 mg/L.
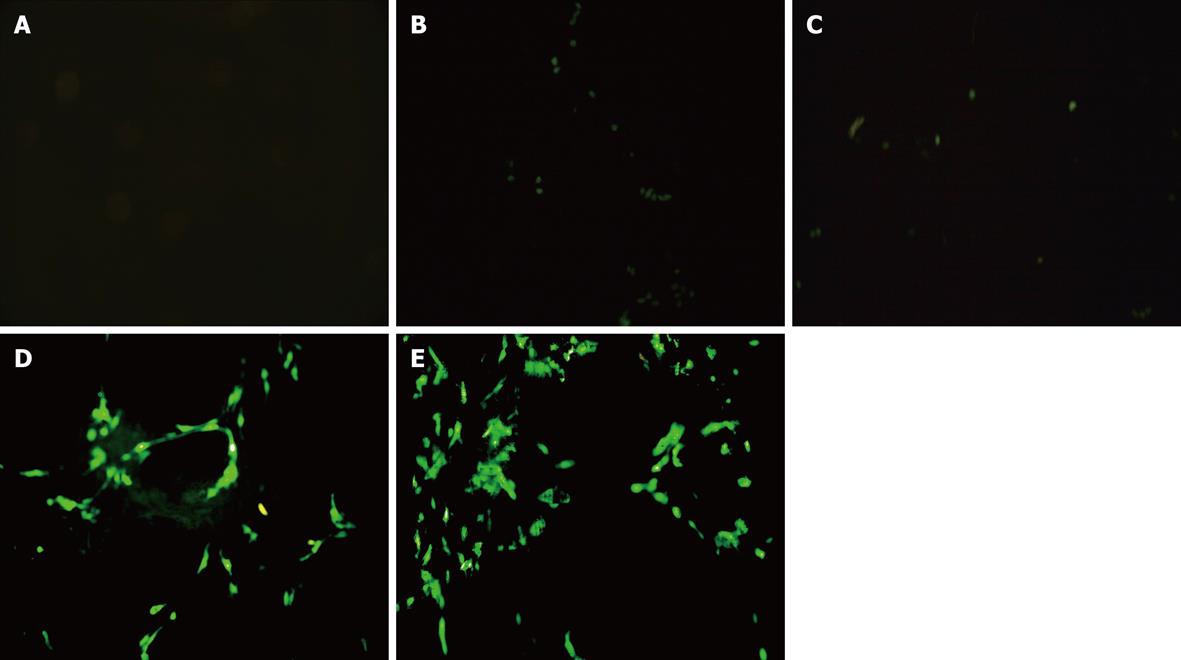
At 48 h after transfection, the cells were analyzed by fluorescence microscopy and flow cytometry (FCM) to obtain the efficiency of transfection.
The expressions of the gene FAK, type I collagen and type III collagen, MMP-13 and TIMP-1 were characterized by semiquantitative real-time quantitative polymerase chain reaction (Q-PCR). Briefly, total RNA was extracted from the cells that had been transfected with the plasmid expressing the FAK or HK shRNA and reversely transcripted into cDNA, which was used as the template for PCR. Using the primer design software, Primer Express 2.0, the specific primers for each gene were synthesized by Beijing Saibaisheng Gene Technique Co., Ltd. and the following primers were generated: FAK-Forward 5′-ACTTGGACGCTGTATTGGAG-3′, FAK-Reverse 5′-CTGTTGCCTGTCTTCTGGAT-3′ (833 bp amplicon); Collagen type I-Forward 5′-TACAGCACGCTTGTGGATG-3′, Collagen type I-Reverse 5′-TTGAGTTTGGGTTGTTGGTC-3′ (256 bp amplicon); Collagen type III-Forward 5′-ATGGTGGCTTTCAGTTCACC-3′, Collagen type III-Reverse 5′-TGGGGTTTCAGAGAGTTTGG-3′ (425 bp amplicon); MMP-13-Forward 5′-GCGGGAATCCTGAAGAAGTCTAC-3′, MMP-13-Reverse 5′-TTGGTCCAGGAGGAAAAGCG-3′ (424 bp amplicon); TIMP-1-Forward 5′-TCCCCAGAAATCATCGACAC-3′, TIMP-1-Reverse 5′-ATCGCTGAACAGGGAAACAC-3′ (329 bp amplicon); GAPDH-Forward 5′-GAGGACCAGGTTGTCTCCTG-3′, GAPDH-Reverse 5′-GGATGGAATTGTGAGGGAGA-3′ (298 bp amplicon). Reaction system: 10 μL 2.5 × real master Mix, 1.25 μL 20 × SYBR solution, 0.5 μL upstream primer, 0.5 μL downstream primer and 2 μL DNA template were brought up to 25 μL with purified water. Reaction conditions: 93°C 5 min, 1 cycle; 93°C 45 s, 55°C 1 min, 10 cycles; 93°C 30 s, 55°C 45 s, 30 cycles. The PCR reactions were subjected to 93°C for 5 min, 1 cycle; and then 10 cycles of 93°C 45 s, 55°C 1 min, and 30 cycles of 93°C 30 s, 55°C 45 s. The size and quantity of amplified products were confirmed by 2% agarose gel electrophoresis. Fluorescent quantitative analysis was performed with the thermal cycler’s software package to calculate the ΔCt value. The expression levels of FAK, type I collagen and type III collagen, MMP-13 and TIMP-1 were calculated by the 2-ΔΔCt analysis. The 2-ΔΔCt was presented as the relative expression of the gene expression[12].
At 24 or 48 h after transfection of FAK shRNA, HSCs were harvested, washed with phosphate-buffered saline (PBS), and lysed in the improved RIPA buffer (50 mmol/L Tris-HCL, pH 7.5; 100 mmol/L NaCl; 1% NP-40; 0.5% sodium deoxycholate; 2 μg/mL leupeptin; 1% SDS; 2 mmol/L EDTA; 1 mmol/L PMSF; 50 mmol/L HEPES; 1 mmol/L sodium orthovanadate). The supernatant was collected and the protein concentration was determined using comassie brilliant blue assay. Cell extracts containing equal quantities of proteins (100-110 μg) were electrophoresed in 8% or 10% polyacrylamide gel. Subsequently, the separated proteins were transferred to nitrocellulose membrane. The membrane was blocked for non-specific binding for 30 min (5% skimmed milk in PBS), and then incubated overnight at 4°C with rabbit anti-FAK polyclonal antibody (1:400), rabbit anti-MMP-13 polyclonal antibody (1:200), rabbit anti-TIMP-1 polyclonal antibody (1:200) or mouse anti–GAPDH monoclonal antibody (1:100). The membrane was subsequently incubated at room temperature for 2 h with goat anti-rabbit IgG (1:2000). Blots were developed with enhanced chemiluminescence detection reagents (Santa Cruz Biotechnology Inc.), exposed on Kodak Xdmat blue XB-1 film and quantified by Bandscan 5.0 software using GAPDH as internal control. Densitometry is reported using the integral optical density value (IOD). The results were represented in the form of IOD ratio of the target protein to GAPDH.
All the data were expressed by mean ± SD and analyzed with SPSS 13.0 software. The comparison of mean variability among all groups was conducted by one-way ANOVA analysis and two group comparison with LSD test. Student’s t test was carried out for independent samples. Statistical significance was considered at P < 0.05.
FAK shRNA plasmids were successfully transfected into HSC. The results from fluorescence microscopy and FCM showed that the transfection efficiency was 40% at 48 h (Figure 1). The levels of FAK mRNA transcripts and protein expression were determined by real-time Q-PCR and Western blotting analysis. The expression of FAK mRNA and FAK protein in the FN group was significantly higher than that of the control group, P = 0.000 and P = 0.024, respectively. There was no difference between the FN group, Sofast group and HK group. In comparison with the HK group, the expression of FAK mRNA and FAK protein in the FAK shRNA plasmid group was significantly decreased (0.37 ± 0.03 vs 1.59 ± 0.06, P = 0.000; 0.77 ± 0.03 vs 2.24 ± 0.20, P = 0.000), and the rates of down-regulation were 70.51% and 72.53%, respectively.
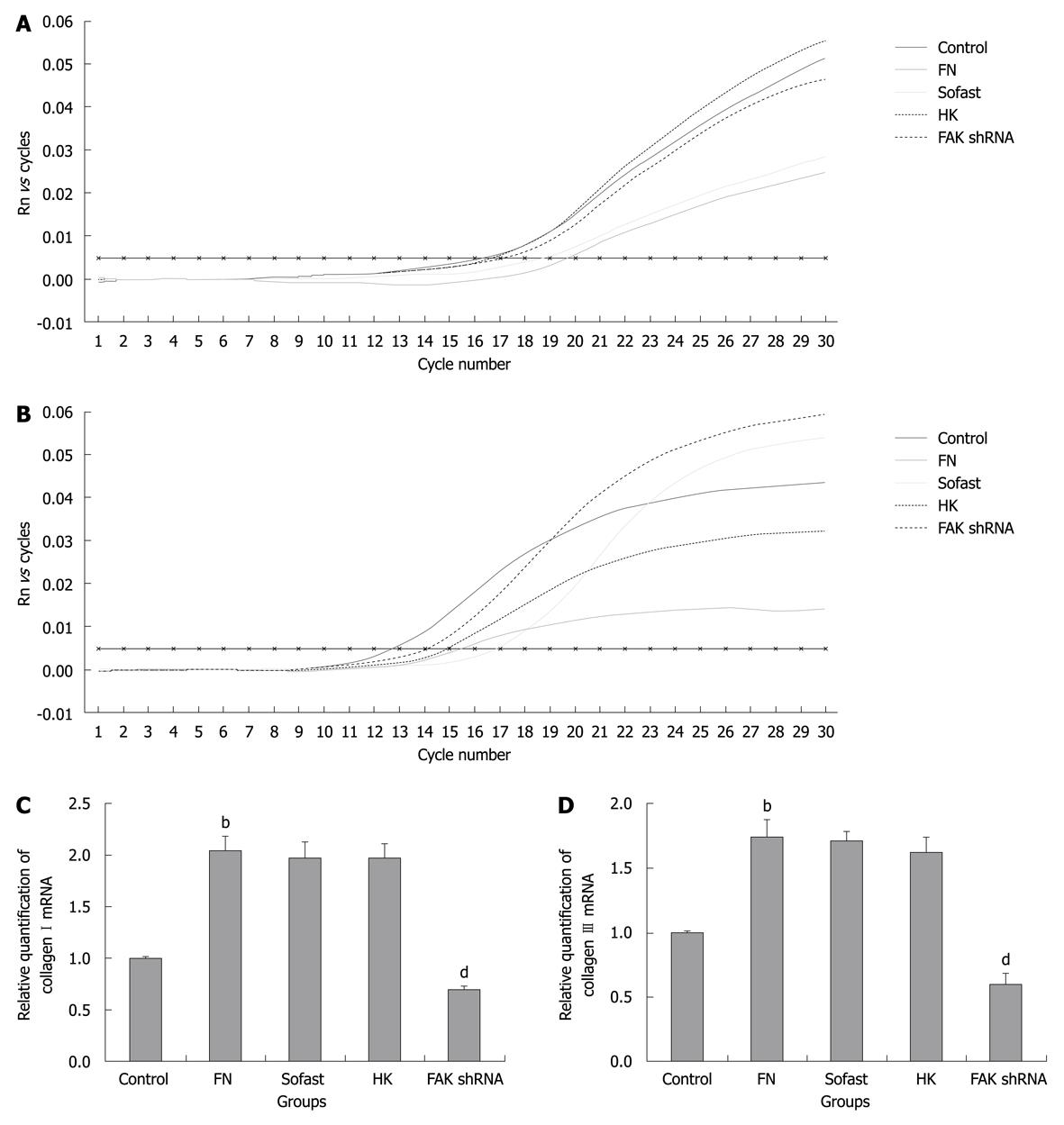
Investigation was carried out in the influence of disruption of FAK expression mediated by FAK shRNA on ECM synthesis in HSC. The levels of type I collagen and type III collagen mRNA transcripts were determined by real-time Q-PCR. The levels of type I collagen and type III collagen mRNA transcripts in FN group were significantly higher than that of the control group. The levels of type I collagen and type III collagen mRNA transcripts in FAK shRNA plasmid group were significantly decreased compared with the HK group (0.69 ± 0.03 vs 1.96 ± 0.15, P = 0.000; 0.59 ± 0.07 vs 1.62 ± 0.12, P = 0.020) and the down-regulated rates were 64.80% and 63.58%, respectively (Figure 2).
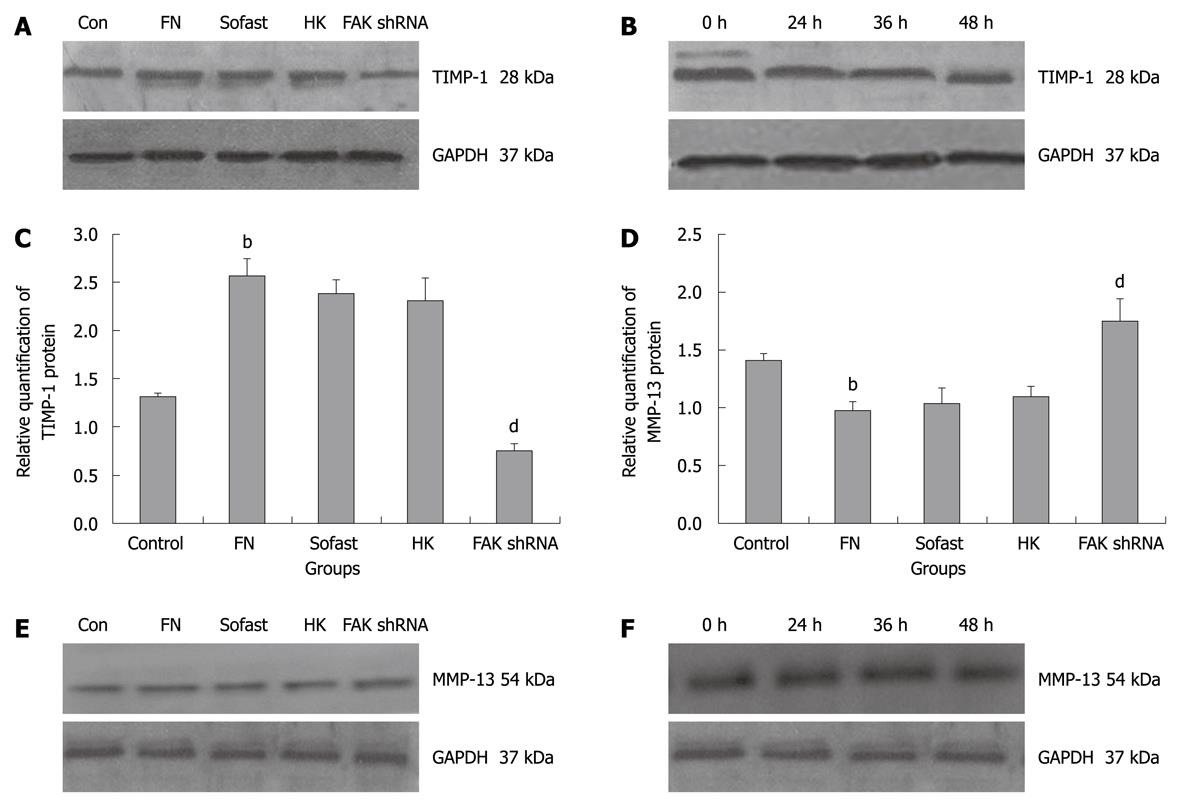
To further explore the effects of FAK shRNA on the ECM degradation in HSC, the levels of MMP-13 and TIMP-1 were determined by real-time Q-PCR and Western blotting analysis. The transfection of HK shRNA did not modulate the levels of MMP-13 and TIMP-1, the cells expressing HK shRNA were similar to that in FN group and Sofast group, P > 0.05. However, the knockdown of FAK expression by the FAK shRNA significantly reduced the levels of TIMP-1 mRNA and TIMP-1 protein (0.49 ± 0.02 vs 1.72 ± 0.10, P = 0.005; 0.76 ± 0.08 vs 2.31 ± 0.24, P = 0.000), and the down-regulated rates were 69.78% and 67.10%, respectively (Figure 3A-C). The results of real-time Q-PCR and Western blotting analysis showed that the levels of MMP-13 of FN group were significantly down-regulated compared with that of control group. Compared with the HK group, the expression of MMP-13 mRNA was significantly up-regulated by 56.96% at 36 h after transfection of FAK shRNA plasmids into HSC (1.24 ± 0.04 vs 0.79 ± 0.03, P = 0.020), and the expression of MMP-13 protein could be increased by 59.63% at 48 h after transfection (1.74 ± 0.20 vs 1.09 ± 0.09, P = 0.000) (Figure 3D-F).
The current knowledge on the pathophysiology of liver fibrogenesis refers to the increased synthesis and decreased degradation of ECM, mainly type I collagen and type III collagen, thereby ECM was overproduced and deposited in the liver. Although several hepatic cell types can synthesize ECM proteins, HSCs are the major source of increased ECM in chronic liver diseases. They can undergo a proliferative and phenotypic change. Excessive deposition of ECM, mainly type I collagen and type III collagen, results in liver fibrosis; and the up-regulation of TIMPs blocks activity of MMPs and inhibits the degradation of ECM, thereby aggravating liver fibrosis.
The interaction of HSC and ECM mainly lies between integrins, and FAK plays an integral role in the integrin signal pathway. Activated FAK has been implicated in a diverse array of cellular behaviors, such as cell proliferation[4,5], apoptosis, cell migration[6], collagen metabolism[7,8] and the transfer of tumor cells. It is closely related to numerous fibrotic diseases and it plays a vital role in the occurrence and development of liver fibrosis[13]. This is consistent with our previous studies, which indicated that FAK phosphorylation could promote collagen synthesis of HSC in vivo. Furthermore, using in vitro cell culture techniques, we found that the synthesis of total collagen and type I collagen in HSC could be inhibited by the endogenous inhibitor FRNK[9]. We hypothesized that FAK gene silencing may represent a novel method for the treatment and reversal of liver fibrosis. Therefore, in this study, FAK shRNA plasmids were transfected into HSC transiently to test our hypothesis, and the expressions of FAK mRNA and FAK protein were significantly decreased, the down-regulation rates being 70.51% and 72.53%, respectively. We have found that FAK shRNA can effectively and specially suppress the expression of FAK.
A substantial change in liver fibrosis or liver cirrhosis is the deposition of ECM, which is mainly composed of type I collagen and type III collagen, covering approximately 80%-90% of the increased total collagen. The increase of type I collagen and type III collagen is an important symbol of liver fibrosis or liver cirrhosis. Therefore, in this study, FAK shRNA plasmids were transfected into FN-stimulated HSC transiently and the expression of type I collagen mRNA and type III collagen mRNA was significantly down-regulated by 64.80% and 63.58%, respectively. These data show that FAK shRNA can effectively suppress the synthesis of collagen and FAK gene silencing may, therefore, represent a novel direction for the treatment and reversal of liver fibrosis.
Furthermore, we attempted to assess the role of FAK in the regulation of collagen metabolism in HSC. In the liver, ECM is regulated by MMPs and their specific inhibitors, TIMPs. A principal feature of hepatic fibrosis is a disturbance in the balance between MMPs and TIMPs. Collagenases such as MMP-1 and MMP-13 are able to degrade fibrillar collagens, mainly type I, II and III collagen. These may be responsible for key events in the degradation of ECM. MMP-13 is the interstitial collagenase in rats and its specific inhibitor is TIMP-1. Although the expression of MMP-13 was increased in the liver tissues of CCl4-induced rat liver fibrosis models, fibrosis still occurred as there was also a corresponding increase in the expression of TIMP-1[14]. This strongly suggests that a disruption in the balance between MMP-13 and TIMP-1 is possibly an important factor in liver fibrogenesis[15]. According to some studies, FAK is closely related to the expression of TIMP-1 and MMP-13[16,17]. In this study, FAK shRNA plasmids were transfected into FN-stimulated HSC transiently and the expression of MMP-13 mRNA and MMP-13 protein was significantly up-regulated by 56.96% and 59.63%. Correspondingly, the levels of TIMP-1 mRNA and TIMP-1 protein were significantly down-regulated by 69.78% and 67.10%, respectively. FAK shRNA inhibited the ratio of TIMP-1/MMP-13 expression in mRNA and protein levels in HSC after transfection. The data indicate that FAK shRNA regulated the collagen metabolism in HSC by disturbing the balance between MMP-13 and TIMP-1.
In summary, we have effectively disrupted the expression of FAK by FAK shRNA. The knockdown of FAK expression significantly reduced the synthesis of type I collagen and type III collagen, which may be related to the up-regulation of MMP-13 and down-regulation of TIMP-1. These data support the hypothesis that FAK disruption by shRNA may be an efficient and specific approach for treatment of liver fibrosis. Future studies will address the signal transduction pathway by which FAK regulates the collagen metabolism in HSC.
Focal adhesion kinase (FAK) plays an essential role in the activation of hepatic stellate cells (HSCs) which are the major source of collagens and matrix metalloproteinases in the fibrotic liver. Liver fibrosis results from excessive deposition of extracellular matrix components, composed of mainly type I collagen produced by HSC.
The central events in the liver fibrogenesis have been proved to be the activation, proliferation and migration of HSC, and their proliferation and collagen synthesis are promoted by phosphorylation of FAK, a non-receptor protein tyrosine kinase. In the area of knockdown or inhibition of FAK with various molecular biological technologies, an area of intense research is to establish a method to knockdown or inhibit FAK expression thoroughly so as to enhance the collagen metabolism.
Recent reports have highlighted the importance of HSC including activation, proliferation and migration in pathogenesis of liver fibrosis. The collagen metabolism in HSC, particular in activated HSC, is currently an area of intense research. This is the first study to report that shRNA-mediated disruption of FAK expression can attenuate extracellular matrix (ECM) synthesis and promote ECM degradation. This represents a potential target for novel anti-fibrosis therapies.
The results of this study indicated that suppression of FAK expression may represent a novel method and direction for the treatment and reversal of hepatic fibrosis.
The study focuses on modification of hepatic stellate cell metabolism by shRNA mediated inhibition of FAK, a non-receptor protein tyrosine kinase involved in proliferation and collagen synthesis. The authors demonstrate that FAK inhibition is associated with a decrease in collagen synthesis by HSCs.
Peer reviewers: Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany; Atsushi Masamune, MD, PhD, Division of Gastroenterology, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan
S- Editor Wang JL L- Editor Ma JY E- Editor Zheng XM
| 1. | Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120-129. |
| 2. | Kato R, Kamiya S, Ueki M, Yajima H, Ishii T, Nakamura H, Katayama T, Fukai F. The fibronectin-derived antiadhesive peptides suppress the myofibroblastic conversion of rat hepatic stellate cells. Exp Cell Res. 2001;265:54-63. |
| 3. | Tsukada S, Parsons CJ, Rippe RA. Mechanisms of liver fibrosis. Clin Chim Acta. 2006;364:33-60. |
| 4. | Zhang XL, Huo XX, Shen JG, Wei J, Jiang HQ. Focal adhesion kinase tyrosine phosphorylation promotes rat hepatic fibrogenesis and its possible mechanism. Jichu Yixue Yu Linchuang. 2007;27:143-147. |
| 5. | Shen JG, Zhang XL, Huo XX, Jiao QH, Wang HF. FRNK inhibited hepatic stellate cells in vitro. Jichu Yixue Yu Linchuang. 2008;28:1151-1155. |
| 6. | Patsenker E, Popov Y, Wiesner M, Goodman SL, Schuppan D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration and activation in vitro. J Hepatol. 2007;46:878-887. |
| 7. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. |
| 8. | Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, Brenner DA, Rippe RA. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278:8083-8090. |
| 9. | Wei J, Zhang XL, Shen JG, Huo XX, Dun ZN. Inhibitory effects of FRNK on collagen synthesis in hepatic stellate cells. Zhongguo Bingli Shengli Zazhi. 2009;25:144-147. |
| 10. | Zhang XL, Wei J, Huo XX, Shen JG. The regulative effects of FRNK on collagen metabolism in hepatic stellate cells. J Gastroenterol Hepatol. 2007;22:A235. |
| 11. | Yang M, Mattes J. Discovery, biology and therapeutic potential of RNA interference, microRNA and antagomirs. Pharmacol Ther. 2008;117:94-104. |
| 12. | Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol. 2006;15:56-61. |
| 13. | Dun ZN, Zhang XL. [Focal adhesion kinase and fibrosis]. Jichu Yixue Yu Linchuang. 2009;29:106-108. |
| 14. | Wasser S, Ho JM, Ang HK, Tan CE. Salvia miltiorrhiza reduces experimentally-induced hepatic fibrosis in rats. J Hepatol. 1998;29:760-771. |
| 15. | Hsiao Y, Zou T, Ling CC, Hu H, Tao XM, Song HY. Disruption of tissue-type plasminogen activator gene in mice aggravated liver fibrosis. J Gastroenterol Hepatol. 2008;23:e258-e264. |
| 16. | Patsenker E, Popov Y, Wiesner M, Goodman SL, Schuppan D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration and activation in vitro. J Hepatol. 2007;46:878-887. |
| 17. | Tan TW, Yang WH, Lin YT, Hsu SF, Li TM, Kao ST, Chen WC, Fong YC, Tang CH. Cyr61 increases migration and MMP-13 expression via alphavbeta3 integrin, FAK, ERK and AP-1-dependent pathway in human chondrosarcoma cells. Carcinogenesis. 2009;30:258-268. |